Abstract
Females are born with a finite number of primordial follicles. 4-Vinylcyclohexene diepoxide (VCD) is a metabolite formed by epoxidation of 4-vinylcyclohexene (VCH) via its two monoepoxides 1,2- and 7,8-4-vinylcyclohexene monoepoxide (VCM). VCD specifically destroys small preantral (primordial and small primary) follicles in the rodent ovary. The phase I enzyme, cytochrome P450 isoform 2E1 (CYP2E1) is involved in ovarian metabolism of VCM to VCD. Further, microsomal epoxide hydrolase (mEH) can detoxify VCD to an inactive tetrol (4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane). This study evaluated the effects of VCD-induced ovotoxicity on mEH in CYP2E1+/+ and −/− mice (129S1/SvImJ background strain) using a postnatal day 4 mouse whole ovary culture system. The hypothesis of our study is that there is a relationship between CYP2E1 and mEH gene expression in the mouse ovary. Relative to control, VCD exposure caused follicle loss (p < 0.05) in ovaries from both genotypes; however, after 15 days, this loss was greater (p < 0.05) in CYP2E1+/+ ovaries. In a time course (2–15 days), relative to control, VCD (5μM) caused an increase (p < 0.05) in mEH mRNA by 0.5-fold (day 10) and 1.84-fold (day 15) in CYP2E1−/− but not +/+ ovaries. 7,12-Dimethylbenz[a]anthracene (DMBA) also destroys ovarian follicles but, unlike VCD, is bioactivated by mEH to an ovotoxic 3,4-diol-1,2-epoxide metabolite. Incubation of ovaries in increasing concentrations of DMBA (0.5–1μM, 15 days) resulted in greater (p < 0.05) follicle loss in CYP2E1−/−, relative to +/+ ovaries. With greater mEH (CYP2E1−/−), increased follicle loss with DMBA (bioactivation) and decreased follicle loss with VCD (detoxification) support that ovarian expression of CYP2E1 and mEH may be linked.
Keywords: VCD-induced ovotoxicity, cytochrome P450 isoform 2E1, microsomal epoxide hydrolase
The ovary is a heterogeneous organ composed of follicles at various stages of growth. At birth, the ovary contains a finite number of small preantral follicles, which can grow and mature toward ovulation. Primordial follicles cannot be regenerated (Hirshfield, 1991); thus, chemical-induced depletion of this follicle pool can lead to premature ovarian failure. 4-Vinylcyclohexene (VCH) is an occupational chemical formed from the dimerization of 1,3-butadiene as a by-product during the manufacture of pesticides, flame retardants, rubber, and plastics (IARC, 1994). VCH can be metabolized via cytochrome P450 (CYP) enzymes to form monoepoxides (VCM) which can be further metabolized to a diepoxide, 4-vinylcyclohexene diepoxide (VCD). VCD has been shown to be the ultimate ovotoxicant in mice and rats, specifically targeting small preantral (primordial and primary) follicles (Kao et al., 1999; Smith et al., 1990; Springer et al., 1996a,b). In rats, VCD-induced ovotoxicity has been shown to require repeated dosing and to occur via acceleration of atresia (apoptosis; Hu et al., 2001a,b, 2002; Springer et al., 1996a,b).
Much research has focused on how the liver metabolizes chemicals such as VCH and VCD. However, a number of studies have shown the potential of the ovary for xenobiotic metabolism (Cannady et al., 2002, 2003; Rajapaksa et al., 2007a,b). The mouse ovary expresses the phase I enzymes CYP2A, CYP2B, cytochrome P450 isoform 2E1 (CYP2E1), and microsomal epoxide hydrolase (mEH; Cannady et al., 2002, 2003). CYP2E1 has been proposed to be involved in ovarian metabolism of VCH/VCM to the ovotoxic form, VCD (Cannady et al., 2003). Further, the potential involvement of ovarian CYP2E1 in metabolism of VCH and VCM was demonstrated using CYP2E1+/+ and −/− mice (Rajapaksa et al., 2007a). The monoepoxide, VCM, induced follicle loss in ovaries cultured from neonatal CYP2E1+/+ but not CYP2E1−/− mice. These findings supported that VCM conversion to the ovotoxic metabolite, VCD, in the ovary could be catalyzed by CYP2E1. Additionally, in this study, there was a nonsignificant trend for greater primary follicle loss in CYP2E1+/+ compared to CYP2E1−/− ovaries following exposure to a high concentration of VCD (30μM). This observation suggested a role for mEH in enhanced detoxification of VCD in the CYP2E1−/− ovary.
mEH has the ability to metabolize a wide range of substrates. This metabolism can detoxify as well as bioactivate xenobiotics (Omiecinski et al., 2000). Following bioactivation of VCH to VCD, mEH can detoxify VCD to an inactive tetrol metabolite (4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane). Previous research has shown that mEH is expressed in the murine ovary and that expression and activity can be enhanced by VCH and VCD exposure in mouse ovarian small preantral follicles (Cannady et al., 2002). Additionally, mEH mRNA and protein were upregulated in cultured postnatal day (PND) 4 B6C3F1 mouse ovaries exposed to VCD (Keating et al., 2008). Therefore, because mEH expression was increased by VCD exposure, this increase might enhance detoxification of VCD and afford a measure of protection against VCD-induced follicle loss. Such a circumstance could contribute to the requirement for repeated dosing to observe ovotoxicity.
In addition to VCD, the polycyclic aromatic hydrocarbon 7,12-dimethylbenz(a)anthracene (DMBA) also acts as an ovotoxicant, targeting follicles of all types (Matikainen et al., 2001). DMBA requires bioactivation via the combined activities of CYP1A1 and/or 1B1 and mEH to form a 3,4-diol-1,2-epoxide to induce follicle loss (Shiromizu and Mattison, 1985). A previous study reported that DMBA exposure caused an increase in mEH mRNA expression in cultured B6C3F1 PND4 mouse ovaries (Rajapaksa et al., 2007b). Thus, mEH can potentially detoxify VCD but bioactivate DMBA.
The hypothesis in the present study is that CYP2E1 has some interaction with mEH expression and function in the murine ovary. To test this, the temporal association between VCD-induced follicle loss and mEH mRNA expression in CYP2E1+/+ and −/− mouse ovaries was evaluated. Additionally, a comparison of the effect of VCD and DMBA on ovotoxicity in cultured ovaries from CYP2E1+/+ and −/− mice was made. An in vitro culture system was used to examine effects of ovotoxicants without the metabolic influence from the liver using ovaries from PND4 mice (enriched in small preantral follicles; Devine et al., 2002b).
MATERIALS AND METHODS
Reagents.
VCD (mixture of isomers, > 99% purity), 2-β-mercaptoethanol, 30% acrylamide/0.8% bis-acrylamide, ammonium persulfate, glycerol, N',N', N', N'-tetramethylethylenediamine, Tris base, Tris–HCL, sodium chloride, Tween-20, bovine serum albumin (BSA), ascorbic acid (vitamin C), and transferrin were purchased from Sigma-Aldrich, Inc. (St Louis, MO). Dulbecco's modified Eagle medium: nutrient mixture F-12 (Ham) 1× (DMEM/Ham's F12), Albumax, penicillin/streptomycin (5000 U/ml, 5000 μg/ml, respectively), Hanks’ Balanced Salt Solution (without CaCl2, MgCl2, or MgSO4), mEH and β-actin custom-designed primers, and Superscript III One-Step reverse transcriptase (RT)-PCR System were obtained from Invitrogen Co. (Carlsbad, CA). Millicell-CM filter inserts were purchased from Millipore (Bedford, MA), and 48-well cell culture plates were obtained from Corning, Inc. (Corning, NY). The mEH antibody (rabbit anti-mEH raised in goat) was purchased from Detroit R and D (Detroit, MI). Donkey anti-goat secondary antibody was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). RNeasy Mini Kit, QIAshredder Kit, RNeasy MinElute Kit, and Quantitect SYBR Green PCR Kit were purchased from Qiagen, Inc. (Valencia, CA). RNAlater was obtained from Ambion, Inc. (Austin, TX). ECL Plus chemiluminescence detection kit was purchased from GE Healthcare, Amersham (Buckinghamshire, UK).
Animals.
CYP 2E1 wild-type (+/+; 129S1/SvImJ background strain; two females, two males) and null (−/−; two females, two males) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the University of Arizona Animal Care Facility. 129S1/SvImJ mice breeding pairs were housed one male and one female per cage. At weaning (21 days), the pups were housed four per cage by sex. All animals were housed in plastic cages and maintained in a controlled environment (22°C ± 2°C; 12-h light/dark cycles). The animals were provided with a standard diet with ad libitum access to food and water and allowed to give birth. All animal experiments were approved by the University of Arizona's Institutional Animal Care and Use Committee.
In vivo animal dosing.
PND28 female offspring of both CYP 2E1 wild-type (+/+) and CYP 2E1 null (−/−) mice were dosed daily (15 days; ip) with sesame oil (vehicle control) or sesame oil containing VCD (0.57 mmol/kg/day). Previously, it was shown that VCD at that concentration causes significant loss of primordial follicles in B6C3F1 female mice following 12 days of daily ip dosing (Kao et al., 1999). Animals were killed by CO2 inhalation 4 h following the final dose.
In vitro ovarian cultures.
Ovaries from PND4 female B6C3F1 mice were cultured as described by Parrott and Skinner (1999). PND4 female CYP2E1+/+ and CYP2E1−/− mice were killed by CO2 inhalation followed by decapitation. Each ovary was removed (oviduct and excess tissue trimmed) and placed on a piece of Millicell-CM membrane floating on 250 μl of DMEM/Ham's F12 medium containing 1 mg/ml BSA, 1 mg/ml Albumax, 50 μg/ml ascorbic acid, 5 U/ml penicillin/5 μg/ml streptomycin, and 27.5 μg/ml transferrin per well in a 48-well plate previously equilibrated to 37°C. Using fine forceps, a drop of medium was placed to cover the top of the ovary to prevent drying. Previous studies have shown in the in vitro neonatal ovary culture system that treatment of rat ovaries for 15 days with VCD (1–100μM) resulted in a dose-dependent loss of healthy primordial and small primary follicles (Devine et al., 2002b). Additionally, incubation of CYP2E1+/+ and −/− cultured mouse ovaries with 30μM VCD for 15 days resulted in loss of almost all healthy primordial and primary follicles (Rajapaksa et al., 2007a). Thus, concentrations of VCD used in this study were within this range (5–30μM). Significant loss of healthy primordial follicles (p < 0.05) occurred in cultured B6C3F1 PND4 mouse ovaries exposed to DMBA (12.5nM–1μM) for 15 days at all concentrations (Rajapaksa et al., 2007b). Also, ovaries from neonatal B6C3F1 mice were less sensitive to VCM than those from CYP2E1+/+ mice (Rajapaksa et al., 2007a), which might be due to higher basal mEH expression in B6C3F1 mouse ovaries. Therefore, a higher starting concentration (> 12.5nM) of DMBA was used in cultures with CYP2E1+/+ and −/− mice (0.5–1μM). Ovaries were incubated with vehicle control, VCD (5–30μM; 2–15 days), or DMBA (0.5–1μM; 15 days). These concentrations have been shown to be in an optimal range for in vitro induction of ovotoxicity (Devine et al., 2002a,b; Rajapaksa et al., 2007b). Plates containing ovaries were cultured at 37°C and 5% CO2 in air. For those cultures lasting more than 2 days, media were removed and fresh media and treatment were replaced every 2 days. A fresh drop of media was placed on top of each ovary with each media change.
Histological evaluation of follicle numbers.
Following incubation, ovaries were placed in Bouin's fixative for 2 h, transferred to 70% ethanol, embedded in paraffin, serially sectioned (5 μm thick), and every sixth section was mounted. All ovarian sections were stained with hematoxylin and eosin. Healthy oocyte-containing follicle populations were classified and counted in every 12th section (n = 4–10 ovaries per treatment). Unhealthy (atretic) follicles were identified by pyknosis of granulosa cells and intense eosinophilic staining of oocytes (Devine et al., 2002a,b). Rarely, there is an ovary in which generalized necrosis as opposed to apoptosis is visible. When this occurs, that ovary is removed from the data set. Follicle population classification was according to the procedure of Flaws et al. (1994) which was adapted from that described by Pedersen and Peters (1968). Briefly, primordial follicles contained the oocyte surrounded by a single layer of squamous-shaped granulosa cells, primary follicles contained the oocyte surrounded by a single layer of cuboidal-shaped granulosa cells, and secondary follicles contained the oocyte surrounded by multiple layers of granulosa cells.
RNA isolation.
After 15 days of repeated in vivo daily dosing of PND28 CYP2E1+/+ or −/− mice with sesame oil or sesame oil containing VCD (0.57 mmol/kg/day), livers were removed and stored in RNAlater at − 80oC. PND4 ovaries, following 2, 5, 10, or 15 days of in vitro culture (10 ovaries per pool) treated with vehicle control or VCD (5μM), were stored in RNAlater at − 80°C. Total RNA was isolated using an RNeasy Mini Kit. Briefly, ovaries were lysed and homogenized using a motor pestle followed by applying the mixture onto a QIAshredder column. The QIAshredder column containing ovarian tissue sample was then centrifuged at 14,000 rpm for 2 min. The resulting flow through was applied to an RNeasy mini column, allowing RNA to bind to the filter cartridge. Following washing, RNA was eluted from the filter and concentrated using an RNeasy MinElute kit. Briefly, isolated RNA was applied to an RNeasy MinElute spin column, and after washing, RNA was eluted using 14 μl of RNase-free water. RNA concentration was determined using an ND-1000 spectrophotometer (λ = 260/280 nm; NanoDrop technologies, Inc., Wilmington, DE).
First-strand cDNA synthesis and real-time PCR.
Total RNA (0.5 μg) was reverse transcribed into cDNA utilizing the Superscript III One-Step RT-PCR System. cDNA was diluted (1:25) in RNase-free water. Diluted cDNA (2 μl) was amplified on a Rotor-Gene 3000 using Quantitect SYBR Green PCR kit and custom-designed primers for mEH (forward primer: 5’ GGG TCA AAG CCA TCA GGC A 3’; reverse primer: 5’ CCT CCA GAA GGA CAC CAC TTT; Cannady et al., 2002) and β-actin (forward primer: 5’ ACG CAG CTC AGT AAC AGT CC 3’; reverse primer: 5’ TCC ATC CTG GCC TCA CTG TC 3’; NCBI Genbank accession number AK 151010). The regular cycling program consisted of a 15-min hold at 95°C and 45 cycles of denaturing at 95°C for 15 s, annealing at 58°C for 15 s, and extension at 72°C for 20 s at which point data were acquired. Product melt conditions were determined using a temperature gradient from 72°C to 99°C with a 1°C increase at each step. There was no difference in β-actin mRNA between vehicle control and VCD-treated ovaries. Therefore, each sample was normalized to β-actin before quantification.
Protein isolation.
Whole ovarian protein homogenates were prepared from cultured ovaries or B6C3F1 adult mouse whole ovary via homogenization in tissue lysis buffer as previously described (Thompson et al., 2005). Briefly, homogenized samples were placed on ice for 30 min, followed by two rounds of centrifugation at 10,000 rpm for 15 min. Supernatant was collected and sample aliquoted until further use and stored at − 80oC. Protein was quantified using a standard bicinchoninic acid protocol on a 96-well assay plate. Emission absorbance values were detected with a λ = 540-nm excitation on a Synergy HT Multi-Detection Microplate Reader using KC4 software (Bio-Tek Instruments, Inc., Winooski, VT). Absorbance values obtained at 540 nm were quantified using comparison to a BSA protein standard curve.
Western blot analysis.
To confirm specificity of the mEH antibody on ovarian protein, a Western blot was performed. SDS–PAGE (10%) was used to separate B6C3F1 adult mouse ovarian proteins in homogenates (10 μg) and subsequently transferred onto nitrocellulose membranes as previously described (Thompson et al., 2005). Briefly, membranes were incubated for blocking (1 h) with shaking at 4oC in 5% milk in Tris-buffered saline with Tween-20 (TTBS). Membranes were incubated with mEH primary antibody (goat anti-rabbit; 1:10,000 dilution) in 5% milk in TTBS overnight at 4oC. Membranes were washed three times for 10 min each with TTBS. HRP-conjugated secondary antibody was added for 1 h at room temperature. Membranes were washed three times for 10 min each in TTBS, followed by a single wash for 10 min in Tris-buffered saline. Positive mEH-containing protein bands on the Western blot gel were detected using chemiluminescence (using ECL Plus detection substrate) and exposed to x-ray film.
Confocal microscopy.
The response to VCD for increased mEH protein quantification by Western blotting was previously shown to correspond with mEH protein as measured by immunofluorescence and confocal microscopy analysis in cultured PND4 B6C3F1 neonatal mouse ovaries (VCD/control = Western blotting 2.14; immunofluorescence 1.47; p < 0.05; Keating et al., 2008 and Supplementary Fig. 1). Additionally, VCD-induced increases in mEH protein in the ovary measured by immunofluorescence staining and confocal microscopy was in good agreement with increased mEH activity in isolated small preantral follicles collected from VCD-dosed mice (VCD/control = immunofluorescence 1.29; enzyme activity 4.84; p < 0.05; Cannady et al., 2002 and Supplementary Fig. 2). Following in vitro culture, ovaries treated with vehicle or VCD (5μM) were fixed in 4% buffered formalin for 2 h, transferred to 70% ethanol, embedded in paraffin, serially sectioned, and every 10th section was mounted. Sections were deparaffinized (approximately six sections/ovary) and incubated with primary antibody directed against mEH (goat anti-rabbit; 1:50 dilution) at 4°C overnight. Specificity for this antibody was determined by Western blotting. Secondary biotinylated antibody (horse anti-goat; 1:75 dilution) was applied for 1 h, followed by CY-5-streptavidin (1 h; 1:50 dilution). Sections were treated with ribonuclease A (100 μg/ml) for 1 h, followed by staining with YOYO-1 (10 min; 5nM). Slides were repeatedly rinsed with phosphate-buffered saline, cover slipped, and stored in the dark (4°C) until visualization. Primary antibody was not added to immunonegative ovarian sections. Immunofluorescence was visualized on a Zeiss (LSM 510 NLO-Meta) confocal microscope with an argon and helium-neon laser projected through the tissue into a photomultiplier at λ = 488 and 633 nm for YOYO-1 (green) and CY-5 (red), respectively. All images were captured using a ×40 objective lens. Briefly, the image of each entire ovarian section was captured using confocal microscopy. Mean integrated density (MID) of fluorescence was determined for all primordial and small primary follicles expressing mEH using Image J software. Using the circular tool of Image J software, follicles were selected and the MID was measured. A background measurement was subtracted from MID for each field. Mean values were calculated for control and VCD-treated follicles, and VCD-treated follicle MID was expressed as a percentage of control treatment.
FIG. 1.
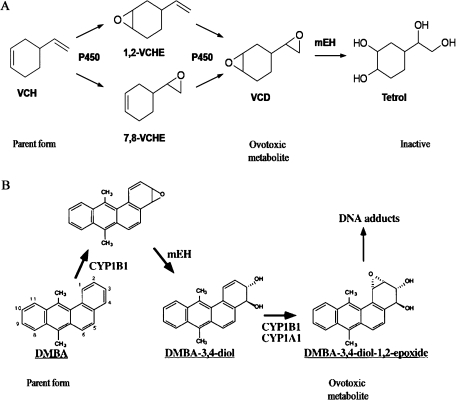
Metabolism of VCH and DMBA. (A) VCH is bioactivated by CYP450 isoforms to form either 1,2- or 7,8-monoepoxide (VCM) metabolites and, subsequently, VCD. The action of mEH metabolizes VCD to an inactive tetrol metabolite. Adapted from Keller et al. (1997). (B) DMBA is bioactivated by CYP450 isoforms to a DMBA-3,4-epoxide and, subsequently, hydrolyzed by mEH to DMBA-3,4-diol. Further activation by CYP450 isoforms results in formation of the bioactive metabolite, DMBA-3,4-diol-1,2-epoxide. Adapted from Miyata et al. (1999).
Statistical analysis.
Comparisons were made between treatments using Statview software ANOVA and Fisher's protected least significant difference multiple range test. The assigned level of significance for all tests was p < 0.05.
RESULTS
CYP2E1 Dependence on VCD-Induced Follicle Loss
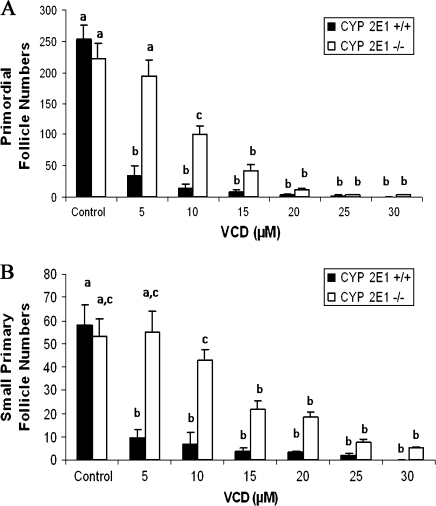
To determine a dose-response for VCD-induced follicle loss, follicles were counted in ovaries from PND4 CYP2E1+/+ and −/− mice that had been cultured with increasing concentrations of VCD (5–30μM) for 15 days (Fig. 2). The concentration range was based on that determined previously to destroy primordial follicles in ovaries from those mice (Rajapaksa et al., 2007a). There was no difference in follicle number between CYP2E1+/+ and −/− when cultured in control medium. Relative to control, incubation with VCD for 15 days depleted (p < 0.05) primordial follicles at concentrations of 10μM and higher in ovaries from both groups of animals (Fig. 2A). In CYP2E1+/+ ovaries, primordial follicle loss was maximal at the lowest concentration, 5μM. Conversely, in CYP2E1−/− ovaries, there was a dose-dependent decrease in follicle numbers with VCD exposure. Additionally, there was greater (p < 0.05) primordial follicle loss in the CYP2E1+/+ compared to CYP2E1−/− ovaries at 5 and 10μM VCD.
FIG. 2.
Effect of CYP2E1 expression on ovotoxicity with VCD. Ovaries from PND4 CYP2E1+/+ and −/− mice were incubated with control medium or increasing concentrations of VCD (5–30μM) for 15 days. Following incubation, ovaries were collected and processed for histological evaluation as described in the Materials and Methods section. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted per ovary, n = 4–9; values with different letters are significantly different at p < 0.05.
Similar to primordial follicles, VCD-induced small primary follicle loss was seen at ≥ 10μM VCD in CYP2E1+/+ and −/− ovaries; however, no small primary follicle loss was observed at 5μM VCD in CYP2E1−/− ovaries. Further, there was less (p < 0.05) small primary follicle loss in CYP2E1−/− compared to CYP2E1+/+ ovaries at 5 and 10μM VCD (Fig. 2B). There was no effect of VCD on large primary or secondary follicles in either genotype at any concentration (data not shown).
CYP2E1 Dependence on Time Course of VCD-Induced Follicle Loss
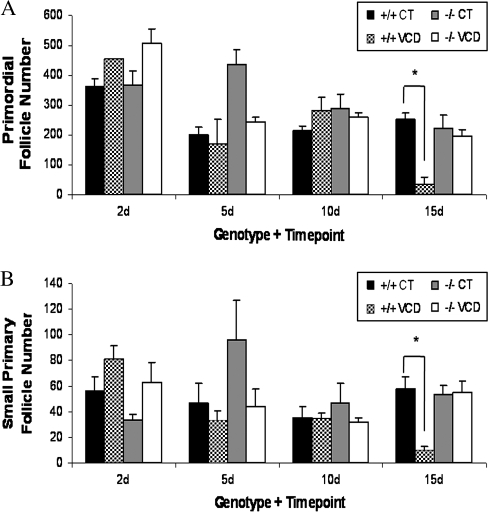
To evaluate a time course of the effect of VCD exposure on follicle loss, ovaries from PND4 CYP2E1+/+ and −/− mice were incubated with a low concentration (5μM) of VCD for 2, 5, 10, and 15 days (Fig. 3). Primordial (Fig. 3A) and small primary (Fig. 3B) follicle loss occurred (p < 0.05) in CYP2E1+/+ mice after 15 days of VCD exposure. In contrast, no loss of any follicle type (p > 0.05) was seen in ovaries from CYP2E1−/− mice at any time (Fig. 3). The large variation seen in CYP2E1−/− in primordial and small primary follicle number on day 5 was due to one outlier animal. There was no effect of VCD on large primary or secondary follicles in either genotype at any time (data not shown).
FIG. 3.
Effect of CYP2E1 expression on VCD-induced ovotoxicity. Ovaries from PND4 CYP2E1+/+ and −/− mice were incubated with control medium or 5μM VCD for 2, 5, 10, or 15 days. Following incubation, ovaries were collected and processed for histological evaluation as described in the Materials and Methods section. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted per ovary, n = 3; *different (p < 0.05) from control.
CYP2E1 Dependence on VCD-Induced mEH mRNA Expression
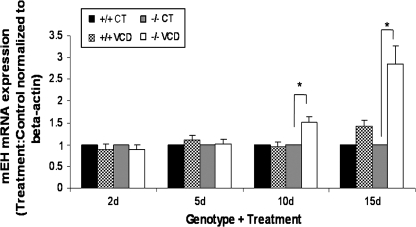
To correlate mEH expression with induction of follicle loss, mEH mRNA was evaluated by real-time PCR and normalized to β-actin expression (Fig. 4). After 2 and 5 days of VCD (5μM) exposure, there was no difference in mEH expression compared to control in ovaries from either CYP2E1+/+ or −/− mice. However, compared to control treatment, in CYP2E1−/− ovaries, VCD caused a 0.5-fold (50%) and 1.84-fold (184%) increase (p < 0.05) in mEH expression at days 10 and 15, respectively. Additionally, relative to controls, there was no increase in mEH expression in CYP2E1+/+ ovaries incubated with VCD at any time point (Fig. 4).
FIG. 4.
Effect of VCD on ovarian mEH mRNA. Ovaries from PND4 CYP2E1+/+ and −/− mice were incubated with control medium or 5μM VCD for 2, 5, 10, or 15 days. Following incubation, total RNA was isolated as described in the Materials and Methods section. One microgram total RNA was reverse transcribed to cDNA and analyzed for mEH and β-actin mRNA by RT-PCR. Values are mean fold change ± SE; n = 3 (8–10 ovaries per pool). *Different (p < 0.05) from control.
mEH Protein Expression
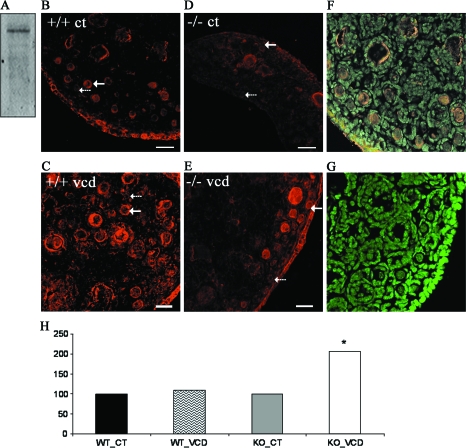
To confirm specificity of an anti-mEH antibody on ovarian protein, a Western blot was performed (Fig. 5A). A single band was observed at the expected 50-KDa molecular weight. To localize basal expression of mEH and determine VCD-induced changes, CYP2E1+/+ and −/− ovaries were cultured in control medium or medium containing 5μM VCD for 15 days (Fig. 5). Ovaries were collected and analyzed by immunofluorescence of mEH protein as visualized by confocal microscopy. mEH is expressed in granulosa cells of primordial and primary follicles and in the ovarian interstitial compartment. Quantification of mEH expression was carried out by determining the MID of mEH staining in primordial and small primary follicles for each treatment as described in the Materials and Methods section. (The number of follicles evaluated were CYP2E1+/+ control treatment, 113; CYP2E1+/+ VCD treatment, 58; CYP2E1−/− control treatment, 146; CYP2E1−/− VCD treatment, 130.) There was no effect of VCD on mEH staining intensity in follicles in the CYP2E1+/+ ovary. However, relative to control treatment, there was an increase (p < 0.05) in mEH protein staining in follicles in the CYP2E1−/− ovary treated with VCD (Fig. 5H).
FIG. 5.
Effect of VCD on mEH protein. (A) Representative Western blotting of adult B6C3F1 mouse ovary protein incubated with anti-mEH antibody. Ovaries from PND4 CYP2E1+/+ (B, C) and CYP2E1−/− (D, E) mice were incubated with control medium (B, D) or 5μM VCD (C, E) for 15 days. Following culture, ovaries were processed for immunofluoresence and confocal microscopy as described in the Materials and Methods section. All images were captured with a ×40 objective lens. Red staining = Cy5-labeled anti-mEH antibody. Green staining = YoYo1 genomic DNA. (F) Merged genomic stain and mEH protein stain. (G) Immunonegative stain (Cy5 and YoYo1 without anti-mEH antibody). (H) Quantification of mEH staining intensity, relative to controls, in small preantral follicles. Broken arrows indicate primordial follicles. Solid arrows indicate small primary follicles. Scale bar = 25μM.
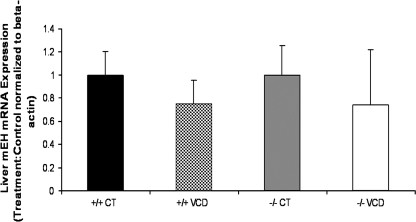
Effect of VCD and CYP2E1 Dependence on Hepatic mEH mRNA Expression
To evaluate possible effects of VCD on hepatic mEH mRNA expression and to determine any differences due to CYP2E1 genotype, PND28 female mice were dosed daily with sesame oil (vehicle control) or sesame oil containing VCD (0.57 mmol/kg/day) for 15 days (n = 3 animals per treatment). mEH mRNA expression in liver did not change in response to VCD, and there was no difference between genotypes (Fig. 6).
FIG. 6.
Effect of VCD on hepatic mEH mRNA. PND28 CYP2E1+/+ and −/− mice were dosed daily with sesame oil (control) or VCD (0.57 mmol/kg/day; ip) for 15 days. Liver was removed and total RNA was isolated as described in the Materials and Methods section. One microgram total RNA was reverse transcribed to cDNA and analyzed for mEH and β-actin mRNA by RT-PCR. Values are mean fold change ± SE; n = 3.
CYP2E1 Dependence on DMBA-Induced Follicle Loss
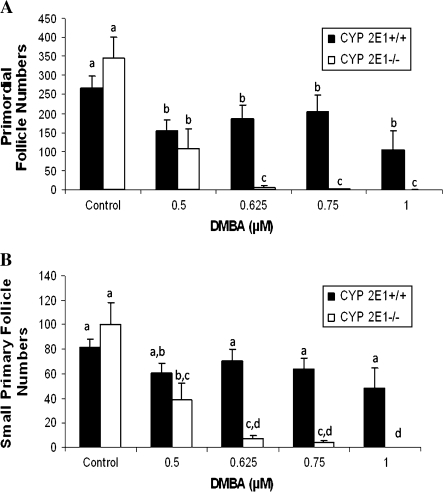
To compare the genotypic response to another known ovotoxicant, the effect of DMBA on ovaries from PND4 CYP2E1+/+ and −/− mice cultured with increasing concentrations of DMBA (0.5–1μM) for 15 days (Fig. 7) was assessed. DMBA is a more potent ovotoxicant than VCD; therefore, concentrations of DMBA (0.5–1μM) that were used were much lower than with VCD (5–30μM; Fig. 2).
FIG. 7.
Effect of CYP2E1 expression on ovotoxicity with DMBA. Ovaries from PND4 CYP2E1+/+ and −/− mice were incubated with control medium or increasing concentrations of DMBA (0.5–1μM) for 15 days. Following incubation, ovaries were collected and processed for histological evaluation as described in the Materials and Methods section. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted per ovary, n = 4–10; values with different letters are significantly different at p < 0.05.
There was no effect of dimethyl sulfoxide (DMBA vehicle) on primordial follicle number (data not shown). There was no difference in follicle numbers between CYP2E1+/+ and −/− ovaries incubated in vehicle control medium. Relative to control medium, there was a loss (p < 0.05) of primordial follicles at all concentrations of DMBA in both CYP2E1+/+ and −/− ovaries. Reduced primordial follicle numbers in CYP2E1+/+ ovaries plateaued at the lowest concentration of DMBA (0.5μM). However, in CYP2E1−/− ovaries, a dose-dependent loss of primordial follicles continued at higher concentrations (p < 0.05; Fig. 7A).
There was no difference in small primary follicle numbers between both CYP2E1+/+ and −/− ovaries incubated in vehicle control medium (Fig. 7B). No loss of small primary follicles occurred in CYP2E1+/+ ovaries at any concentration of DMBA. However, as with primordial follicles, there was a dose-dependent loss of primary follicles in CYP2E1−/− ovaries. Thus, in contrast to VCD treatment, DMBA treatment resulted in a greater (p < 0.05) loss of both primordial and small primary follicles in CYP2E1−/− as compared with CYP2E1+/+ ovaries.
DISCUSSION
A previous study evaluating the effects of VCH and VCM on ovotoxicity in the mouse ovary supported that ovarian CYP2E1 is capable of converting the monoepoxide metabolite, VCM, to the ovotoxic form, VCD, which does not require bioactivation (Rajapaksa et al., 2007a). In the present study, the ovotoxic effects of VCD, which should not be a substrate for CYP2E1, were evaluated in CYP2E1 wild-type (+/+) and null (−/−) neonatal mouse ovaries. The initial assumption was that there should be no differential effect on ovotoxicity between CYP2E1+/+ and −/− ovaries. There was a dose-dependent loss of primordial and primary follicles in ovaries from CYP2E1+/+ and −/− mice. Surprisingly, however, at the lowest concentrations, VCD-induced follicle loss was greater (p < 0.05) in CYP2E1+/+ as compared with CYP2E1−/− ovaries. Additionally, during the time course of exposure to a low concentration of VCD (5μM), follicle loss was observed on day 15 in CYP2E1+/+ but not CYP2E1−/− ovaries.
mEH is highly expressed in the mouse ovary, and ovarian expression of mEH mRNA in B6C3F1 mice was increased in response to VCD (Cannady et al., 2002; Keating et al., 2008). Unlike CYP2E1, mEH detoxifies VCD by converting it to an inactive tetrol metabolite. Therefore, the differing sensitivities to VCD between genotypes in the present study could be that detoxification of VCD was increased in CYP2E1−/− ovaries. Therefore, mEH mRNA expression was measured in VCD-exposed ovaries from CYP2E1+/+ versus CYP2E1−/− mice. There was no effect of a low concentration of VCD (5μM) on mEH mRNA expression in either genotype on day 2 or 5. However, mEH expression was increased (p < 0.05) on days 10 and 15 in CYP2E1−/− ovaries. In contrast, mEH expression was not affected in CYP2E1+/+ ovaries at any time. Thus, VCD-induced increases in mEH expression on day 10 is likely to have protected follicles on day 15 in CYP2E1−/− ovaries due to increased detoxification of VCD. On the other hand, with no increase in mEH mRNA expression in CYP2E1+/+ ovaries, follicle loss on day 15 could not be prevented. This finding was supported by immunofluorescence staining of mEH protein in which there was an increase of mEH protein in response to VCD in CYP2E1−/− but not CYP2E1+/+ ovaries. Taken together, these observations demonstrate a relative resistance of CYP2E1−/− ovaries to VCD-induced ovotoxicity resulting from higher levels of mEH activity, to provide increased detoxification.
In contrast to the effect of continuous exposure to VCD in cultured PND4 CYP2E1+/+ and −/− ovaries, there was no effect of repeated daily dosing in vivo with VCD on hepatic mEH mRNA expression, regardless of CYP2E1 genotype. This agrees with another study in which there was no effect of VCD dosing on mEH mRNA expression in rat liver (Springer et al., 1996b). Therefore, based on this finding, regulation of mEH expression in the ovary appears to be relatively unique since similar regulation does not occur in the liver.
Whereas mEH detoxifies VCD, it bioactivates DMBA to its ovotoxic 3,4-diol-1,2-epoxide metabolite (Shiromizu and Mattison, 1985). In a previous study, a time course of follicle loss was compared with that of changes in mEH mRNA expression in ovaries from PND4 B6C3F1 mice cultured with DMBA (Rajapaksa et al., 2007b). A large increase in mEH mRNA expression was observed on day 2 of incubation. This was followed by a substantial increase in follicle loss on day 4. From this, it was concluded that the large increase in follicle loss was the result of increased mEH activity for bioactivation of DMBA. Therefore, to further explore the hypothesis that differential mEH expression occurs in CYP2E1+/+ and −/− ovaries, a DMBA dose-response study was performed.
Like VCD, DMBA is not a substrate for CYP2E1. As a polycyclic aromatic hydrocarbon, it is metabolized by CYP1B1, CYP1A1, and mEH to form the ultimate ovotoxic metabolite, DMBA-3,4-diol-1,2-epoxide (Miyata et al., 1999). In contrast to VCD, DMBA caused less (p < 0.05) follicle loss in CYP2E1+/+ as compared to −/− ovaries. These observations support that with higher mEH expression in CYP2E1−/− relative to +/+ ovaries, increased detoxification of VCD retards follicle loss, whereas increased bioactivation of DMBA enhances follicle loss. As a result, the markedly greater ovarian sensitivity to DMBA relative to VCD in causing ovotoxicity in the present and previous studies (Borman et al., 2000) may, in part, result from relatively high induced levels of ovarian mEH expression.
In addition to the role of mEH in mediating ovotoxicity induced by VCD and DMBA, the findings presented here support that in the mouse ovary, expression of CYP2E1 and mEH genes may be inversely associated. That is, in ovaries lacking CYP2E1, the responsiveness of mEH expression to induction by xenobiotics may be greater than in CYP2E1 intact ovaries. It is possible that CYP2E1 directly regulates mEH expression or may affect induction or inhibition of transcriptional activating proteins that differentially regulate mEH expression. Alternatively, it is possible that the enhanced mEH response to induction compensates for the lack of CYP2E1. CYP2E1 expression is regulated by HNF1α (Akiyama and Gonzalez, 2003) and β-catenin (Sekine et al., 2006), while mEH is regulated by NRF2 (Hu et al., 2006). Interestingly, hepatic CYP2E1 activates NRF2 (Gong and Cederbaum, 2006); thus, a lack of CYP2E1 would be expected to have the opposite effect on mEH expression of that described here. Insulin-induced hepatic expression of both CYP2E1 and mEH, however, follow a different pattern of expression (Kim and Novak, 2007). Insulin-resistant diabetes is associated with upregulation of CYP2E1 while mEH is downregulated. Insulin treatment causes a shifting of these patterns of expression (reduced CYP2E1; increased mEH). While no common mechanisms of regulation for both CYP2E1 and mEH have been reported in the literature, analysis of CYP2E1 and mEH gene promoters in the rat using MatInspector which interrogates the TRANSFAC database of transcription factors identified a number of common transcription factor binding sites between the promoters (data not shown). These transcription factors include HNF1 (CYP2E1-1 site; mEH-3 sites), NRF2 (CYP2E1-4 sites; mEH-2sites), and PPAR (CYP2E1-1 site; mEH-1 site). Furthermore, in view of the differences in VCD-induced expression between the ovary and the liver reported here, conclusions from studies of the hepatic genes may not be relevant for the ovary. It is therefore possible that expression of common transcription factors regulate both CYP2E1 and mEH. To our knowledge, this is the first report that suggests there may be such a relationship between the two enzymes. Therefore, the mechanism(s) by which this occurs is/are unknown and will be the subject of further investigation.
SUPPLEMENTARY DATA
Supplementary Figures 1 and 2 are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (grant number ES09246) and Center Grant 06694.
Supplementary Material
Acknowledgments
We wish to thank Andrea Grantham for histological processing of ovarian tissue and Patricia Christian for assistance with immunohistochemistry and confocal analysis.
References
- Akiyama TE, Gonzalez FJ. Regulation of P450 genes by liver-enriched transcription factors and nuclear receptors. Biochim. Biophys. Acta. 2003;1619:223–234. doi: 10.1016/s0304-4165(02)00480-4. [DOI] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: Comparison through calculation of an ovotoxic index. Toxicol. Appl. Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries. Toxicol. Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of cytochrome P450 2E1, 2A, and 2B in the mouse ovary: The effect of 4-vinylcyclohexene and its diepoxide metabolite. Toxicol. Sci. 2003;73:423–430. doi: 10.1093/toxsci/kfg077. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Rajapaksa KS, Hoyer PB. In vitro ovarian tissue and organ culture: A review. Front. Biosci. 2002a;7:1979–1989. doi: 10.2741/devine. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol. Appl. Pharmacol. 2002b;184:107–115. [PubMed] [Google Scholar]
- Gong P, Cederbaum AI. NRF2 is increased in CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:114–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod. Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol. Reprod. 2001a;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol. Reprod. 2001b;65:87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol. Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol. Cell Biol. 2006;26:940–954. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Vol. 60. IARC Press, Lyon, France: 1994. 4-Vinylcyclohexene. IARC monographs on the evaluation of carcinogenic risks to humans: Some industrial chemicals; p. 347. [Google Scholar]
- Kao SW, Sipes IG, Hoyer PB. Early effects induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod. Toxicol. 1999;13:67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Keating AF, Sipes IG, Hoyer PB. Expression of ovarian microsomal epoxide hydrolase and glutathione S-transferase during onset of VCD-induced ovotoxicity in B6C3F1 mice. Toxicol. Appl. Pharmacol. 2008;230:109–16. doi: 10.1016/j.taap.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DA, Carpenter SC, Cagen SZ, Reitman FA. In vitro metabolism of 4-vinylcyclohexene in rat and mouse liver, lung, and ovary. Toxicol. Appl. Pharmacol. 1997;144:36–44. doi: 10.1006/taap.1996.8098. [DOI] [PubMed] [Google Scholar]
- Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol. Ther. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu H, Laine J, Sakai T, Korsmeyer S, Casper R, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazard environmental chemicals. Nat. Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Miyata M, Judo G, Lee Y, Yang TJ, Gelboni HV, Fernandez-Salguero P, Kimura S, Gonzalez FJ. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J. Biol. Chem. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Hassett C, Hosagrahara V. Epoxide hydrolase-polymorphism and role in toxicology. Toxicol. Lett. 2000;112–113:365–370. doi: 10.1016/s0378-4274(99)00235-0. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Rajapaksa KS, Cannady EA, Sipes IG, Hoyer PB. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol. Appl. Pharmacol. 2007a;221:215–221. doi: 10.1016/j.taap.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa KS, Sipes IG, Hoyer PB. Involvement of microsomal epoxide hydrolase in ovotoxicity caused by 7,12-dimethylbenz(a)anthracene. Toxicol. Sci. 2007b;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome P450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- Shiromizu K, Mattison DR. Murine oocyte destruction following intraovarian treatment with 3-methylcholanthrene or 7,12-dimethylbenz(a)anthracene: Protection by alpha-naphthoflavone. Teratog. Carcinog. Mutagen. 1985;5:463–472. doi: 10.1002/tcm.1770050609. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol. Appl. Pharmacol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol. Appl. Pharmacol. 1996a;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer LA, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol. Appl. Pharmacol. 1996b;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol. Appl. Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.