Abstract
The use of nanotechnology in medicine and more specifically drug delivery is set to spread rapidly. Currently many substances are under investigation for drug delivery and more specifically for cancer therapy. Interestingly pharmaceutical sciences are using nanoparticles to reduce toxicity and side effects of drugs and up to recently did not realize that carrier systems themselves may impose risks to the patient. The kind of hazards that are introduced by using nanoparticles for drug delivery are beyond that posed by conventional hazards imposed by chemicals in classical delivery matrices. For nanoparticles the knowledge on particle toxicity as obtained in inhalation toxicity shows the way how to investigate the potential hazards of nanoparticles. The toxicology of particulate matter differs from toxicology of substances as the composing chemical(s) may or may not be soluble in biological matrices, thus influencing greatly the potential exposure of various internal organs. This may vary from a rather high local exposure in the lungs and a low or neglectable exposure for other organ systems after inhalation. However, absorbed species may also influence the potential toxicity of the inhaled particles. For nanoparticles the situation is different as their size opens the potential for crossing the various biological barriers within the body. From a positive viewpoint, especially the potential to cross the blood brain barrier may open new ways for drug delivery into the brain. In addition, the nanosize also allows for access into the cell and various cellular compartments including the nucleus. A multitude of substances are currently under investigation for the preparation of nanoparticles for drug delivery, varying from biological substances like albumin, gelatine and phospholipids for liposomes, and more substances of a chemical nature like various polymers and solid metal containing nanoparticles. It is obvious that the potential interaction with tissues and cells, and the potential toxicity, greatly depends on the actual composition of the nanoparticle formulation. This paper provides an overview on some of the currently used systems for drug delivery. Besides the potential beneficial use also attention is drawn to the questions how we should proceed with the safety evaluation of the nanoparticle formulations for drug delivery. For such testing the lessons learned from particle toxicity as applied in inhalation toxicology may be of use. Although for pharmaceutical use the current requirements seem to be adequate to detect most of the adverse effects of nanoparticle formulations, it can not be expected that all aspects of nanoparticle toxicology will be detected. So, probably additional more specific testing would be needed.
Keywords: drug delivery, cancer therapy, nanoparticles, toxicology, pharmaceuticals
Introduction
Recent years have witnessed unprecedented growth of research and applications in the area of nanoscience and nanotechnology. There is increasing optimism that nanotechnology, as applied to medicine, will bring significant advances in the diagnosis and treatment of disease. Anticipated applications in medicine include drug delivery, both in vitro and in vivo diagnostics, nutraceuticals and production of improved biocompatible materials (Duncan 2003; De Jong et al 2005; ESF 2005; European Technology Platform on Nanomedicine 2005; Ferrari 2005). Engineered nanoparticles are an important tool to realize a number of these applications. It has to be recognized that not all particles used for medical purposes comply to the recently proposed and now generally accepted definition of a size ≤100 nm (The Royal Society and Royal Academy of Engineering 2004). However, this does not necessarily has an impact on their functionality in medical applications. The reason why these nanoparticles (NPs) are attractive for medical purposes is based on their important and unique features, such as their surface to mass ratio that is much larger than that of other particles, their quantum properties and their ability to adsorb and carry other compounds. NPs have a relatively large (functional) surface which is able to bind, adsorb and carry other compounds such as drugs, probes and proteins. However, many challenges must be overcome if the application of nanotechnology is to realize the anticipated improved understanding of the patho-physiological basis of disease, bring more sophisticated diagnostic opportunities, and yield improved therapies. Although the definition identifies nanoparticles as having dimensions below 0.1 μm or 100 nm, especially in the area of drug delivery relatively large (size >100 nm) nanoparticles may be needed for loading a sufficient amount of drug onto the particles. In addition, for drug delivery not only engineered particles may be used as carrier, but also the drug itself may be formulated at a nanoscale, and then function as its own “carrier” (Cascone et al 2002; Baran et al 2002; Duncan 2003; Kipp 2004). The composition of the engineered nanoparticles may vary. Source materials may be of biological origin like phospholipids, lipids, lactic acid, dextran, chitosan, or have more “chemical” characteristics like various polymers, carbon, silica, and metals. The interaction with cells for some of the biological components like phospholipids will be quite different compared to the non biological components such as metals like iron or cadmium. Especially in the area of engineered nanoparticles of polymer origin there is a vast area of possibilities for the chemical composition.
Although solid NPs may be used for drug targeting, when reaching the intended diseased site in the body the drug carried needs to be released. So, for drug delivery biodegradable nanoparticle formulations are needed as it is the intention to transport and release the drug in order to be effective. However, model studies to the behavior of nanoparticles have largely been conducted with non-degradable particles. Most data concerning the biological behavior and toxicity of particles comes from studies on inhaled nanoparticles as part of the unintended release of ultrafine or nanoparticles by combustion derived processes such as diesel exhaust particles (reviewed by Oberdörster 1996; Donaldson et al 2001, 2004; Borm 2002; Donaldson and Stone 2003; Dreher 2004; Kreyling et al 2004; Oberdörster, Oberdörster et al 2005). Research has demonstrated that exposure to these combustion derived ultrafine particles/nanoparticles is associated with a wide variety of effects (Donaldson et al 2005) including pulmonary inflammation, immune adjuvant effects (Granum and Lovik 2002) and systemic effects including blood coagulation and cardiovascular effects (Borm and Kreyling 2004; Oberdorster, Oberdörster et al 2005). Since the cut-off size for both ultrafine and nanoparticles (100 nm) is the same, now both terms are used as equivalent. Based on the adverse effects of ultrafine particles as part of environmental pollution, engineered nanoparticles may be suspected of having similar adverse effects. It is the purpose of this review to use this database on combustion derived nanpoarticles (CDNP) obtained by inhalation toxicology and epidemiology and bridge the gap to engineered nanoparticles.
Nanoparticles and drug delivery
Drug delivery and related pharmaceutical development in the context of nanomedicine should be viewed as science and technology of nanometer scale complex systems (10–1000 nm), consisting of at least two components, one of which is a pharmaceutically active ingredient (Duncan 2003; Ferrari 2005), although nanoparticle formulations of the drug itself are also possible (Baran et al 2002; Cascone et al 2002; Duncan 2003; Kipp 2004). The whole system leads to a special function related to treating, preventing or diagnosing diseases sometimes called smart-drugs or theragnostics (LaVan et al 2003). The primary goals for research of nano-bio-technologies in drug delivery include:
More specific drug targeting and delivery,
Reduction in toxicity while maintaining therapeutic effects,
Greater safety and biocompatibility, and
Faster development of new safe medicines.
The main issues in the search for appropriate carriers as drug delivery systems pertain to the following topics that are basic prerequisites for design of new materials. They comprise knowledge on (i) drug incorporation and release, (ii) formulation stability and shelf life (iii) biocompatibility, (iv) biodistribution and targeting and (v) functionality. In addition, when used solely as carrier the possible adverse effects of residual material after the drug delivery should be considered as well. In this respect biodegradable nanoparticles with a limited life span as long as therapeutically needed would be optimal.
Table 1 presents some of the types of chemical structures and possibilities for the preparation of nanoscale materials used as pharmaceutical carrier system (reviewed in Borm and Muller-Schulte 2006). Certainly none of the so far developed carriers fulfill all the parameters mentioned above to the full extent; the progress made in nanotechnology inter alia emerging from the progress in the polymer-chemistry, however, can provide an intriguing basis to tackle this issue in a promising way.
Table 1.
Overview of nanoparticles and their applications in Life Sciences
| Particle class | Materials | Application |
|---|---|---|
| Natural materials or derivatives | Chitosan Dextrane Gelatine Alginates Liposomes Starch |
Drug/Gene delivery |
| Dendrimers | Branched polymers | Drug delivery |
| Fullerenes | Carbon based carriers | Photodynamics Drug delivery |
| Polymer carriers | Polylactic acid Poly(cyano)acrylates Polyethyleinemine Block copolymers Polycaprolactone |
Drug/gene delivery |
| Ferrofluids | SPIONS USPIONS |
Imaging (MRI) |
| Quantum dots | Cd/Zn-selenides | Imaging In vitro diagnostics |
| Various | Silica-nanoparticles Mixtures of above |
Gene delivery |
The aims for nanoparticle entrapment of drugs are either enhanced delivery to, or uptake by, target cells and/or a reduction in the toxicity of the free drug to non-target organs. Both situations will result in an increase of therapeutic index, the margin between the doses resulting in a therapeutic efficacy (eg, tumor cell death) and toxicity to other organ systems. For these aims, creation of long-lived and target-specific nanoparticles is needed. Chemical formulations under investigation are shown in Table 2. Most of the compounds are biodegradable polymers resulting in drug release after degradation. One of the problems in the use of particulate drug carriers including nanomaterials is the entrapment in the mononuclear phagocytic system as present in the liver and spleen (Lenaerts et al 1984; Gibaud et al 1996; Demoy et al 1997; Moghimi et al 2001). However, liver targeting of nanoparticles may be favorable when treating liver diseases like tumor metastasis or hepatitis. Surface modification with polyethylene glycol (PEG) resulted in prolonged presence in the circulation by inhibiting recognition and phagocytosis by the mononuclear phagocytic system (Bazile et al 1995; Peracchia et al 1999; Niidome et al 2006). In addition to altering the distribution the PEG modification also reduced in vitro toxicity when gold nanorods were modified using PEG (Niidome et al 2006). Coating of NP may also be needed to prevent agglomeration. Several coatings can be used to prevent agglomeration and keeping the particles in colloidal suspension including various polymers like polyethylene glycol (PEG), poly(vinylpyrrolidone) (PVP) etc, natural polymers like dextran, chitosan, pullulan etc, and surfactants like sodium oleate, dodecylamine etc (reviewed by Gupta and Gupta 2005).
Table 2.
Chemicals under investigation for drug delivery
| Albumin | Damascelli et al 2003 |
| Cetyl alcohol/polysorbate | Koziara et al 2004 |
| Chitosan |
Dyer et al 2002; Huang et al 2004 |
| Gelatin | Cascone et al 2002 |
| Gold |
Hainfield et al 2004; Paciotti et al 2004 |
| Hydrogels | Gupta and Gupta 2004 |
| Magnetic iron oxide | Gupta and Gupta 2005 |
| Methoxy | Kim et al 2003 |
| poly(ethylene glycol)/poly(ε-caprolactone) | |
| Polyalkylcyanoacrylate composites |
Alyautdin et al 1997; Kreuter et al 2003 |
| Poly(D,L-lactic-co-glycolic)acid (PLGA) |
Panyam et al 2002; Weissenbrock et al 2004 |
| Solid lipid formulations |
Muller et al 2000; Wissing et al 2004 |
NP size can influence the NP distribution as was demonstrated for lipid vesicles for which a lower liver uptake was found for the smaller vesicles (200/300 nm versus 25/50 nm) (Seki et al 2004). Even small size differences may be of influence for the actual distribution and thus bioavailability (Saez et al 2000; Fishbein et al 2001; Shim et al 2004; Zhang et al 2004; Fang et al 2006). For liposomes with sizes >100 nm the clearance rate by the mononuclear phagocytic system increased with increasing size, while for sizes below 100 nm charge was more important (Senior and Gregoriadis 1982; Senior et al 1985). However, not all particles with sizes below 100 nm will behave similarly and composition will be important as well. Analogous to earlier findings on asbestiform and mineral fibers, the actual size and shape of nanomaterials will be of importance.
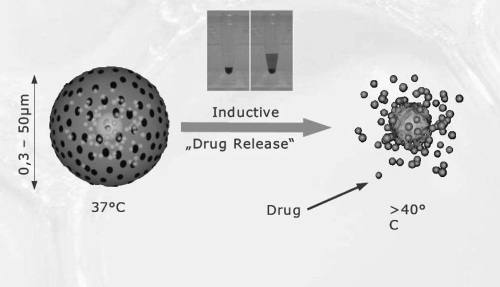
Besides degradation physical means such as heating and light may be used to provoke the therapeutic effect (cell death) or for local drug release, respectively. Thermosensitive nanoparticles may be used for selective release of the content after specific localization. An example of this principle is presented in Figure 1. For doxorubicin an enhanced cytotoxicity was observed in vitro at 42 °C compared to 37 °C using copolymers of polyethylene glycol (PEG) and poly-L-lactide (PLLA) (Na et al 2006). In addition, the release of photosensitizers from nanoformulations by light, so called photodynamic therapy, was able to induce cytotoxicity as demonstrated for PLGA nanoparticles containing zinc(II) phthalocyanine (Ricci-Junior and Marchetti 2006) and indocyanine green (Gomes et al 2006).
Figure 1.
Graph illustrating contactless controllable drug carrying system based on thermosensitive magnetic nano- and micro particles. The insert shows the application of the system with Rhodamine B encapsulated beads that is released after heating up to 45 °C.
Use of NP formulations in drug delivery
One of the major challenges in drug delivery is to get the drug at the place it is needed in the body thereby avoiding potential side effects to non diseased organs. This is especially challenging in cancer treatment where the tumor may be localized as distinct metastases in various organs. The non restricted (cyto)toxicity of chemotherapeutics thus limits the full use of their therapeutic potential. Local drug delivery or drug targeting results in increased local drug concentrations and provides strategies for more specific therapy. Nanoparticles have specific particles as tools to enable these strategies. These include benefits such as their small size which allows penetration of cell membranes, binding and stabilization of proteins, and lysosomal escape after endocytosis.
The entrapment of chemotherapeutics in nanosized formulations like liposomes has been already subject of study for considerable time (reviews: Crommelin and Storm 2003; Metselaar and Storm 2005; Minko et al 2006). Liposomes as nanosized phospholipid “fatty” structures have the advantage of being small, flexible and biocompatible thus being able to pass along the smallest arterioles and endothelial fenestrations without causing clotting. Now also other materials, including various (co-)polymers and dendrimers at the nanosize range have become available to alter the distribution of encapsulated or attached drugs.
One of the therapeutics under intensive study is paclitaxel (taxol). For paclitaxel the nanoparticle formulation resulted in enhanced cytotoxicity for tumor cells in vitro, and at the same time an increased sustainable therapeutic efficacy in an in vivo animal model (Win and Feng 2006). The paclitaxel was encapsulated in vitamin E TPGS-emulsified poly (D,L-lactic-co-glycolic acid) (PLGA) nanoparticles, and this system resulted in a higher and prolonged level above the effective concentration in vivo, reflected in an increased area under the curve (AUC)
Apart from size the surface chemistry of particles is of crucial importance in particle uptake, distribution and effects. This was shown extensively with acute and chronic models of surface modified micro quartzes (Schins et al 2002; Albrecht et al 2005). Quartz which was coated with PVNO-polymer was taken op by macrophages without toxicity and showed no genotoxicity in epithelial cells or acute and chronic inflammation. On the other hand naïve quartz caused these effects to a large extent. An altered body distribution was demonstrated for two types of polymer particles (Tomazic-Jezic et al 2001). Only PMMA (about 1.4 μm and about 6.4 μm) particles but not PS (about 1.2 μm, 5.2 μm and 12.5 μm) particles could be recovered form the spleen after intraperitoneal administration (Tomazic-Jezic et al 2001). Whether a similar situation exists for nanoparticles is unknown, but studies with surface modified polystyrene particles do suggest different effects on blood coagulation (Nemmar et al 2003), mitochondrial ROS formation and cellular oxidative burst (Xia et al 2006). In addition, as mentioned above the coating of nanoparticles with polyethylene glycol (PEG) increases the time in circulation for the nanoparticles (Bazile et al 1995; Peracchia et al 1999; Niidome et al 2006).
The aims for nanoparticle entrapment of drugs are either enhanced delivery to, or uptake by, target cells and/or a reduction in the toxicity of the free drug to non-target organs. For these aims, creation of long-lived and target-specific nanoparticles is needed. One of the problems is the entrapment of nanoparticles in the mononuclear phagocytic system as present in the liver and spleen (Lenaerts et al 1984; Gibaud et al 1996; Demoy et al 1997; Moghimi et al 2001). However, liver targeting of nanoparticles may be favorable when treating liver diseases like tumor metastasis or hepatitis. Also oligonucleotides for modification of gene expression were demonstrated to migrate into the liver when bound to biodegradable polyalkylcyanoacrylate nanoparticles (Fattal et al 1998). Surface modification with PEG resulted in prolonged presence in the circulation by inhibiting recognition and phagocytosis by the mononuclear phagocytic system (Bazile et al 1995; Peracchia et al 1999; Niidome et al 2006). Besides reduction of therapeutic efficacy, liver entrapment may also have an adverse effect on liver function. For cyanoacrylate and polystyrene nanoparticles (about 214 nm and about 128 nm, respectively) transient liver alterations were observed after acute and chronic intravenous administration (Fernandez-Urrusuno et al 1995, 1997). Inflammatory responses were characterized by secretion of acute phase protein α1-acid glycoprotein by hepatocytes (Fernandez-Urrusuno et al 1995). In addition, antioxidant defenses of hepatocytes were depleted probably as a result of local release of oxidative species (Fernandez-Urrusuno et al 1997).
Although nanoformulation is aimed at enhancing drug delivery without loss of drug activity, in a study comparing insulin-chitosan nanoparticles to chitosan solution and chitosan powder formulations the insulin-chitosan nanoparticles were less effective in terms of bioavailability and lowering blood glucose level in both a rat and sheep model (Dyer et al 2002).
Cellular and intracellular targets
For drug delivery not only organ or cellular targeting is of importance but also the fate of the nanoparticles within the cells. Particles generally end intracellularly in endosomes or lysosomes followed by degradation. For activity of the encapsulated drugs release into the cytosol is needed. However, for nanoparticles of about 20 nm also cellular uptake without contribution by endocytic mechanisms was demonstrated (Edetsberger et al 2005). Chemical characteristics such as surface charge may also determine the fate of nanoparticles in cells. Surface functionalization of gold nanoparticles with PEG resulted in efficient internalization in endosomes and cytosol, and localized in the nuclear region (Shenoy et al 2006). Poly(DL-lactide-co-glycolide) nanoparticles were found to be ingested by cells by endocytosis (Panyam et al 2002; Konan et al 2003). The escape from these endosomes into the cellular cytoplasm was suggested to be caused by a change in surface charge form negative to positive of the PLGA nanoparticles resulting in cytoplasmic delivery of the incorporated drugs. The hypothesis that the positive surface charge influenced the escape of the endosomes was supported by data obtained with negatively charged polystyrene nanoparticles which did not reach the cytosol but remained in the endosomal compartment of the smooth muscle cells used in this study (Panyam et al 2002).
Specific targeting to retinal pigment epithelium cells in the eye is possible (Bourges et al 2003). Very small quantum dots (<10 nm) have been used for specific targeting of peptide coated dots to the vasculature of lungs and tumors (Åkerman et al 2002). In addition, polymer shells on the quantum dots might be linked to targeting molecules. For example quantum dots cores can be coated with hydrophilic polyethylene glycol (PEG) to increase the half life time (Ballou et al 2004). However, also uptake by lymph nodes was demonstrated in which the quantum dots could be observed up until 4 months after administration, so accumulation seems likely (Ballou et al 2004). PEG coating abrogated uptake by the reticuloendothelial system of liver and spleen. In contrast about 40–50 nm magnetic nanoparticles coated with PEG were quite well taken up by endocytosis (Gupta and Curtis 2004).
Surface modifications of nanoparticles offer possibilities for medical applications like drug targeting in terms of cellular binding, uptake and intracellular transport. Carbohydrate binding ligands on the surface of biodegradable and biocompatible poly(D,L-lactic-co-glycolide)acid (PLGA) nanospheres were found to increase cellular binding (Weissenböck et al 2004). Such increased adherence may lead to an enhanced activity of the drug presented as or incorporated in nanoparticles. Coupling specific proteins such as antibodies to the nanoparticle surface may enable a more specific immunologically directed targeting of the particles (Nobs et al 2004; Prinzen et al 2007).
The Brain – the ultimate target for drug delivery
From several perspectives the brain is a challenging organ for drug delivery. First, the incidence of degenerative diseases in the brain will increase with the aging population. Secondly, the blood brain barrier (BBB) is well-known as the best gatekeeper in the body toward exogenous substances (review Pardridge 2007). Generally pharmaceuticals including most small molecules do not cross the BBB. The endothelial barrier is specifically tight at the interface with the brain astrocytes and can in normal conditions only be passed using endogeneous BBB transporters resulting in carrier mediated transport, active efflux transport and/or receptor mediated transport. However the barrier properties may be compromised intentionally or unintentionally by drug treatment allowing passage of nanoparticles (Olivier et al 1999; Kreuter et al 2003; Lockman et al 2003; Koziara et al 2006). The delivery of drugs by nanocarrier was recently reviewed (Koziara et al 2006; Tiwari and Amiji 2006).
Passage of the BBB was suggested to be possible by the toxic effect of nanoparticles (about 200 nm) on cerebral endothelial cells (Olivier et al 1999), although for similar nanoparticles (about 300 nm) this was contradicted in another study (Kreuter et al 2003). In addition this effect was not found for a different type of nanoparticles (Lockman et al 2003). Physical association of the drug to the nanoparticles was necessary for drug delivery to occur into the brain (Kreuter et al 2003). When nanoparticles with different surface characteristics were evaluated, neutral nanoparticles and low concentrations of anionic nanoparticles were found to have no effect on BBB integrity, whereas high concentrations of anionic nanoparticles and cationic nanoparticles were toxic for the BBB. The extent of brain uptake of anionic nanoparticles at lower concentrations was superior to neutral or cationic formulations at the same concentrations. So, nanoparticle surface charges must be considered for toxicity and brain distribution profiles (Lockman et al 2004). Especially coating of the nanoparticles with the polysorbate (Tween) surfactants resulted in transport of drugs across the blood brain barrier (Kreuter 2004). The mechanism for transport was suggested to be endocytosis via the Low Density Lipoprotein (LDL) receptor of the endothelial cells after adsorption of lipoproteins form blood plasma to the nanoparticles (Kreuter 2001, 2004). Additional investigations revealed the role of apolipoprotein-E for transport of drugs across the BBB while apolipoprotein-E variants that did not recognized lipoprotein receptors failed in transporting the drug across the BBB (Michaelis et al 2006). It was suggested that the recognition and interaction with lipoprotein receptors on brain capillary endothelial cells was responsible for the brain uptake of the drug.
Passage of the BBB may also be achieved by masking certain drug characteristics preventing or limiting binding to cellular efflux systems like p-glycoprotein, a cellular transporter associated with drug removal from cells. P-glycoprotein is one of the ATP dependent efflux transporters that has an important physiological role in limiting drug entry into the brain (Girardin 2006; Sharom 2006). In addition, p-glycoprotein also designated the multidrug resistance protein may be highly expressed in drug resistant tumor cells. Surfactant coated poly(butyl) cyanoacrylate nanoparticles have been used to deliver drugs to the CNS (Alyautdin et al 1997) The effect of entrapment of a cytotoxic drug paclitaxel (PX) in cetyl alcohol/polysorbate nanoparticles (PX NP) was evaluated in an in situ rat brain perfusion model (Koziara et al 2004). The results suggest that entrapment of paclitaxel in nanoparticles significantly increases the brain drug uptake and its toxicity towards p-glycoprotein expressing tumor cells (p-glycoprotein is an efflux transporter associated with drug removal from the cells). It was hypothesized that PX nanoparticles limit paclitaxel binding to p-glycoprotein and subsequent efflux from the cells, which consequently would lead to higher brain and tumor cell levels.
Other routes for reaching the brain, circumventing the BBB, may be via migration along the olfactory or trigeminal nerve endings after deposition on the olfactory mucosa in the nasal region (Oberdörster et al 2004). Translocation of ultrafine 13C particles (35 nm) was detected by using this isotope measurement in the brain olfactory bulb after inhalation exposure. Also other solid NP like manganese oxide was shown to translocate to the brain by the olfactory route (Elder et al 2006), based on measurements of manganese in different parts of the brain. In order to increase the specific uptake via the inhalation route nanoparticles have been functionalized by conjugation with bioactive ligands-lectins to the surface of poly (ethylene glycol)- poly (lactid acid) (PEG-PLA) nanoparticles. Wheat germ agglutinin (WGA) was used which binds to N-acetyl-D-glucosamine and sialic acid both of which are abundantly present in the nasal cavity. There was a twofold increase in the brain uptake of such functionalized NP (Gao et al 2006). However, it needs to be stated that both passage of the BBB and the olfactory route only account for up to 2% nanoparticles uptake, and its efficacy with regard to drug delivery needs to make considerable increments before use.
Toxicological hazards of nanoparticles
General concepts
To use the potential of Nanotechnology in Nanomedicine, full attention is needed to safety and toxicological issues. For pharmaceuticals specific drug delivery formulations may be used to increase the so called therapeutic ratio or index being the margin between the dose needed for clinical efficacy and the dose inducing adverse side effects (toxicity). However, also for these specific formulations a toxicological evaluation is needed. This is particularly true for the applications of nanoparticles for drug delivery. In these applications particles are brought intentionally into the human body and environment, and some of these new applications are envisaged an important improvement of health care (Buxton et al 2003; European Technology Platform on Nanomedicine 2005; Ferrari 2005). Opinions started to divert when toxicologists claimed that new science, methods and protocols are needed (Borm 2002; Nel et al 2006). However, the need for this is now underlined by several expert reports (Oberdörster, Maynard et al 2005; SCENIHR 2006) and more importantly by the following concepts:
Nanomaterials are developed for their unique (surface) properties in comparison to bulk materials. Since surface is the contact layer with the body tissue, and a crucial determinant of particle response, these unique properties need to be investigated from a toxicological standpoint. When nanoparticles are used for their unique reactive characteristics it may be expected that these same characteristics also have an impact on the toxicity of such particles. Although current tests and procedures in drug and device evaluation may be appropriate to detect many risks associated with the use of these nanoparticles, it cannot be assumed that these assays will detect all potential risks. So, additional assays may be needed. (SCENIHR 2006) This may differ depending on the type of particles used, ie, biological versus non-biological origin.
Nanoparticles are attributed qualitatively different physico-chemical characteristics from micron-sized particles, which may result in changed body distribution, passage of the blood brain barrier, and triggering of blood coagulation pathways. In view of these characteristics specific emphasis should be on investigations in (pharmaco)kinetics and distribution studies of nanoparticles. What is currently lacking is a basic understanding of the biological behavior of nanoparticles in terms of distribution in vivo both at the organ and cellular level.
Effects of combustion derived nanoparticles in environmentally exposed populations mainly occur in diseased individuals. Typical pre-clinical screening is almost always done in healthy animals and volunteers and risks of particles may therefore be detected at a very late stage.
It may be argued that some if not all of these specific effects will be detected during routine testing and post marketing evaluation after clinical use. All would depend on the types of assays used in the preclinical evaluation, which should be considered in the light of the use of the final products. In addition, one cannot rely on the toxicological profile of the bulk material when that material is used in a nanoformulation. What is clear is that the safety evaluation and the risk benefit analysis need to be performed on a case by case basis.
The use of nanoparticles as drug carrier may reduce the toxicity of the incorporated drug. In general the toxicity of the whole formulation is investigated while results of the nanoparticles itself are not described. So, discrimination between drug and nanoparticle toxicity cannot be made. So, there should be a specific emphasis on the toxicity of the “empty” non-drug loaded particles. This is especially important when slowly or non degradable particles are used for drug delivery which may show persistence and accumulation on the site of the drug delivery, eventually resulting in chronic inflammatory reactions.
Evidence for nanoparticle toxicity
The largest database on the toxicity of nanoparticles has originated from inhalation toxicology including the PM10 literature (particulate matter with a size below 10 mm), where the ‘NP hypothesis’ has proved to be a powerful drive for research (Donaldson et al 2002, 2004; Oberdörster, Oberdörster et al 2005; Borm et al 2006). An overview of particle terminology in relation to ambient effects is given in Table 3. Therefore it relevant to discuss this evidence in the expectation that it will shed light on the toxicity of engineered NPs. The idea that combustion-derived NPs are an important component that drives the adverse effects of environmental particulate air pollution or PM10 comes from several sources:
Table 3.
Various denominations of particles in inhalation toxicology and drug delivery in relation to their source (ambient, bulk, engineered)
| Particle type | Description |
|---|---|
| PM10, PM2.5 | Particle mass fraction in ambient air with a mean diameter of 10 or 2.5 μm respectively. Basis of current standards for ambient particles in Europe and USA |
| Coarse particles | The mass fraction of PM10, which is bigger than 2.5 μm |
| Ultrafine particles (PM0.1) | The fraction of PM10 with a size cut-off at 0.1 μm. Contains primary particles and agglomerates smaller than 100 nm |
| PSP | Poorly soluble particles with low specific toxicity. Maybe be fine or ultrafine. Terminology used in relation to bulk synthetic particles. Examples TiO2, carbon blacks, Amorphous silica, Iron oxides (Fe2O3), Zinc oxides (ZnO) |
| CDNP | Combustion derived nanoparticles, such as diesel exhaust particles (DEP) |
| DEP | Diesel exhaust particles |
Much of the mass of PM10 is considered to be non-toxic and so there has arisen the idea that there is a component(s) of PM10 that actually drives the pro-inflammatory effects and combustion-derived NP seems a likely candidate.
Nanoparticles are the dominant particle type by number suggesting that they may be important and their small size means that they have a large surface area per unit mass. Particle toxicology suggests that, for toxic particles generally, more particle surface equals to more toxicity.
Substantial toxicological data and limited data from epidemiological sources support the contention that NPs in PM10 are important drivers of adverse effects.
The adverse health effects of particulate matter (PM) are measurable as exacerbations of respiratory disease and deaths as well as hospitalizations and deaths from respiratory and cardiovascular disease (Dockery et al 1993; Brooke et al 2004; Pope et al 2004). Inflammation is the common factor that binds together these adverse effects and the ability of NPs to cause inflammation can be seen as an important property. It is not clear what effects of NPs have pulmonary inflammation as a prerequisite and what effects could potentially be driven by exposures below those causing inflammation. There is also the potential for pulmonary inflammation to result in changes in membrane permeability that in turn may impact the potential for particles to distribute beyond the lung. Some NPs may have the extra potential of affecting cardiovascular disease directly. Vascular function was impaired after inhalation of diesel exhaust particles (Mills et al 2005). However, data to date are limited and not all studies of nanoparticles have shown significant translocation from lung to the blood. In some studies translocation has been rather minimal (Kreyling et al 2002; Takenaka et al 2006). Understanding clearance kinetics of inhaled ambient air nanoparticles will also be important in understanding their potential for adverse effects.
The current paradigm in particle toxicology is that ultrafine ambient air particles have the potential of affecting cardiovascular disease both indirectly via pulmonary inflammation and directly through particle distribution. Although important, this property of redistribution has yet to be demonstrated for NPs present in real PM10. It should be noted that there are several mechanisms whereby NPs could lead to inflammatory effects, as is the case for larger particles. These mechanisms are either based on the large surface area of particle core or on soluble components released by the NPs. In addition various chemicals including those of biological origin like endotoxin may be adsorbed onto the NP and released (Carty et al 2003; Kreyling et al 2004; Schins et al 2004). Several toxicological studies support the contention that NPs in PM10 could drive inflammatory effects. There are a number of components of PM10 that contribute to the mass but have little toxicity – these include salts such as sulfates, chlorides and ammonium salts and nitrates, but also wind-blown or crustal dust. In fact within PM10 there are only few components that toxicologists would identify as likely mediators of adverse effects – ie, particle surfaces, organics, metals and endotoxin (in some PM10 samples). In fact, a large surface area, organics and metals are all characteristic of combustion–derived particles and so these have attracted considerable toxicological attention (Donaldson et al 2005). However, it is difficult to untangle, in a combustion particle sample, the relative roles of surface, organics and metals, although this has been most attempted in vitro. The aggregation of multiple chemical species including biological compounds like endotoxin limits the extrapolation of the results on the toxicological effects of such particles.
Toxicological effects of nanoparticles
As already mentioned above, NPs exert some very special properties that are very relevant in the further design of toxicity testing of engineered nanomaterials. An overview of most striking effects of (nano) particles that have been observed over the last decades is given in Table 4 along with the particle type that have been tested in this response. Several effects are just quantitatively different from fine particles. In this case nanoparticles may cause the same effects as ‘traditional’ particles (eg, inflammation, lung cancer) but they may be more potent because of their greater surface area.
Table 4.
Toxicity of engineered and combustion (nano) particles as illustrated by their most unique adverse effects in vivo and in vitro
| Description of finding, in vivo | Particle types |
|---|---|
| NPs cause pulmonary inflammation in the rat | All PSP |
| Later studies show that inflammation is mediated by surface area dose | SWCNT, MWCNT |
| NPs cause more lung tumors than fine particles in rat chronic studies. Effect is surface area mediated | PSP only |
| NPs cause progression of plague formation (ApoE -/- mice) | SWCNT, PM2.5 |
| NPs affect immune response to common allergens | Polystyrene, CB, DEP |
| NsP can have access to systemic circulation upon inhalation and instillation | Specific NP, dependent on surface coating |
| Description of finding, in vitro | |
| NPs cause oxidative stress in vivo and in vitro, by inflammatory action and generation of surface radicals | PSP, NP general, CNT |
| NPs inhibit macrophage phagocytosis, mobility and killing | CB, TiO2 |
| NPs cause platelet aggregation | PM, SWCNT, fullerenes, latex-COOH surface |
| NPs exposure adversely affects cardiac function and vascular homeostasis | PM, SWCNT |
| NPs interfere with Ca-transport and cause increased binding of pro-inflammatory transcription factor NF-kB | CB (< 100 nm), ROFA, PM2.5 |
| NPs can affect mitochondrial function | Ambient NP, |
| NPs can translocate to the brain from the nose | MnO2, Au, carbon |
| NPs do affect rolling in hepatic tissue | CB |
However, nanoparticles could also cause new types of effects not previously seen with larger particles (eg, mitochondrial damage, uptake through olfactory epithelium, platelet aggregation, cardiovascular effects). These effects depicted in Table 4 clearly need a new way of handling their toxicology. In addition, epidemiological evidence suggests that these effects occur predominantly in subjects that have an impaired health. This finding should be considered in developing toxicological testing models.
Effects on blood and cardiovascular system
As we discussed earlier, ligand coated engineered nanoparticles are being explored and used as agents for molecular imaging or drug delivery tools. This has led to a considerable understanding of particle properties that can affect penetration in tissue without affecting tissue function. Cationic NPs, including gold and polystyrene have been shown to cause hemolysis and blood clotting, while usually anionic particles are quite non-toxic. This conceptual understanding maybe used to prevent potential effects of unintended NP exposure. Similarly, drug loaded nanoparticles have been used to prolong half-life or reduce side-effects and have shown which particle properties need to be modified to allow delivery, while being biocompatible (Gupta and Gupta 2005).
On the other hand, one is trying to find explanations for the increased risk of patients with cardiovascular diseases upon exposure to PM and/or traffic. Several toxicological studies have demonstrated that combustion and model NPs can gain access to the blood following inhalation or instillation and can enhance experimental thrombosis but it is not clear whether this was an effect of pulmonary inflammation or particles translocated to the blood (Nemmar et al 2002, 2003; Mills et al 2005). High exposures to DEP by inhalation caused altered heart rate in hypertensive rats (Campen et al 2003) interpreted as a direct effect of DEP on the pacemaker activity of the heart. Inflammation in distal sites has long been associated with destabilization of atheromatous plaques and both instillation and inhalation of PM cause morphological evidence of atheromatous plaque increase and destabilization in rabbits (Suwa et al 2002) and mice (Chen and Nadziejko 2005). Ultrafine carbon black instilled into the blood has been reported to induce platelet accumulation in the hepatic microvasculature of healthy mice in association with prothrombotic changes on the endothelial surface of the hepatic microvessels (Khandoga et al 2004). Recent studies with carbon derived nanomaterials showed that platelet aggregation was induced by both single and multi-wall carbon nanotubes, but not by the C60-fullerenes that are used as building blocks for these CNT (Radomski et al 2005). These data show that not all nanomaterials act similar in this test, and that surface area is not the only factor playing a role here. The data also corroborate the earlier concept developed in medicine that mainly cationic species have an effect on blood clotting. Interestingly, this is the first study that allows bridging of data, since also a real life PM10 sample (SRM1648) was included in the test-series. Actually the PM sample showed a lower effect compared to the carbon nanotubes (Radomski et al 2005).
Uptake and effects of nanoparticles in the brain
Nanoparticles can get access to the brain by two different mechanisms, ie, (1) transsynaptic transport after inhalation through the olfactory epithelium, and (2) uptake through the blood-brain barrier. The first pathway has been studied primarily with model particles such as carbon, Au and MnO2 in experimental inhalation models in rats (Oberdörster et al 2004; Oberdörster, Oberdörster et al 2005). The second pathway has been the result of extensive research and particle surface manipulation in drug delivery (Kreuter 2001; Koziara et al 2006; Tiwari and Amiji 2006). The latter studies suggest that the physiological barrier may limit the distribution of some proteins and viral particles after transvascular delivery to the brain, suggesting that the healthy BBB contains defense mechanisms protecting it from blood borne nanoparticle exposure. When nanoparticles with different surface characteristics were evaluated, neutral nanoparticles and low concentrations of anionic nanoparticles were found to have no effect on BBB integrity, whereas high concentrations of anionic nanoparticles and cationic nanoparticles were toxic for the BBB. Nanoparticles have been shown to induce the production of reactive oxygen species and oxidative stress (Nel et al 2006) and this has been confirmed in the brain after inhalation of MnO2 nanoparticles (Elder et al 2006). Oxidative stress has been implicated in the pathogenesis of neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases. Evidence for the involvement of ambient air nanoparticles in these effects is presented by studies in biopsies from city dwellers. Alzheimer’s like pathology was demonstrated in brain sections by increased markers of inflammation and AB42-accumulation in frontal cortex and hippocampus in association with the presence of nanoparticles (Calderon-Garciduenas et al 2004). Also inhalation exposure of BALB/c mice to particulate matter showed activation of pro-inflammatory cytokines in the brain (Campbell et al 2005). Whether this is due to the fraction of combustion nanoparticles remains to be investigated.
Current data on the toxicology engineered nanoparticles
In the past few years a number of papers have described the toxicology of newly engineered nanomaterials, including fullerenes (Sayes et al 2005), carbon nanotubes (Donaldson et al 2006), quantum dots (Hardman 2006) and have illustrated that apart from size and surface area, many more parameters describing the material (surface) properties have to be included. In a recent report Costigan (2006) reviewed the evidence for toxicity of NPs used in healthcare products. Her conclusions again stressed the limited availability of toxicity data of the NPs in use.
Carbon nanotubes
Carbon nanotubes are long carbon-based tubes that can be either single- or multiwalled and have the potential to act as biopersistent fibers. Nanotubes have aspect ratios >100, with lengths of several mm and diameters of 0.7 to 1.5 nm for single-walled carbon nanotubes (SWCNT) and 2 to 50 nm for multiwalled carbon nanotubes (MWCNT). In vitro incubation of keratinocytes and bronchial epithelial cells with high doses of SWCNT results in ROS generation, lipid peroxidation, oxidative stress, mitochondrial dysfunction, and changes in cell morphology (Shvedova et al 2003; Sayes et al 2006). Recent studies with carbon derived nanomaterials showed that platelet aggregation was induced by both single and multi-wall carbon nanotubes, but not by the C60-fullerenes that are used as building blocks for these CNT (Radomski et al 2005). MWCNT also elicit pro-inflammatory effects in keratinocytes (Monteiro-Riviere et al 2005). Several studies using intratracheal instillation of high doses of nanotubes in rodents demonstrated chronic lung inflammation, including foreign-body granuloma formation and interstitial fibrosis (Warheit et al 2004; Muller et al 2005). In two in vivo studies SWCNTs were demonstrated to induce lung granulomas after intratracheal administration (Lam et al 2004; Warheit et al 2004) indicating that these nanotubes can not be classified as a new form of graphite on material safety data sheets. On a dose per mass basis the nanotubes were more toxic than quartz particles well known for their lung toxicity. Carbon black, carbonyl iron and graphite produced no significant adverse effects (Lam et al 2004; Warheit et al 2004). These studies also reveal the tendency of the nonphysiologic administration route and the unrealistic high doses to lead to asphyxiation through nanotube clumping in the airways (Warheit et al 2004; Muller et al 2005). Although it has been suggested that the granulomatous inflammation could be a biopersistent fiber effect, the high dose of the aggregated nanotubes and the presence of metal impurities (eg, Fe) could account for artificial toxicity.
Fullerenes
Fullerenes are being explored as potential new antimicrobial agents in view of their potency for induction of reactive oxygen species after photoexcitation (Yamakoshi et al 2003). However, this may have an impact on microbial communities if they are released into the environment via effluents. Therefore, various studies with fullerenes have been published with regard to the ecotoxicity of these important building blocks in nanomaterials. Tests with un-coated, water soluble, colloidal fullerenes (nC60) show that the 48-hour LC50 in Daphnia magna varied form 460 to 800 ppb (Lovern and Klaper 2006; Zhu et al 2006), using standard EPA protocols. However, for sonicated C-60 fullerenes the LC50 was one order of magnitiude higher with 7.9 ppm (Lovern and Klaper 2006). In largemouth brass, although no mortality was seen, lipid peroxidation was seen in the brain and glutathione depletion in the gill after exposure to 0.5 ppm nC60 for 48 hours (Oberdörster 2004). There are several hypotheses as to how lipid damage may have occurred in the brain, including direct redox activity by fullerenes reaching the brain via circulation or axonal translocation and dissolving into the lipid-rich brain tissue, oxygen radical production by microglia, or production of reactive fullerene metabolites by cytochrome P450 metabolism.
Dendrimers
Because of their specific nature dendrimers are specifically suited for drug delivery purposes. Although their small size (up to 10 nm) limits extensive drug incorporation into the dendrimers, their dendritic nature and branching allows for drug loading onto the outside surfaces of the polymeric structure (Svenson and Tomalia 2005). Functionalization of the surface with specific antibodies may further enhance potential targeting. Apart from application in drug-delivery, dendrimers are being investigated for many other uses including bacterial cell killing, as gene transfer agents and trans-membrane transport. Little published data is available on the toxicity of this class of particles. A recent review on this topic (Duncan and Izzo 2005) concluded that it will only ever be possible to designate a dendrimers as “safe” when related to a specific application. The so far limited clinical experience with dendrimers makes it impossible to designate any particular chemistry intrinsically “safe” or “toxic”.
Quantum dots
Quantum dots are a heterogeneous group of nanoparticles (reviewed by Hardman 2006). Quantum dot absorption, distribution, metabolism and excretion, and therefore also quantum dot toxicity, depend on multiple factors derived from both inherent physicochemical properties and environmental conditions. Quantum dots may vary in size ranges from 2.5 up to 100 nm, depending on coating thickness. Studies specifically performed to investigate quantum dot toxicity are few (Hardman 2006). In vitro studies have indicated that quantum dots may be toxic (Hoshino et al 2004; Shiohara et al 2004; Lovric, Bazzi et al 2005) of which some toxicity could be attributed to the surface coating (Hoshino et al 2004, 2007). Choi et al (2007) demonstrated that quantum dot toxicity was reduced after surface modification with N-acetylcysteine, while the non modified cadmium telluride quantum dots induced lipid peroxidation in the cells. Lovric, Cho et al (2005) showed “naked” quantum dots to be cytotoxic by induction of reactive oxygen species resulting in damage to plasma membranes, mitochondria and nucleus. As it is the bioactive coating which allows the use of quantum dots for specific targeting to cells and/or cell organelles, attention is warranted in using the surface molecules in terms of induction of toxic effects. However, also the quantum dot core material has an effect on the toxic potential of the quantum dots as for cadmium containing quantum dots the toxicity was suggested to be due to release of highly toxic free Cd2+ ions (Derfus et al 2004; Kirchner et al 2005). For quantum dots composed of cadmiun/telluride cellular toxicity was found but not for cadmium selenium/zinc sulfate quantum dots (Cho et al 2007). On the other hand Hardman (2006) also reported on studies demonstrating a lack of both in vitro and in vivo toxicity. However, before there can be a responsible development of quantum dots with minimal risks more information on toxicological risks needs to be provided.
Gold nanoparticles/nanoshells
In the summary of evaluations performed by the Joint FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) Expert Committee on Food Additives (JECFA) gold was not considered to present a hazard when used as coloring agent and food additive (JECFA 2001). However, such evaluations did not consider nanoformulations of gold. Metallic colloidal gold nanoparticles are widely used, can be synthesized in different forms (rods, dots), are commercially available in various size ranges and can be detected at low concentrations. Cells can take up gold nanoparticles without cytotoxic effects (Connor et al 2005; Shenoy et al 2006). For biomedical applications, they are used as potential carriers for drug delivery, imaging molecules and even genes (Kawano et al 2006), and for the development of novel cancer therapy products (Hirsch et al 2003; Hainfeld et al 2004; Loo et al 2004; O’Neal et al 2004; Radt et al 2004). For gold nanorods the cytotoxicity could be attributed to the presence of the stabilizer CTAB of which even residual presence after washing resulted in considerable cytotoxicity. PEG-modified gold nanorods with removing the excess CTAB did not show cytotoxicity (Niidome et al 2006). In an acute oral toxicity study no signs of gross toxicity or adverse effects were noted when a nanogold suspension (nanoparticle diameter ca. 50 nm) was evaluated, the single dose for acute oral LD50 being greater than 5000 mg/kg body weight (Lai et al 2006).
Gold solutions are also used to prepare nanoshells composed of gold and copper, or gold and silver to function as contrast agents in Magnetic Resonance Imaging (RMI) (Su et al 2006), and gold-silica for photothermal ablation of tumor cells (Bernardi et al 2007; Stern et al 2007). In vitro the non targeted nanoshells did not show cytotoxicity for the tumor cells, whereas after binding to the tumor cells cell death could be obtained after laser activation (Lowery et al 2006; Bernardi et al 2007; Stern et al 2007). Also in vivo positive results were obtained with photothermal ablation therapy in a mouse model for colon carcinoma after intraveneous administration of PEG coated gold nanoshells of approximately 130 nm (O’Neal et al 2004).
Silica
For silica nanoparticles both in vitro toxic and non toxic responses were observed. Both 15 nm and 46 nm silica nanoparticles showed similar dose dependent cytotoxicity in vitro (Lin et al 2006). There was an increase in toxicity both at increasing doses and at increasing exposure time (24, 48, and 72 h). SiO2 exposure resulted in an increased ROS levels and reduced glutathione levels indicating an increase in oxidative stress. Also Chang et al (2007) found silica nanoparticles to be toxic at high dosages as shown by a reduction in cell viability/cell proliferation and by lactate dehydrogenase (LDH) release from the cells indicating membrane damage. Cells with a long doubling time were more susceptible for the cytotoxic effects of the silica nanoparticles than cells with short doubling times (Chang et al 2007). In another study only at concentrations above 0.1 mg/ml a significant reduction in cell viability was observed (Jin et al 2007). In addition, an alveolar macrophage cell line (MHS) was found to be more susceptible for nanaoparticle induced cytotoxicity than a lung epithelial cell line (A549) which was suggested to be due to the phagocytic properties of the macrophage cell line. Cell death was probably not caused by apoptosis (Jin et al 2007). In contrast for cationic silica nanoparticles using amino-hexyl-amino-propyltrimethoxysilane as a surface modification low or no cell toxicity was observed (Ravi Kumar et al 2004).
Nanomaterials in medicine: needs
Although there is a considerable amount of data on the toxicity of NPs, this data is mainly based on a small panel of NPs (combustion derived NPs, TiO2, CB) and the assumption that a lot of effects by particulate matter are driven by the ultrafine particle fraction in it (Donaldson et al 2002; Oberdörtster, Oberdörster et al 2005; Borm and Muller-Schulte 2006). In most studies the nanoparticles were used as a model for ambient air particle toxicity. One of the more general conclusions is that indeed there is a clear tendency for very small (nano) particles to be more toxic than larger particles with the same chemical composition.
For nanoformulations used in drug delivery the focus in most papers is mainly on obtained reduction of toxicity of the incorporated drug, whereas the possible toxicity of the carrier used is not considered. Especially possible residues of such a treatment may harbor potential local and/or systemic toxic responses.
For medical applications certain routine assays need to be performed which will detect a number of potential hazards. However, it can be anticipated that not all hazards are at this moment known for the use of nanoparticles. In a recent report Costigan (2006) reviewed the evidence for toxicity of NPs used in healthcare products. Her conclusions again stressed the limited availability of toxicity data of the NPs in use. However, in NPs for healthcare products most if not all mechanisms of toxicity could be identified by conventional hazard identification testing as currently required to comply with the regulations for healthcare products (Costigan 2006). Costigan identified four possible mechanisms of NP toxicity, being chemical toxicity of one of the constituents with the same mode of action as the bulk chemical, toxicity due to degradation products, toxicity due to endocytosis of the NPs, and membrane lysis due to the NPs possibly via chemical toxicity.
Although hazard identification is the general approach for safety evaluation of healthcare products, it is recommended to add testing driven by the anticipated application and classification by risk. Some engineered NPs, which get airborne will pose inhalation hazards, while cosmetics with NPs provide dermal exposures. For parenteral use interactions with blood components, systemic distribution and kinetics are of importance, when engineered NPs are being used as devices to target drugs to specific tissues, to increase their biological half time, or for imaging purposes. Each nanoparticle formulation should be tested on a case by case basis in the requisite ways focusing on their portal of entry. In this respect also the potential adverse (toxic) effects of empty particles should be considered. In developing testing procedures and protocols a number of basic issues need to be considered:
Which effects are specific for nanomaterials, and which effects are merely stronger? Nanoparticles may cause the same effects as ‘traditional’ particles (eg, inflammation, lung cancer) but they may be more potent because of their greater surface area. Nanoparticles could also cause new types of effects not previously seen with larger particles or bulk chemicals.
Can we extrapolate available data and concepts? The epidemiological evidence on ultrafine particles has revealed several effects, mechanisms of action and susceptible groups upon inhalation of ultrafine particles. Whether these concepts can be used for nanoparticles released from manufactured nanomaterials is yet unknown.
Is our current regulation robust enough to handle risks of nanomaterials? We deal with a growing set of materials of which the properties are largely unknown and for which current testing procedures and legislation might produce false negatives and/or false positives. The central question here is whether current testing and classification protocols are appropriate or sufficient. These will detect certain toxic effects as demonstrated by the studies already published. However, it can be anticipated that not all hazards will be detected and additional specific testing may be needed. Nanotechnology also promotes convergence of technologies, and for example similar materials may be applied in the automotive and the life sciences sector. To stimulate production and marketing of safe nanomaterials exchange of data between sectors is recommended. Sharing of information on the toxicity of nanomaterials, will significantly reduce time to market for many products and producers.
Should we use the precautionary principle in current regulatory testing? The precautionary principle (PP) is a highly debated issue in international politics and was first added in EU environmental regulation in the Maastricht treaty in Article 174 (ex Art 130r). The PP points out that scientific uncertainty is no reason for inaction if there might be strong adverse effects. It has been criticized for being too vague and too arbitrary to form a basis for rational decision-making. On the other hand the PP does not necessarily imply a complete ban of substances but may be applied in steps of decreasing uncertainty or perceived risk. It is typically applied where scientific information is insufficient, inconclusive or uncertain whilst at the same time there are indications that potential effects may occur. Approaches in risk governance range from early explorations by Swiss RE (Hett 2004) to more recent detailed reviews such by Renn and Roco (2006) and the Health Council of the Netherlands (2006). A crucial difference between both reports is that Renn and Roco place the nanotechnology products themselves into the risk issue categories rather than the risk issues that accompany the use of these products. This difference may seem trivial, but is important since it is the purpose and application rather than the device itself that connect hazard to exposure, and therefore creates a risk. For example, it fails to distinguish between brain implants for enhancement purposes and those for fighting tremor in Parkinson patients. This may pave the way to one nanotechnology product compromising or allowing all the others. Furthermore, it can convey the impression that the broader societal implications and accompanying ethical issues of nanotechnologies are as new as the nanotechnology products themselves, or even are still in the future. However, most of them are not.
Conclusions
The use of Nanotechnology in medicine and more specifically drug delivery is set to spread rapidly. For decades pharmaceutical sciences have been using nanoparticles to reduce toxicity and side effects of drugs. Up to recently it was not realized that these carrier systems themselves may impose risks to the patient. The type of hazards that are introduced by using nanoparticles for drug delivery are beyond that posed by conventional hazards imposed by chemicals in delivery matrices. However, so far, the scientific paradigm for the possible (adverse) reactivity of nanoparticles is lacking and we have little understanding of the basics of the interaction of nanoparticles with living cells, organs and organisms. A conceptual understanding of biological responses to nanomaterials is needed to develop and apply safe nanomaterials in drug delivery in the future. Furthermore a close collaboration between those working in drug delivery and particle toxicology is necessary for the exchange of concepts, methods and know-how to move this issue ahead.
References
- Albrecht C, Knaapen AM, Becker, et al. The crucial role of particle surface reactivity in respirable quartz induced reactive oxygen/nitrogen species formation and APE/Ref-1 induction in rat lung. Respir Res. 2005;6:129. doi: 10.1186/1465-9921-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerman ME, Chan WCW, Laakkonen P, et al. Nonocrystal targeting in vivo. PNAS. 2002;99:12617–21. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyautdin RN, Petrov VE, Langer K, et al. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. J Pharm Res. 1997;14:325–8. doi: 10.1023/a:1012098005098. [DOI] [PubMed] [Google Scholar]
- Ballou B, Lagerholm BC, Ernst LA, et al. Non-invasive imaging of quantum dots in mice. Bioconjug Chem. 2004;15:79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- Baran ET, Özer N, Hasirci V. In vivo half life of nanoencapsulated L-asparaginase. J Mat Sc: Mat in Med. 2002;13:1113–21. doi: 10.1023/a:1021125617828. [DOI] [PubMed] [Google Scholar]
- Bazile D, Prud’Homme C, Bassoullet M-T, et al. Stealth PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84:493–8. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- Benardi RJ, Lowery AR, Thompson PA, et al. Immunoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neurooncol. 2007 Sep 6; doi: 10.1007/s11060-007-9467-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Borm PJ. Particle toxicology: from coal mining to nanotechnology. Inhal Toxicol. 2002;14:311–24. doi: 10.1080/08958370252809086. [DOI] [PubMed] [Google Scholar]
- Borm PJ, Kreyling W. Toxicological hazards of inhaled nanoparticles – potential implications for drug delivery. J Nanosc Nanotechnol. 2004;4:521–31. doi: 10.1166/jnn.2004.081. [DOI] [PubMed] [Google Scholar]
- Borm PJ, Muller-Schulte D. Nanoparticles in drug delivery and environmental exposure: same size, same risks? Nanomedicine. 2006;1:235–49. doi: 10.2217/17435889.1.2.235. [DOI] [PubMed] [Google Scholar]
- Borm PJA, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fiber Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourges JL, Gautier SE, Delie F, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–9. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, et al. Air pollution and vascular disease. A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Buxton DB, Lee SC, Wickline SA, et al. Recommendations of the National Heart, Lung, and Blood Institute Nanotechnology Working Group. Circulation. 2003;108:2737–42. doi: 10.1161/01.CIR.0000096493.93058.E8. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–8. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–40. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Campen MJ, McDonald JD, Gigliotti AP, et al. Cardiovascular effects of inhaled diesel exhaust in spontaneously hypertensive rats. Cardiovasc Toxicol. 2003;3:353–61. doi: 10.1385/ct:3:4:353. [DOI] [PubMed] [Google Scholar]
- Carty CL, Gehring U, Cyrys J, et al. Seasonal variability of endotoxin in ambient fine particulate matter. J Environm Monit. 2003;5:953–8. doi: 10.1039/b308488d. [DOI] [PubMed] [Google Scholar]
- Cascone MG, Lazzeri L, Carmignani C, et al. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J Mat Sc: Mat in Med. 2002;13:523–6. doi: 10.1023/a:1014791327253. [DOI] [PubMed] [Google Scholar]
- Chang JS, Chang KLB, Hwang DF, et al. In vitro cytotoxicity of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 2007;41:2064–8. doi: 10.1021/es062347t. [DOI] [PubMed] [Google Scholar]
- Chen LC, Nadziejko C. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice V CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhal Toxicol. 2005;17:217–24. doi: 10.1080/08958370590912815. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Maysinger D, Jain M, et al. Long term exposure to CdTe quantum dots causes functional impairment in living cells. Langmuir. 2007;23:1974–80. doi: 10.1021/la060093j. [DOI] [PubMed] [Google Scholar]
- Choi AO, Cho SJ, Desbarats J, et al. Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. J Nanobiotechnology. 2007;12:1. doi: 10.1186/1477-3155-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Costigan S. The toxicology of nanoparticles used in health care products. 2006 Available at the website of the Medicines and Healthcare products Regulatory Agency, Department of Health, UK. Accessed 20 November 2006 URL: http://www.mhra.gov.uk/home/idcplg?IdcService=SS_GET_PAGE&nodeId=996.
- Crommelin DJ, Storm G. Liposomes: from the bench to the bed. J Liposome Res. 2003;13:33–6. doi: 10.1081/lpr-120017488. [DOI] [PubMed] [Google Scholar]
- Damascelli B, Patelli GL, Lanocita R, et al. A novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: preliminary findings. Am J Roentgenol. 2003;181:253–603. doi: 10.2214/ajr.181.1.1810253. [DOI] [PubMed] [Google Scholar]
- De Jong WH, Geertsma RE, Roszek B. Possible risks for human health. Report 265001002/2005. Bilthoven, The Nethrlands: National Institute for Public Health and the Environment (RIVM); 2005. Nanotechnology in medical applications. [Google Scholar]
- Demoy M, Gibaud S, Andreux JP, et al. Splenic trapping of nanoparticles: complementary approaches for in situ studies. Pharm Res. 1997;14:463–8. doi: 10.1023/a:1012095431931. [DOI] [PubMed] [Google Scholar]
- Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Letters. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Brown D, Clouter A, et al. The pulmonary toxicology of ultrafine particles. J Aerosol Med. 2002;15:213–20. doi: 10.1089/089426802320282338. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V. Current hypotheses on the mechanism of toxicity of ultrafine particles. Ann Ist Super Sanità. 2003;39:405–10. [PubMed] [Google Scholar]
- Donaldson K, Stone V, Clouter A, et al. Ultrafine particles. Occup Environ Med. 2001;58:211–16. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Tran CL, et al. Nanotoxicology. Occup Environ Med. 2004;61:727–28. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Tran L, Jimenez LA, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher KL. Toxicological highlight. Health and environmental impact of nanotechnology: Toxicological assessment of manufactured nanoparticles. Toxicol Sc. 2004;77:3–5. doi: 10.1093/toxsci/kfh041. [DOI] [PubMed] [Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Disc. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Dyer M, Hinchcliffe M, Watts P, et al. Nasal delivery of insulin using novel chitosan based formulations: a comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res. 2002;19:998–1008. doi: 10.1023/a:1016418523014. [DOI] [PubMed] [Google Scholar]
- Edetsberger M, Gaubitzer E, Valic E, et al. Detection of nanometer-sized particles in living cells using modern fluorescence fluctuation methods. Biochem Biophys Res Commun. 2005;332:109–16. doi: 10.1016/j.bbrc.2005.04.100. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–8. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Technology Platform on Nanomedicine. 2005. Vision paper and basis for a strategic research agenda for nanomedicine. European Commission Luxembourg, Office for Official Publications of the European Commission, ISBN 92-894-9599-5
- European Science Foundation. 2005. Policy Briefing (ESF), ESF Scientific Forward Look on Nanomedicine IREG Strasbourg, France, ISBN 2-912049-520, 2005.
- Fang C, Shi B, Pei YY, et al. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Fattal E, Vauthier C, Aynie I, et al. Biodegradable polyalkylcyanoacrylate nanoparticles for the delivery of oligonucleotides. J Control Release. 1998;53:137–43. doi: 10.1016/s0168-3659(97)00246-0. [DOI] [PubMed] [Google Scholar]
- Fernández-Urrusuno R, Fattal E, Porquet D, et al. Evaluation of liver toxicological effects induced by polyalkylcyanoacrylate nanoparticles. Toxicol Appl Pharmacol. 1995;130:272–9. doi: 10.1006/taap.1995.1032. [DOI] [PubMed] [Google Scholar]
- Fernández-Urrusuno R, Fattal E, Féger J, et al. Evaluation of hepatic antioxidant systems after intravenous administration of polymeric nanoparticles. Biomaterials. 1997;18:511–17. doi: 10.1016/s0142-9612(96)00178-0. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Chorny M, Banai S, et al. Formulation and delivery mode affect disposition and activity of tyrphostin-loaded nanoparticles in the rat carotid model. Arterioscler Thromb Vasc Biol. 2001;21:1434–9. doi: 10.1161/hq0901.095567. [DOI] [PubMed] [Google Scholar]
- Gao X, Tao W, Lu W, et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27:3482–90. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Gibaud S, Demoy M, Andreux JP, et al. Cells involved in the capture of nanoparticles in hematopoietic organs. J Pharm Sci. 1996;85:944–50. doi: 10.1021/js960032d. [DOI] [PubMed] [Google Scholar]
- Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Cliin Neurosci. 2006;8:311–21. doi: 10.31887/DCNS.2006.8.3/fgirardin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AJ, Lunardi LO, Marchetti JM, et al. Indocyanine green nanoparticles useful for photomedicine. Photomed Laser Surg. 2006;24:514–21. doi: 10.1089/pho.2006.24.514. [DOI] [PubMed] [Google Scholar]
- Granum B, Lovik M. The effect of particles on allergic immune responses. Toxicol Sci. 2002;65:7–17. doi: 10.1093/toxsci/65.1.7. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Curtis ASG. Surface modified supermagnetic nanoparticles for drug delivery: interaction studies with human firbroblasts in culture. J Mater Sci: Mat in Med. 2004;15:493–6. doi: 10.1023/b:jmsm.0000021126.32934.20. [DOI] [PubMed] [Google Scholar]
- Gupta M, Gupta AK. In vitro cytotoxicity studies of hydrogel pullulan nanoparticles prepared by AOT/N-hexane micellar system. J Pharm Pharmaceut Sci. 2004;7:38–46. [PubMed] [Google Scholar]
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:309–15. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- Hardman R. A toxicological review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–72. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Council of the Netherlands. The Hague: Health Council of the Netherlands; 2006. Health significance of nanotechnologies. publication no 2006 /06E http://www.healthcouncil.nl. [Google Scholar]
- Hett A. Nanotechnology. Small matter, many unknowns. Risk perception. Swiss Re, Zurich, Switzerland: 2004. [Google Scholar]
- Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Fujioka K, Oku T, et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–9. [Google Scholar]
- Hoshino A, Manabe N, Fujioka K, et al. Use of fluorescent quantum dot bioconjugates for cellular imaging of immune cells, cell organelle labeling, and nanomedicine: surface modification regulates biological function, including cytotoxicity. J Artif Organs. 2007;10:149–57. doi: 10.1007/s10047-007-0379-y. [DOI] [PubMed] [Google Scholar]
- Huang M, Khor E, Lim L-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res. 2004;21:344–53. doi: 10.1023/b:pham.0000016249.52831.a5. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food additives) Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives. INS 175. 2001 http://jecfa.ilsi.org/evaluation.cfm?chemical=GOLD%20 (METALLIC) &keyword=GOLD.
- Jin Y, Kanna S, Wu M, et al. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007;20:1126–33. doi: 10.1021/tx7001959. [DOI] [PubMed] [Google Scholar]
- Kawano T, Yamagata M, Takahishi H, et al. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J Control Release. 2006;111:382–9. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Stampfl A, Takenaka S, et al. Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation. 2004;109:1320–5. doi: 10.1161/01.CIR.0000118524.62298.E8. [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee YM, Baik DJ, et al. Toxic characteristics of methoxy poly(ethylene glycol)/poly(ε-caprolactone) nanospheres; in vitro and in vivo studies in the normal mice. Biomaterials. 2003;24:55–63. doi: 10.1016/s0142-9612(02)00248-x. [DOI] [PubMed] [Google Scholar]
- Kipp JE. The role of solid nanoparticle technonogy in the parental delivery of poorly water-soluble drugs. Int J Pharm. 2004;284:109–22. doi: 10.1016/j.ijpharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Letters. 2005;5:331–8. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- Konan YN, Chevallier J, Gurny R, et al. Encapsulation of p-THPP into nanoparticles: cellular uptake, subcellular localization and effect of serum on photodynamic activity. Photochem Photobiol. 2003;77:638–44. doi: 10.1562/0031-8655(2003)077<0638:eopinc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Koziara JM, Lockman PR, Allen DD, et al. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 2004;99:259–69. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Koziara JM, Lockman PR, Allen DD, et al. The blood brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6:2712–35. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brian. J Nanosci Nanotechnol. 2004;4:484–8. doi: 10.1166/jnn.2003.077. [DOI] [PubMed] [Google Scholar]
- Kreuter J, Ramge P, Petrov V, et al. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs of the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003;20:409–16. doi: 10.1023/a:1022604120952. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Möller W. Dosimetry and toxicology of ultrafine particles. J Aerosol Med. 2004;17:140–52. doi: 10.1089/0894268041457147. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health Part A. 2002;65:1513–30. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Lai M-K, Chang C-Y, Lien Y-W, et al. Application of gold nanoparticles to microencapsulation of thioridazine. J Control Release. 2006;111:352–61. doi: 10.1016/j.jconrel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Lam C-W, James JT, McCluskey R, et al. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Tox Sc. 2004;77:126–34. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- LaVan DA, McGuire T, Langer R. Small scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21:1184–91. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- Lenaerts V, Nagelkerke JF, Van Berkel TJ, et al. In vivo uptake of polyisobutyl cyanoacrylate nanoparticles by rat liver Kupffer, endothelial, and parenchymal cells. J Pharm Sci. 1984;73:980–2. doi: 10.1002/jps.2600730730. [DOI] [PubMed] [Google Scholar]
- Lin W, Huang YW, Zhou XD, et al. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217:252–9. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Koziara JM, Mumper RJ, et al. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12:635–41. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Koziara JM, Roder KE, et al. In vivo and in vitro assessment of baseline blood-brain-barrier parameters in the presence of novel nanoparticles. Pharm Res. 2003;20:705–13. doi: 10.1023/a:1023492015851. [DOI] [PubMed] [Google Scholar]
- Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3:33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- Lovern SB, Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem. 2006;25:1132–7. doi: 10.1897/05-278r.1. [DOI] [PubMed] [Google Scholar]
- Lovric J, Bazzi HS, Cuie Y, et al. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005;83:377–85. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- Lovric J, Cho SJ, Winnik FM, et al. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol. 2005;12:1159–61. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lowery AR, Gobin AM, Day Es, et al. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine. 2006;1:149–54. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metselaar JM, Storm G. Liposomes in the treatment of inflammatory disorders. Expert Opin Drug Deliv. 2005;2:465–76. doi: 10.1517/17425247.2.3.465. [DOI] [PubMed] [Google Scholar]
- Michaelis K, Hoffmann MM, Dreis S, et al. Covalent linkage of apolipoprotein E to albumin naniparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–53. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- Minko T, Pakunlu RI, Wang Y, et al. New generation of liposomal drugs for cancer. Anticancer Agents Med Chem. 2006;6:537–52. doi: 10.2174/187152006778699095. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–36. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target specific nanoparticles: theory and practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Nemanich RJ, Inman AO, et al. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol Lett. 2005;155:377–84. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–31. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Na K, Lee KH, Lee DH, et al. Biodegradable thermo-sensitive nanoparticles from poly(L-lactic acid)/poly(ethylene glycol) alternating multiblock copolymer for potential anti-cancer drug carrier. Eur J Pharm Sci. 2006;27:115–22. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, et al. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–14. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, et al. Size effect of intraytracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol Appl Pharmacol. 2003;186:38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–7. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Nobs L, Buchegger F, Gurny R, et al. Poly(lactic acid) nanoparticles labeled with biologically active Neutravidin™ for active targeting. Eur J Pharm Biopharm. 2004;58:483–90. doi: 10.1016/j.ejpb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Oberdörster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect. 2004;112:1058–62. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal Toxicol. 1996;8(Suppl):73–89. [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol. 2004;16:437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Olivier JC, Fenart L, Chauvet R, et al. Indirect evidence that drug brain targeting using polysorbate 80-coated polybutylcyanoacrylate nanoparticles is related to toxicity. Pharm Res. 1999;16:1836–42. doi: 10.1023/a:1018947208597. [DOI] [PubMed] [Google Scholar]
- O’Neal DP, Hirsch LR, Halas NJ, et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Paciotti GF, Meyer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–83. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- Panyam J, Zhou WZ, Prabha S, et al. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–26. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug Discovery Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Peracchia MT, Fattal E, Desmaele D, et al. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–8. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long term exposure to particulate air pollution: epidemiological evidence of genearal pathophysiological pathways of disease. Circulation. 2004;109:71–7. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Prinzen L, Miserus R-JJHM, Dirksen A, et al. Optical and magnetic resonance imaging of cell death and platelet activation using annexin A5-functionalized quantum dots. Nano Lett. 2007;7:93–100. doi: 10.1021/nl062226r. [DOI] [PubMed] [Google Scholar]
- Radomski A, Jurasz P, Alonso-Escolano D, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–93. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radt B, Smith TA, Caruso F. Optically addressable nanostructured capsules. Adv Mater. 2004;16:2184–9. [Google Scholar]
- Ravi Kumar MN, Gameti M, Mohapatra SS, et al. Cationic silica nanoparticles as gene carriers: synthesis, characterization and transfection efficiency in vitro and in vivo. J Nanosci Nanotechnol. 2004;4:876–81. doi: 10.1166/jnn.2004.120. [DOI] [PubMed] [Google Scholar]
- Renn O, Roco MC. Nanotechnology and the need for risk governance. J Nanoparticle Research. 2006;8:153–91. [Google Scholar]
- Ricci-Junior E, Marchetti JM. Preparation, characterization, photocytotoxicity assay of PLGA nanoparticles containing zinc(II) phthalocyanine for photodynamic therapy use. J Microencapsul. 2006;23:523–38. doi: 10.1080/02652040600775525. [DOI] [PubMed] [Google Scholar]
- Saez A, Guzman M, Molpeceres J, et al. Freeze-drying of polycaprolactone and poly(D,L-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur J Pharm Biopharm. 2000;50:379–87. doi: 10.1016/s0939-6411(00)00125-9. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Gobin AM, Ausman KD, et al. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–95. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Liang F, Hudson JL, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–42. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- [SCENIHR] EU Scientific Committee on Emerging and Newly Identified Health Risks. Modified Opinion (after public consultation) on the appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologies. 2006 SCENIHR/002/05 Accessed 23 June 2006 URL: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_003b.pdf.
- Schins RP, Duffin R, Hohr D, et al. Surface modification of quartz inhibits toxicity, particle uptake, and oxidative DNA damage in human lung epithelial cells. Chem Res Toxicol. 2002;15:1166–73. doi: 10.1021/tx025558u. [DOI] [PubMed] [Google Scholar]
- Schins RPF, Lightbody JH, Borm PJA, et al. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Seki J, Sonoke S, Saheki A, et al. A nanometer lipid emulsion, lipid nano-sphere (LNS), as a parenteral drug carrier for passive drug targeting. Int J Pharm. 2004;273:75–83. doi: 10.1016/j.ijpharm.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Senior J, Crawley JCW, Fisher D, et al. Influence of surface hydrophobicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly(ethylene glycol)-coated vesicles. Biochim Biophys Acta. 1985;1062:77–82. doi: 10.1016/0005-2736(91)90337-8. [DOI] [PubMed] [Google Scholar]
- Senior J, Gregoriadis G. Is half-life of circulating small unilamellar liposomes determined by changes in their permeability? FEBS Lett. 1982;145:109–14. doi: 10.1016/0014-5793(82)81216-7. [DOI] [PubMed] [Google Scholar]
- Shenoy D, Fu W, Li J, et al. Surface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and delivery. Int J Nanomedicine. 2006;1:51–7. doi: 10.2147/nano.2006.1.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Seok Kang H, Park WS, et al. Transdermal delivery of minoxidil with block copolymer nanoparticles. J Control Release. 2004;97:477–84. doi: 10.1016/j.jconrel.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Shiohara A, Hoshino A, Hanaki K, et al. On the cytotoxicity of quantum dots. Microbiol Immunol. 2004;48:669–75. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environm Health Part A. 2003;66:1909–26. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- Stern JM, Stanfield J, Lotan Y, et al. Efficacy of laser-activated gold nanoshells in ablating prostate cancer cells in vitro. J Endourol. 2007;21:939–43. doi: 10.1089/end.2007.0437. [DOI] [PubMed] [Google Scholar]
- Su C-H, Sheu H-S, Lin C-Y, et al. Nanoshell resonance imaging contrast agents. J Am Chem Soc. 2006;129:2139–46. doi: 10.1021/ja0672066. [DOI] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, Quinlan KB, et al. Particulate air pollution induces progression of atherosclerosis. Am Coll Cardiol. 2002;39:943–5. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- Svenson S, Tomalia DA. Dendrimers in biomedical applications – reflections on the field. Adv Drug Del Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Takenaka S, Karge E, Kreyling WG, et al. Distribution pattern of inhaled ultrafine gold nanoparticles in the rat lung. Inhalation Toxicol. 2006;18:733–40. doi: 10.1080/08958370600748281. [DOI] [PubMed] [Google Scholar]
- The Royal Society and The Royal Academy of Engineering. Nanoscience and nanotechnologies: opportunities and uncertainties. London, UK: 2004. [Google Scholar]
- Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Current Drug Delivery. 2006;3:219–32. doi: 10.2174/156720106776359230. [DOI] [PubMed] [Google Scholar]
- Tomazic-Jezic VJ, Merritt K, Umbreit TH. Significance of the type an dsize of biomaterial particles on phagocytosis and tissue distribution. J Biomed Mater Res. 2001;55:523–9. doi: 10.1002/1097-4636(20010615)55:4<523::aid-jbm1045>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Laurence BR, Reed KL, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–25. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Weissenbock A, Wirth M, Gabor F. WGA grafted PLGA-nanospheres: preparation and association with Caco-2 cells. J Contr Release. 2004;99:383–92. doi: 10.1016/j.jconrel.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Win KY, Feng SS. In vitro and in vivo studies on vitamin E TPGS-emulsified poly(D,L-lactic-co-glycolic acid) nanoparticles for paclitaxel formulation. Biomaterials. 2006;27:2285–91. doi: 10.1016/j.biomaterials.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parental drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Brant J, et al. Comparison of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Umezawa N, Ryu A, et al. Active oxygen species generated from photo-excited fullerene (C-60) as potential medicines: O2− versus 1O2 . J Am Chem Soc. 2003;125:12803–9. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu Y, Jiang X, et al. Camptothecin derivative-loaded poly(caprolactone-co-lactide)-b-PEG-b-poly(caprolactone-co-lactide) nanoparticles and their biodistribution in mice. J Control Release. 2004;96:135–48. doi: 10.1016/j.jconrel.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Zhu S, Oberdörster E, Haasch ML. Toxicity of an engineered nanoparticle (fullerene, C60) in to aquatic species, Daphnia and flathead minnow. Mar Environ Res. 2006;62(Suppl):S5–9. doi: 10.1016/j.marenvres.2006.04.059. [DOI] [PubMed] [Google Scholar]