Abstract
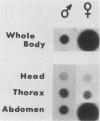
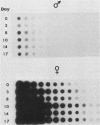
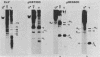
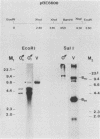
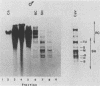
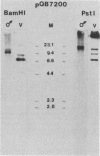
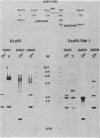
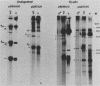
Campoletis sonorensis virus (CsV) (Polydnaviridae) previously was detected only in the calyx epithelial cells and lumen of the oviducts from female C. sonorensis (Ichneumonidae) endoparasitic wasps (Norton et al., Cell Tissue Res. 162:195-208, 1975). Using dot-blot hybridizations, we detected low amounts of CsV DNA in male and female wasp head and thorax tissues and in male abdominal tissues. Low amounts of extrachromosomal viral DNA were detected in Southern blots of undigested male wasp DNA and in male DNA purified by isopycnic centrifugation. High-molecular-weight male wasp DNA digested with any of several restriction endonucleases and hybridized with cloned viral DNAs from CsV superhelices B and Q under stringent conditions contained CsV-specific DNA fragments that differed significantly in size and number from the hybridizing fragments detected in comparably digested viral DNA. Identical offsize restriction fragments were detected in digested female head and thorax DNA. These data suggest that at least CsV DNAs B and Q are integrated in C. sonorensis cellular DNA and that the virus may be transmitted through the germline.
Full text
PDF



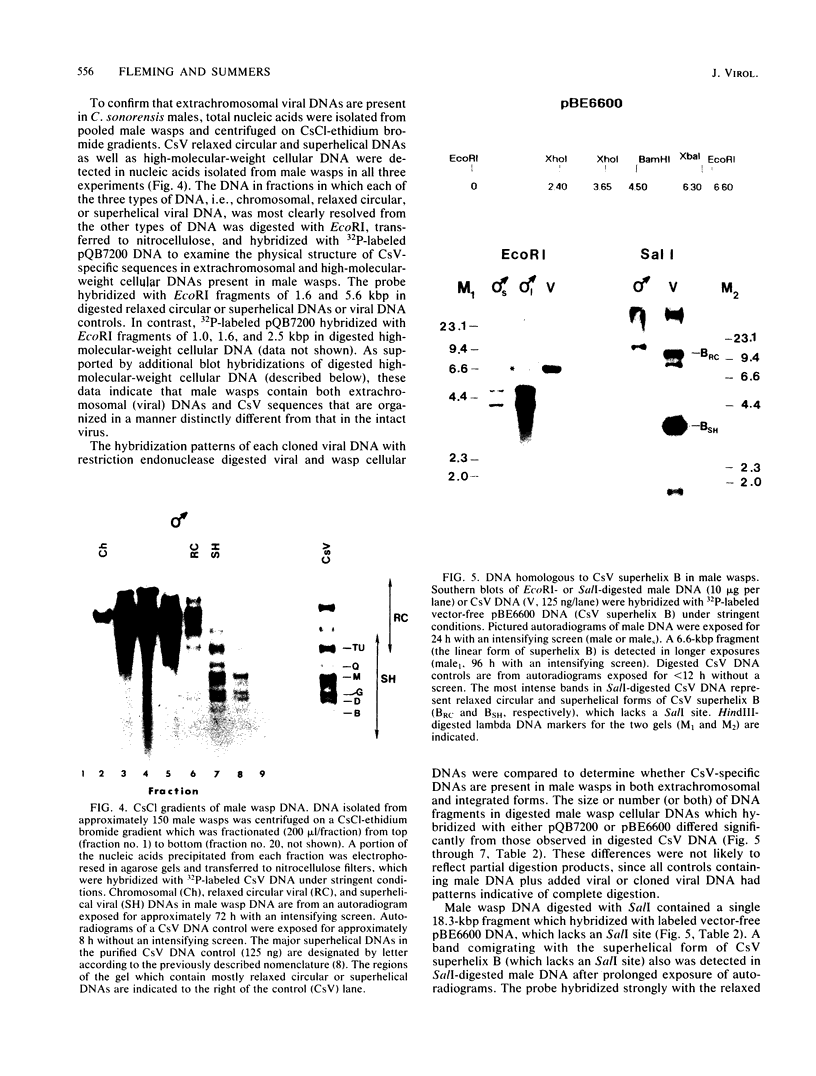
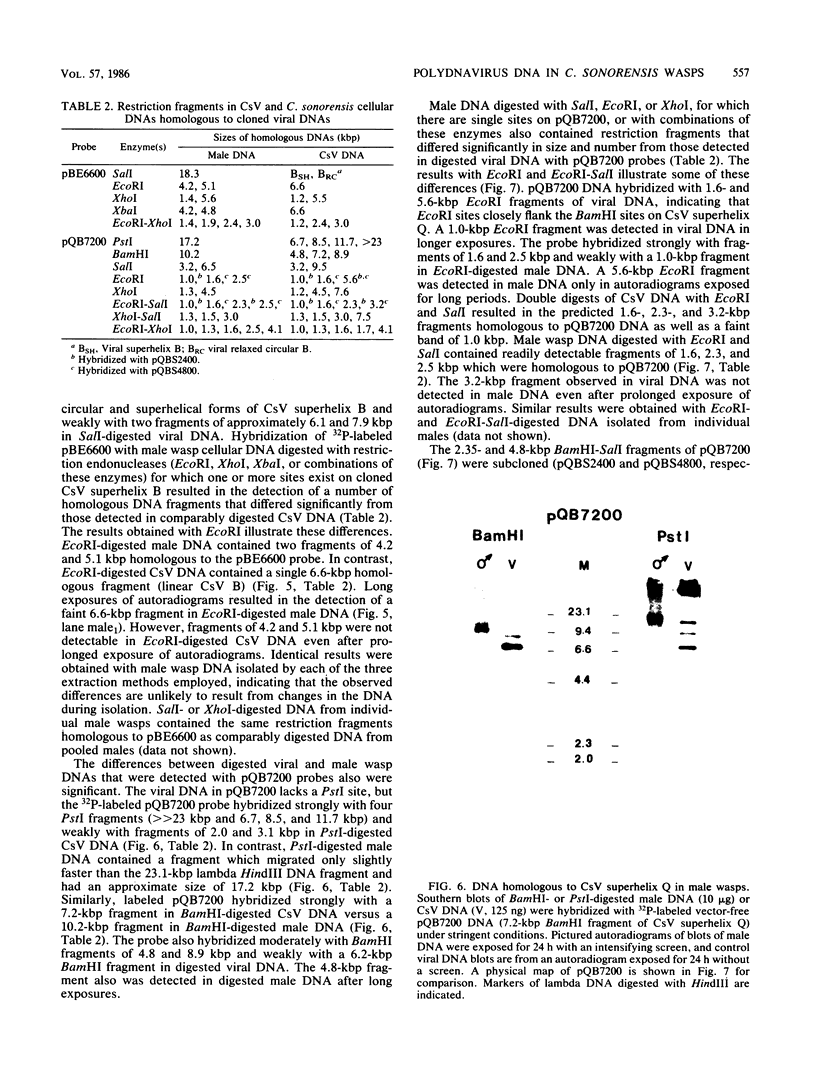
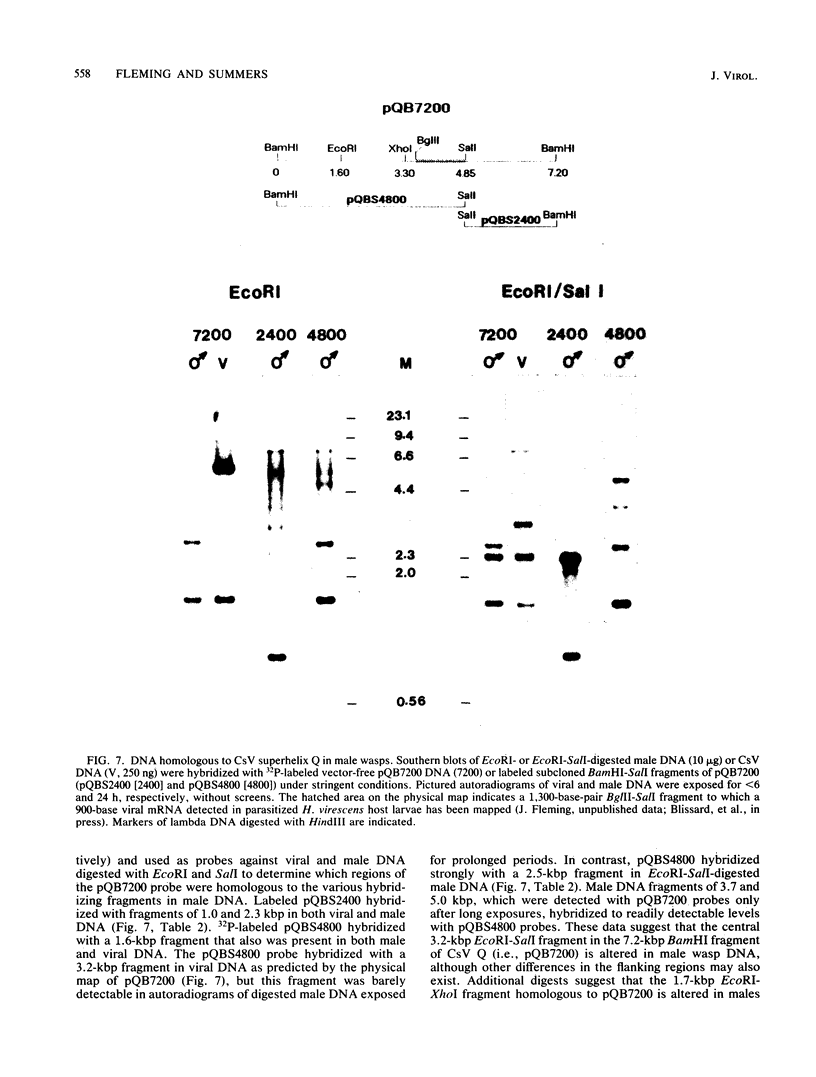
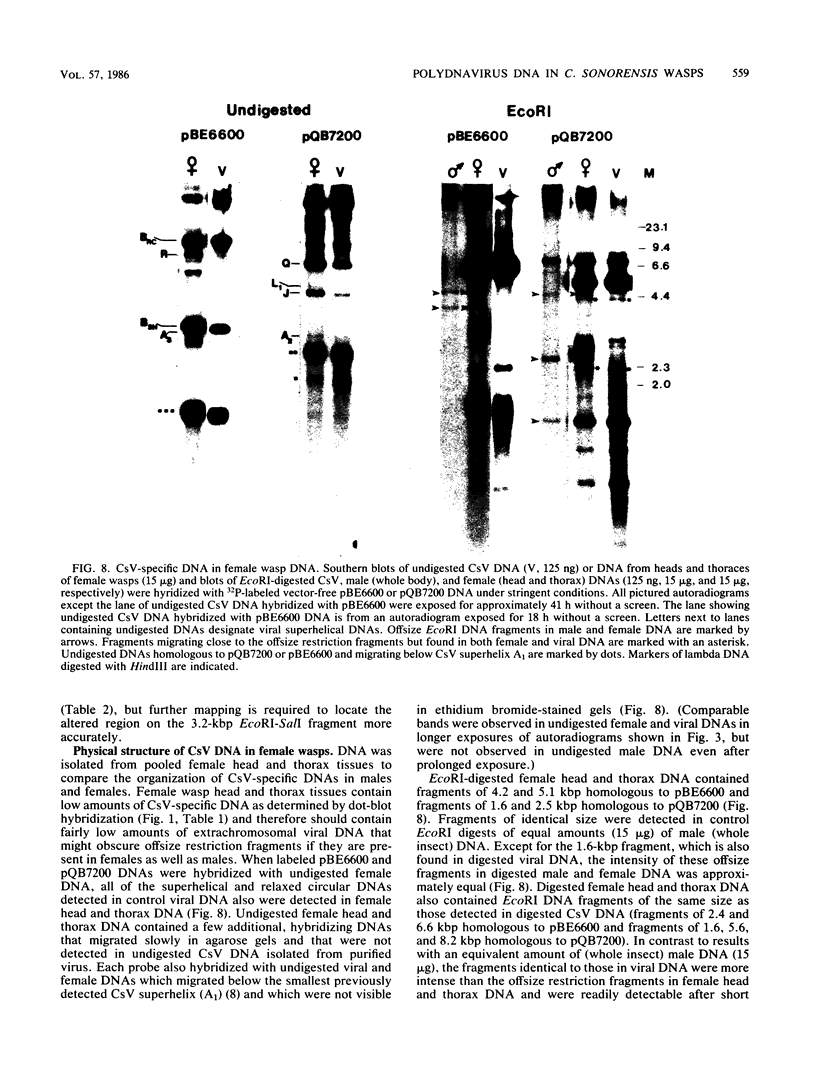



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crain W. R., Davidson E. H., Britten R. J. Contrasting patterns of DNA sequence arrangement in Apis mellifera (honeybee) and Musca domestica (housefly). Chromosoma. 1976 Dec 6;59(1):1–12. doi: 10.1007/BF00327705. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Uptake, fixation, and expression of foreign DNA in mammalian cells: the organization of integrated adenovirus DNA sequences. Curr Top Microbiol Immunol. 1982;101:127–194. doi: 10.1007/978-3-642-68654-2_6. [DOI] [PubMed] [Google Scholar]
- Edson K. M., Vinson S. B., Stoltz D. B., Summers M. D. Virus in a parasitoid wasp: suppression of the cellular immune response in the parasitoid's host. Science. 1981 Feb 6;211(4482):582–583. doi: 10.1126/science.7455695. [DOI] [PubMed] [Google Scholar]
- Fleming J. A., Blissard G. W., Summers M. D., Vinson S. B. Expression of Campoletis sonorensis Virus in the Parasitized Host, Heliothis virescens. J Virol. 1983 Oct;48(1):74–78. doi: 10.1128/jvi.48.1.74-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell P. J., Summers M. D., Vinson S. B. Virus with a Multipartite Superhelical DNA Genome from the Ichneumonid Parasitoid Campoletis sonorensis. J Virol. 1982 Sep;43(3):859–870. doi: 10.1128/jvi.43.3.859-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. N., Vinson S. B. Correlating the initiation of virus replication with a specific pupal developmental phase of an ichneumonid parasitoid. Cell Tissue Res. 1983;231(2):387–398. doi: 10.1007/BF00222189. [DOI] [PubMed] [Google Scholar]
- Norton W. N., Vinson S. B., Stoltz D. B. Nuclear secretory particles associated with the calyx cells of the ichneumonid parasitoid Campoletis sonorensis (Cameron). Cell Tissue Res. 1975 Sep 17;162(2):195–208. doi: 10.1007/BF00209207. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Vinson S. B. Viruses and parasitism in insects. Adv Virus Res. 1979;24:125–171. doi: 10.1016/s0065-3527(08)60393-0. [DOI] [PubMed] [Google Scholar]
- Vinson S. B. Microplitis croceipes: inhibitions of the Heliothis zea defense reaction to Cardiochiles nigriceps. Exp Parasitol. 1977 Feb;41(1):112–117. doi: 10.1016/0014-4894(77)90136-9. [DOI] [PubMed] [Google Scholar]