Abstract
Integral proteins of the nuclear envelope inner membrane have been proposed to reach their sites by diffusion after their co-translational insertion in the rough endoplasmic reticulum. They are then retained in the inner nuclear membrane by binding to nuclear structures. One such structure is the nuclear lamina, an intermediate filament meshwork composed of A-type and B-type lamin proteins. Emerin, MAN1 and LBR are three integral inner nuclear membrane proteins. We expressed these proteins fused to green fluorescent protein in embryonic fibroblasts from wild-type mice and Lmna -/- mice, which lack A-type lamins. We then studied the diffusional mobilities of emerin, MAN1 and LBR using fluorescence recovery after photobleaching. We show that emerin and MAN1, but not LBR, are more mobile in the inner nuclear membrane of cells from Lmna -/- mice than in cells from wild-type mice. In cells from Lmna -/- mice expressing exogenous lamin A, the protein mobilities were similar to those in cells from wild-type mice. This supports a model where emerin and MAN1 are at least partly retained in the inner nuclear membrane by binding to A-type lamins, while LBR depends on other binding partners for its retention.
Integral membrane proteins synthesized on endoplasmic reticulum (ER)1-bound ribosomes are partially translocated through intramembrane channels and incorporated into the membrane with topologies determined by their primary structures (1, 2). Many of the integral proteins synthesized on the ER membrane reach the plasma membrane by vesicular transport in the secretory pathway (3-5). Others contain specific sequences retaining them in or targeting them to intermediate secretory compartments or organelles such as the ER itself, pre-Golgi vesicles, Golgi apparatus, lysosomes and endosomes (5, 6).
Some integral membrane proteins synthesized on the rough ER are targeted to the membranes of the nuclear envelope, which are divided into three morphologically distinct but interconnected domains: outer, pore and inner membranes. As the membrane domains of the nuclear envelope are continuous with each other and the rough ER, integral proteins synthesized on ER-bound ribosomes can potentially reach all of the nuclear membrane domains by lateral diffusion in the interconnected proteolipid bilayers. The results of most targeting studies of integral nuclear membrane proteins are consistent with a diffusion-retention model (7-12). In this model, proteins diffuse laterally after co-translational insertion into the rough ER membrane. They are then retained and immobilized at their destinations by binding to other proteins or structures.
The outer nuclear membrane has ribosomes on its cytoplasmic surface and is similar in composition to the rough ER, with which it is directly continuous (13, 14). A few integral proteins may, however, be specifically localized to the outer nuclear membrane and excluded from the bulk ER by associating with luminal domains of integral proteins of the inner nuclear membrane in the perinuclear space (15). The pore membranes connect the inner and outer membranes at numerous points and are associated with the nuclear pore complexes. Unique integral proteins have been localized to the pore membranes (16-18). They are most likely structural components of the nuclear pore complexes. These proteins are targeted to the pore membrane either by sequences in their nucleocytoplasmic domains, which presumably bind to other pore complex proteins, or by sequences in the transmembrane domains, which are likely to laterally associate with resident transmembrane domains in the pore membrane (19, 20).
Most of the integral proteins of the inner nuclear membrane contain targeting and retention signals in the parts of the proteins directed towards the nucleoplasmic side of the nuclear membrane (7, 8, 10, 11, 21). These domains likely contain regions that bind to nuclear proteins, such as the nuclear lamins, which form a meshwork of intermediate filaments associated with the inner nuclear membrane on its nucleoplasmic face (22-25). Two general types of lamins have been identified in somatic cells, A-type and B-type. The somatic cell A-type lamins, lamin A, lamin C and lamin Adelta10, are alternative splice isoforms encoded by the LMNA gene (26, 27). Two different genes encode the somatic cell B-type lamins, lamin B1 and lamin B2 (28, 29). While B-type lamins are expressed in most cell types, A-type lamin expression is developmentally regulated (30). Because the lamina is a discontinuous structure (31), the inner nuclear membrane proteins may also be able to directly associate with the chromatin at certain points. Approximately eighty different integral proteins are localized to the inner nuclear membrane in interphase cells (32). Several of these proteins have been shown to bind components of the lamina and chromatin (33-45).
Studies of several inner nuclear membrane proteins have shown that they diffuse more rapidly in the ER than in the inner nuclear membrane, giving support to the diffusion-retention model (9-12). To determine the contribution of A-type lamins in retention of integral proteins in inner nuclear membrane, we studied the targeting and diffusion of three inner nuclear membrane proteins: emerin (46, 47), MAN1 (48), and LBR (34, 35). Emerin is known to bind A-type lamins (41, 42, 44) and requires these proteins for efficient retention in inner nuclear membrane (49-51). MAN1 co-fractionates with (52) and binds to (45) lamins. LBR has very weak affinity for A-type lamins (34) and binds B-type lamins (34, 38), heterochromatin proteins (39, 40) and DNA (38, 43).
EXPERIMENTAL PROCEDURES
Plasmid construction
Plasmids encoding nucleoplasmic inner nuclear membrane targeted domains of LBR (amino acids 1-238) and MAN1 (amino acids 1-538) and full-length emerin, fused via their C-termini to the F64L, S65T, H231L variant of green fluorescent protein (GFP), have been previously described (9-11). RFP-C1, a vector encoding monomeric red fluorescent protein (RFP; 53) inserted in the place of GFP in an EGFP-C1 vector (Clontech Laboratories, Inc., Mountain View, CA) was a gift from Dr. Eugene Marcantonio, Columbia University, New York. Lamin A-RFP was constructed by inserting lamin A cDNA generated by PCR with a XhoI restriction site engineered at the 5' end and a BamHI restriction site at the 3' end into these restriction sites in the RFP-C1 vector. All cloning procedures were performed according to standard methods (54). The resulting cDNA was then sequenced using an ABI 3100 capillary sequencer (Applied Biosystems, Foster City, CA).
Cell culture and transfection
Immortalized mouse embryonic fibroblasts (MEFs) from wild-type and Lmna −/− mice (49) were grown in Dulbecco's modified Eagle medium containing 10% fetal bovine serum at 37°C and 5% CO2. For fluorescence recovery after photobleaching (FRAP), cells were transfected using Lipofectamine PLUS (Invitrogen, Carlsbad, CA), following the manufacturer's instructions. Cells were overlaid with the lipid-DNA complexes for approximately 23 h, the 5 first of which were in serum free OPTI-MEM media (Invitrogen). The cells were transfected in dishes and split to chambered coverglasses approximately 29 h post transfection. They were then grown for an additional 16-40 h before the photobleaching experiments.
Immunofluorescence microscopy
Cells were grown in chamberslides for approximately 24 h before being prepared for immunofluorescence microscopy. They were washed 3 times with phosphate-buffered saline (PBS) and then fixed with methanol for 6 min at −20°C. The cells were permeabilized with 0.5% Triton X-100 in PBS for 2 min at room temperature, washed 3 times with 0.1% Tween-20 in PBS (solution A) and incubated with the primary antibodies diluted in PBS containing 0.1% Tween-20 and 2% bovine serum albumin (solution B) for 1 h at room temperature. The primary antibodies used were mouse monoclonal anti-emerin antibody (Novocastra, Newcastle upon Tyne, UK) at a dilution of 1:30, rabbit polyclonal anti-lamin B1 antibody (55) at a dilution of 1:2,000, rabbit polyclonal anti-MAN1 antibody 3509 (56; a gift from Dr. Kunxin Luo, University of California, Berkeley) at a dilution of 1:200 and guinea pig polyclonal anti-LBR antibody (57; a gift from Dr. Harald Herrmann, German Cancer Research Center, Heidelberg) at a dilution of 1:1,000. After 4 washes with solution A, the cells were incubated with the secondary antibodies diluted 1:200 as described for the primary antibodies. Secondary antibodies used were lissamine rhodamine B-conjugated goat anti-rabbit IgG, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated donkey anti-guinea pig IgG. The cells were then washed 4 times with solution A and 3 times with PBS. The slides were dipped in methanol, air-dried and coverslips were mounted using SlowFade Light Anti-fade Kit (Molecular Probes, Eugene, OR). Immunofluorescence microscopy was performed on a Zeiss Axiovert 200 M microscope attached to a Zeiss LSM 510 confocal laser scanning system (Carl Zeiss, Inc., Thornwood, NY).
Fluorescence recovery after photobleaching experiments
FRAP experiments were performed on a Zeiss Axiovert 200 M microscope attached to a Zeiss LSM 510 confocal laser scanning system using the 488 nm line of a 30 mW argon laser in conjunction with a 40 × 0.9 N.A. objective. The bleached area was photobleached at full laser power (100% transmission) for 25 iterations and recovery of photobleaching monitored by scanning at low power (5% transmission) in 2 s intervals. The average intensity of the fluorescence signal was measured in the region of interest using NIH Image J software (http://rsb.info.nih.gov/ij/). It was then normalized to the change in total fluorescence as Irel=T0It/TtI0 where T0 is total cellular intensity during prebleach, Tt total cellular intensity at time point t, I0 the average intensity in the bleach-region during prebleach and It the average intensity in the bleach-region at time point t (58). The normalized fluorescence was then plotted against time after bleach.
As the immobile fraction (the difference between the fluorescence intensity in the bleached area prebleach and the intensity at infinity after bleach) was different for the different proteins and cell types, we used a modified time of half-recovery value (t1/2), where t1/2 is the time after bleach required for the fluorescence levels to reach the median between levels immediately after bleach and prebleach, rather than using the median between prebleach levels and steady state levels. To determine t1/2, we used a modification of the method described by Harrington et al. (59). We plotted ln (1-it) versus time after bleach, where it is the average normalized fluorescence intensity in the bleach-region at time t and 1 the average normalized fluorescence intensity in the bleach-region prebleach. The curves were fitted using MacCurveFit 1.5 (http://www.krs.com.au/mcf.html) and t1/2 calculated as t1/2=ln2 × (−1/slope). Data from the first 31 s after bleach were used in all experiments.
RESULTS
Emerin and MAN1 are partly mislocalized to the ER in MEFs lacking A-type lamins while LBR is not
Previous work has shown that emerin is mislocalized to the ER in embryonic fibroblasts that lack A-type lamins from Lmna −/− mice (49). Several other proteins, such as B-type lamins, are still exclusively localized to the nuclear envelope, although they exhibit abnormalities such as exclusion from one pole of the nucleus in a subset of the cells. Immunofluorescence microscopy studies on immortalized MEFs from wild-type and Lmna −/− mice (Figure 1, rows 1 and 4) confirmed previous data (49) on emerin and lamin B1. We also studied the localization of MAN1 and LBR. MAN1 was, similarly to emerin, partly mislocalized to the ER in cells from Lmna −/− mice (Figure 1, row 2). The MAN1 antibodies available to us exhibited some nonspecific cytoplasmic background, also seen in wild-type MEFs, which complicated our analysis of the protein localization. However, in a “blinded” study where two experienced persons looked at randomly taken confocal micrographs, they identified MAN1 as partly mislocalized to the ER in Lmna −/− and as retained in the nuclear envelope in wild-type cells in 100% of the cases (6 micrographs of each cell type). LBR did not mislocalize to the ER in Lmna −/− MEFs, although some cells exhibited abnormalities as previously described for lamin B1 (49) (Figure 1, row 3).
FIGURE 1.
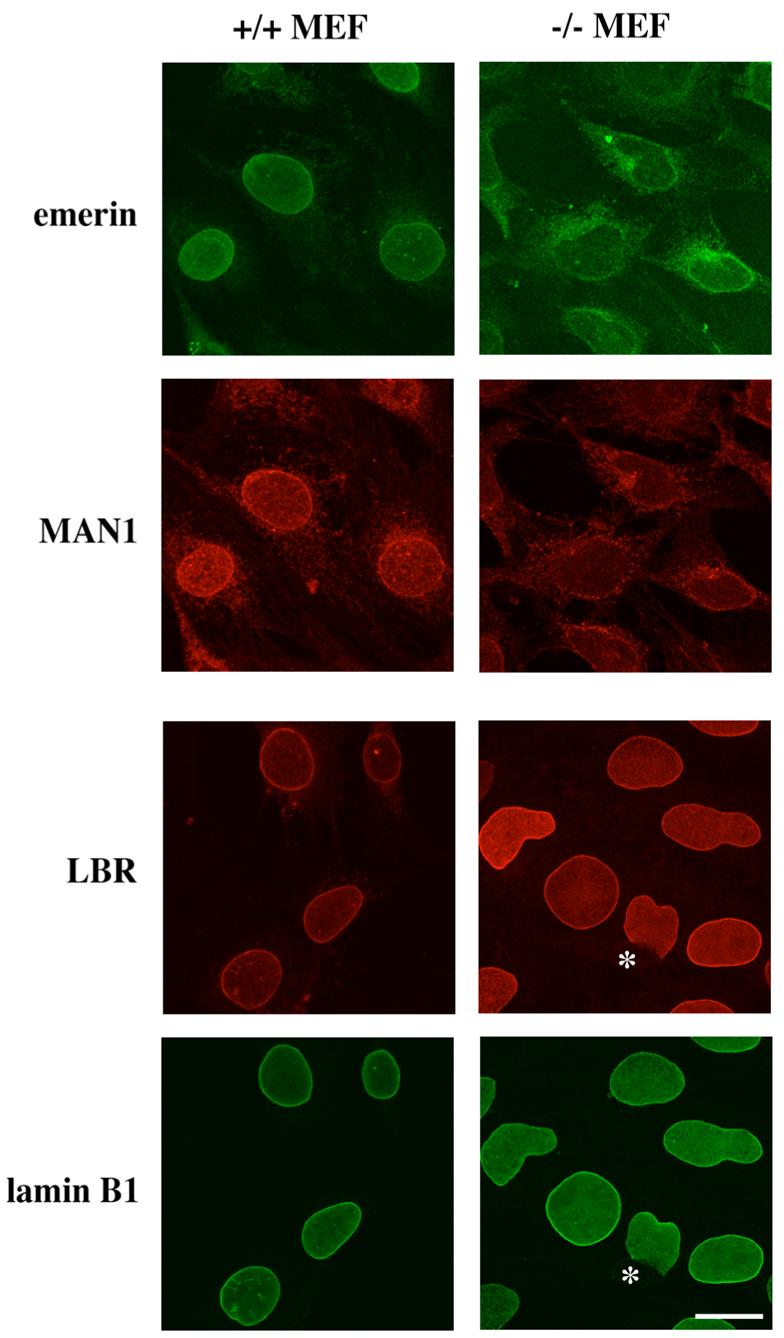
Emerin and MAN1 are partly mislocalized to the ER in MEFs lacking A-type lamins, while LBR and lamin B1 are not. Panels show laser scanning confocal immunofluorescence microscopy images of immortalized wild-type MEFs (left panels) or MEFs lacking A-type lamins (right panels). Cells shown in the first and second rows were double-labeled with monoclonal antibodies against emerin recognized by FITC-conjugated secondary antibodies (first row) and polyclonal antibodies against MAN1 recognized by lissamine rhodamine B-conjugated secondary antibodies (second row). Cells shown in the third and fourth rows were double-labeled with polyclonal antibodies against LBR recognized by TRITC-conjugated secondary antibodies (third row) and polyclonal antibodies against lamin B1 recognized by FITC-conjugated secondary antibodies (fourth row). Asterisks show a cell where both LBR and lamin B1 are excluded from an area of the nuclear envelope in MEFs lacking A-type lamins. Bar: 10 μm.
Emerin and MAN1 are more mobile in the nuclear envelope in MEFs lacking A-type lamins than in wild-type MEFs, while LBR is not
Previous studies have shown that emerin-GFP, MAN1-GFP and LBR-GFP are less mobile in the nuclear envelope than in the ER (9-11). While the majority of LBR-GFP is essentially immobilized within the nuclear envelope (9), emerin-GFP and MAN1-GFP can diffuse slowly, compared to the ER, within the nuclear envelope (10, 11). Based on our results shown in Figure 1, and previous studies showing that A-type lamins bind emerin and MAN1 (41, 42, 44, 45), we hypothesized that these proteins would be more mobile in the nuclear envelope of cells lacking A-type lamins than in wild-type cells. To investigate the diffusional mobility of emerin, MAN1 and LBR, we performed FRAP. In these experiments, GFP-tagged proteins in an area in the nuclear envelope or ER of transiently transfected MEFs were irreversibly bleached using an argon laser at high power. The fluorescence recovery in the bleached area, corresponding to the influx of unbleached molecules from other areas, was then monitored. Figure 2 shows the results from FRAP experiments on emerin-GFP. Figure 2A shows fluorescence recovery in representative cells after photobleaching. As has previously been noted (10), emerin-GFP was not restricted to the nuclear envelope in cells overexpressing the proteins but was also present in the ER. There was also often some protein accumulation in an area most likely representing the Golgi apparatus. Although an exclusive localization of protein to the nuclear envelope was more frequent in wild-type MEFs than in Lmna −/− MEFs, wild-type MEFs also often exhibited some fluorescence in the ER. There was also often a clear accumulation of fusion protein in the nuclear envelope in Lmna −/− MEFs. Only cells with rim fluorescence and a moderate protein expression level were used in these experiments. Fluorescence in cells expressing emerin-GFP recovered more rapidly and to a higher degree in the nuclear envelope of MEFs lacking A-type lamins than in wild-type cells (Figure 2B). In the ER, the recovery rates were very similar in the two cell types, and higher than in the nuclear envelope experiments (Figure 2C).
FIGURE 2.
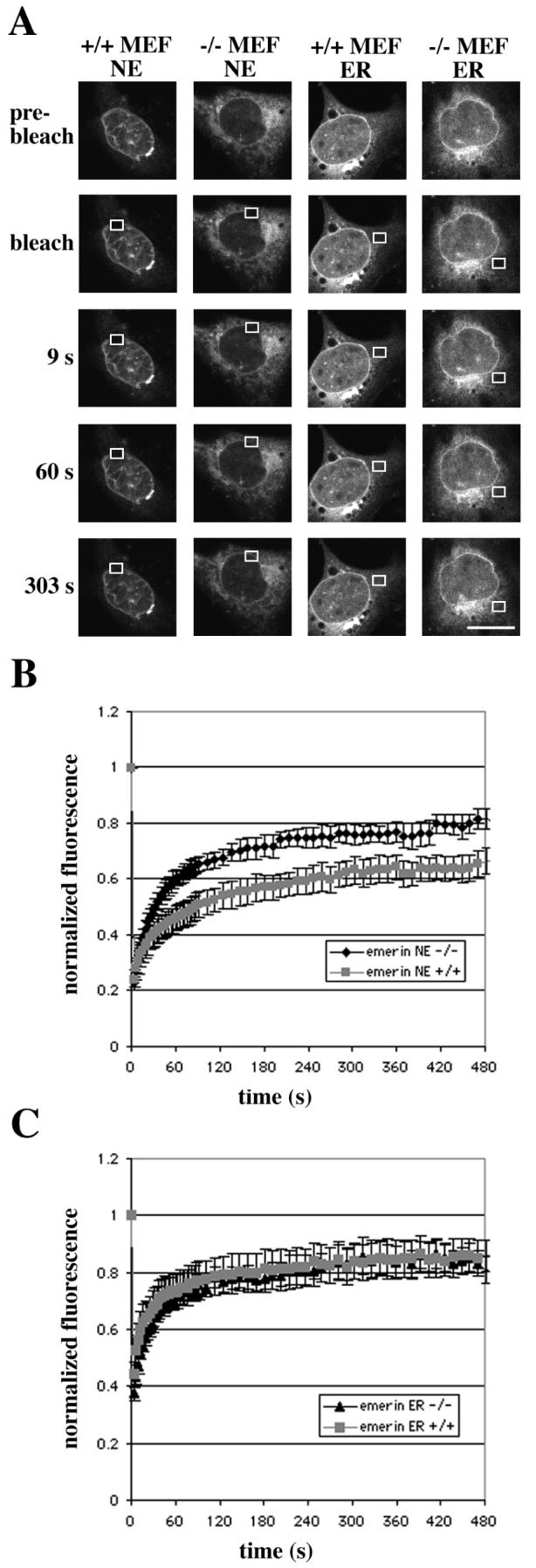
The mobility of emerin-GFP in the nuclear envelope (NE) is higher in MEFs lacking A-type lamins (−/− MEF) than in wild-type MEFs (+/+ MEF), while the mobility in the ER is the same in both cell types. (A) FRAP measurements of mobility of emerin-GFP showing fluorescence recovery in representative, transiently transfected MEFs. The boxed regions were bleached and fluorescence recovery shown 9 s, 60 s and 303 s after bleaching. Bar: 10 μm. (B and C) Quantitative experiments showing normalized fluorescence recovery after photobleaching regions of the nuclear envelope (B) or ER (C), where 1 is the fluorescence level before bleaching. The fluorescence intensity in the bleached region was measured and expressed as relative recovery (see Experimental Procedures). Error bars indicate SEM, n = 7.
FRAP experiments were performed as described for emerin-GFP on cells expressing MAN1-GFP. Similar fluorescence patterns were seen as those for emerin (Figure 3A). The difference in nuclear envelope recovery rates between wild-type and Lmna −/− cells was, however, less pronounced for MAN1-GFP (Figures 3B and 3C).
FIGURE 3.
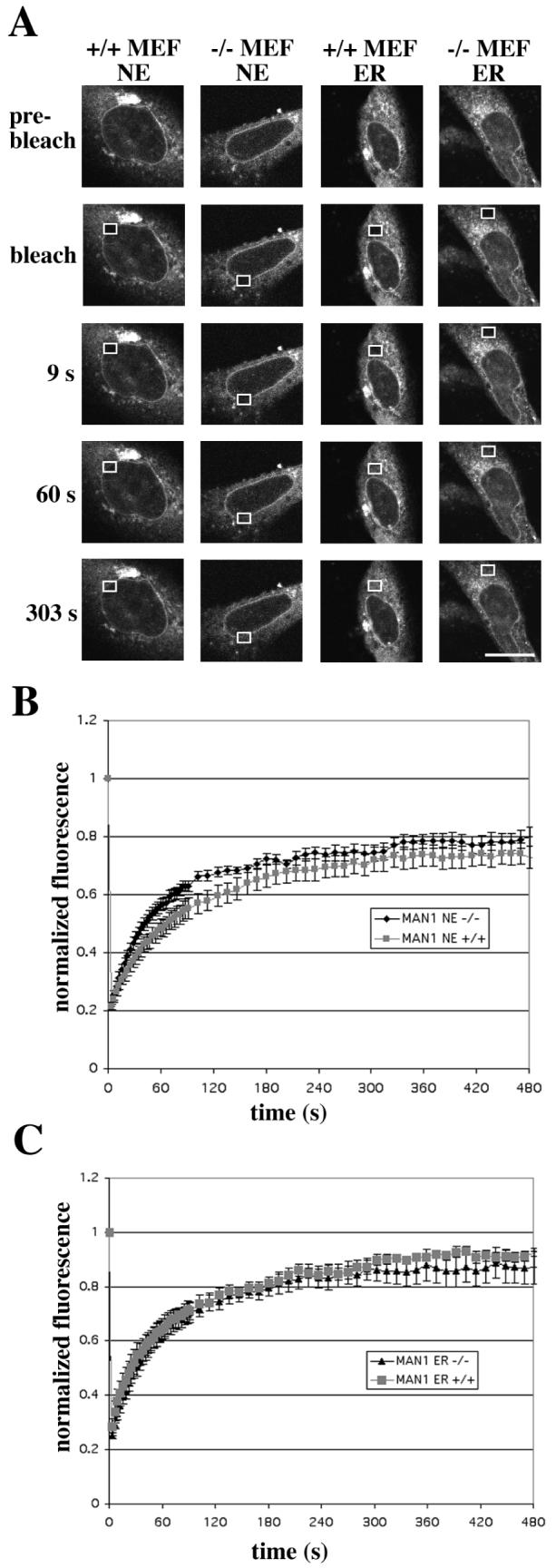
The mobility of MAN1-GFP in the nuclear envelope (NE) is higher in MEFs lacking A-type lamins (−/− MEF) than in wild-type MEFs (+/+ MEF), while the mobility in the ER is the same in both cell types. (A) FRAP measurements of mobility of MAN1-GFP showing recovery of fluorescence in representative, transiently transfected MEFs. The fluorescence in the boxed regions was bleached and recovery shown after 9 s, 60 s and 303 s after bleaching. Bar: 10 μm. (B and C) Quantitative experiments showing normalized fluorescence recovery after photobleaching regions of the nuclear envelope (B) or ER (C), where 1 is the fluorescence level before bleaching. The fluorescence intensity in the bleached region was measured and expressed as relative recovery (see Experimental Procedures). Error bars indicate SEM, n = 13 for nuclear envelope experiments, n = 8 for ER experiments.
We also performed FRAP experiments on wild-type and Lmna −/− cells transiently transfected to express LBR-GFP (Figure 4). This protein localized almost exclusively to the nuclear rim, with very little fluorescence in the ER in cells with or without A-type lamins. However, some fluorescence was seen in the ER of cells expressing the protein at high levels (Figure 4A). There were no differences in the fluorescence recovery rates between wild-type MEFs and Lmna −/− MEFs (Figures 4B and 4C). The recovery rates in the nuclear envelope were much lower than those seen for emerin or MAN1, while the rates in the ER were similar for all three proteins.
FIGURE 4.
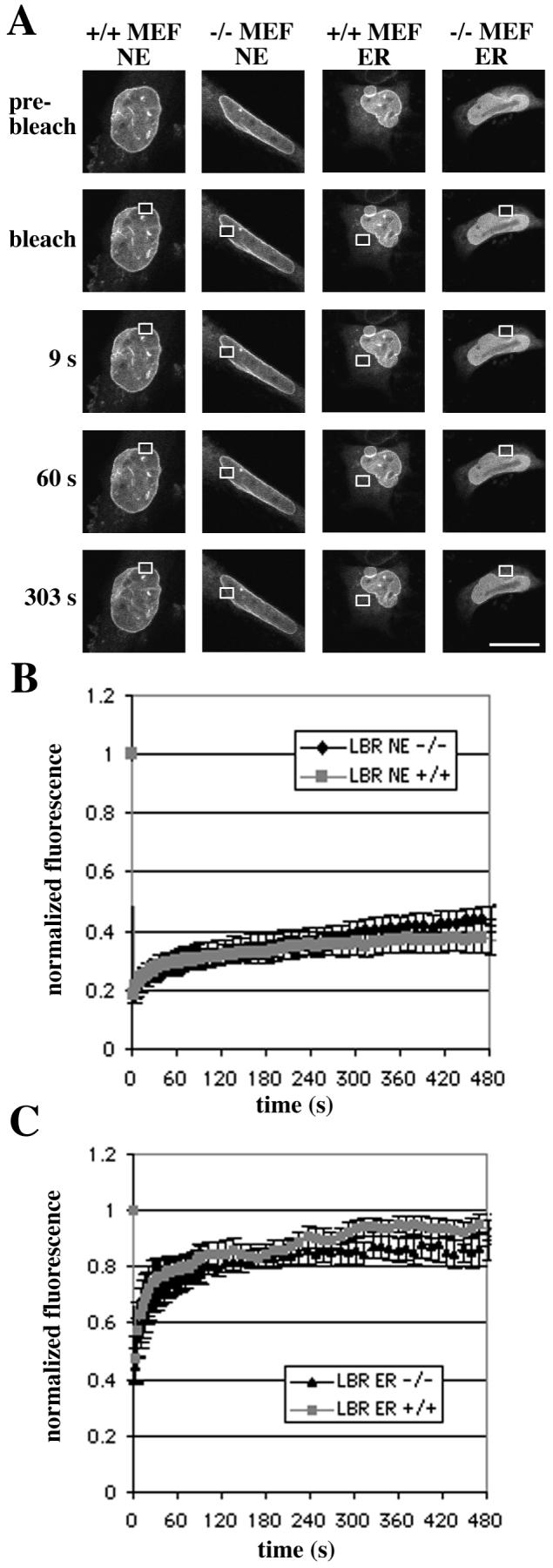
The mobility of LBR-GFP is the same in MEFs lacking A-type lamins (−/− MEF) and in wild-type MEFs (+/+ MEF), both in the nuclear envelope (NE) and in the ER. (A) FRAP studies of mobility of LBR-GFP showing fluorescence recovery in representative transiently transfected MEFs. The fluorescence in the boxed regions was bleached and recovery shown after 9 s, 60 s and 303 s after bleaching. Bar: 10 μm. (B and C) Quantitative experiments showing normalized fluorescence recovery after photobleaching regions of the nuclear envelope (B) or ER (C), where 1 is the fluorescence level before bleaching. The fluorescence intensity in the bleached region was measured and expressed as relative recovery (see Experimental Procedures). Error bars indicate SEM, n=7 for nuclear envelope experiments, n = 6 for ER experiments.
Expression of recombinant lamin A decreases the mobility of emerin-GFP and MAN1-GFP in the nuclear envelope of MEFs without endogenous A-type lamins
To verify that the increased diffusional mobility of emerin in the nuclear envelope of Lmna −/− MEFs was due to the lack of A-type lamins, we cotransfected Lmna −/− MEFs with plasmids encoding lamin A-RFP and emerin-GFP (Figure 5A) and performed FRAP as described above. As a control, we also cotransfected cells with a plasmid encoding RFP. Lamin A-RFP localized to the nuclear rim, similarly to endogenous lamin A, while RFP alone was present in both nuclei and cytoplasm (Figure 5A). To compare the initial recovery rates between the different experiments, we calculated the time after bleach required for the fluorescence levels to reach the median between levels immediately after bleach and prebleach levels (t1/2; see Experimental Procedures for further details). The t1/2 values for emerin-GFP fluorescence were significantly different between wild-type MEFs and Lmna −/− MEFs (Figure 5B). The t1/2 values for emerin-GFP fluorescence in Lmna −/− MEFs expressing emerin-GFP and lamin A-RFP were similar to those in wild-type MEFs and significantly different from those in Lmna −/− MEFs not expressing lamin A-RFP (Figure 5B). The t1/2 values in cells expressing emerin-GFP and RFP were similar to those in Lmna −/− MEFs with no expressed lamin A-RFP.
FIGURE 5.
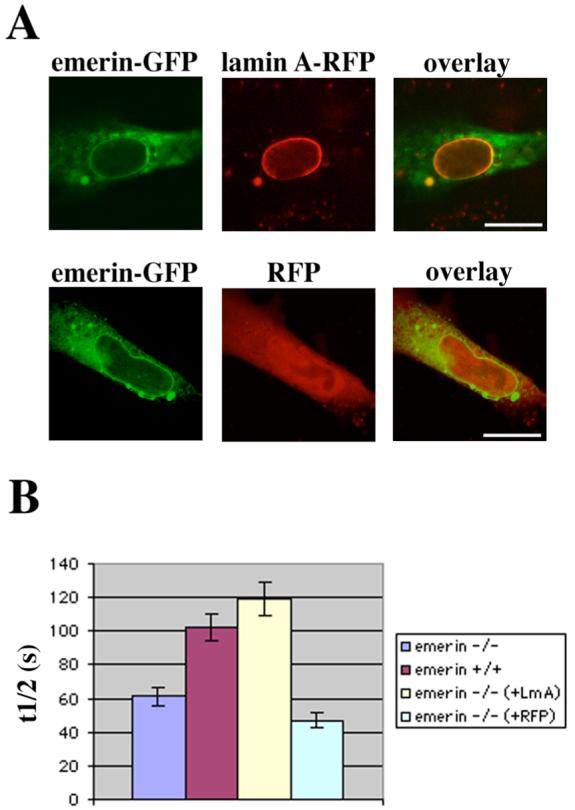
The mobility of emerin-GFP in the nuclear envelope of MEFs lacking endogenous A-type lamins expressing emerin-GFP and lamin A-RFP is similar to the mobility in wild-type MEFs. (A) Panels show laser scanning confocal microscopy images of representative MEFs lacking endogenous A-type lamins cotransfected with plasmids encoding emerin-GFP and lamin A-RFP (upper panels) or emerin-GFP and RFP (lower panels). Bars: 10 μm. (B) Diagram showing the time after bleaching (t1/2) required for fluorescence from emerin-GFP in regions of the nuclear envelope bleached as in Figure 2 to recover to the median between the prebleach value and the value immediately after bleaching. Columns show t1/2 in MEFs lacking A-type lamins (−/−, blue), wild-type MEFs (+/+, red) and MEFs lacking endogenous A-type lamins expressing either lamin A-RFP (−/− (+LmA), yellow) or RFP alone (−/− (+RFP), green). Error bars indicate SEM, n = 17 for MEFs lacking A-type lamins and wild-type MEFs, n = 19 for MEFs expressing lamin A-RFP and n = 14 for MEFs expressing RFP. T-test showed significant differences in t1/2 between +/+ cells and −/− cells (p = 0.00013) and between −/− cells and −/− (+LmA) cells (p = 2.5 × 10−5). There were no significant differences between t1/2 from +/+ cells and −/− (+Lma) cells (p = 0.20) or −/− cells and −/− (+RFP) cells (p = 0.057).
We also performed FRAP experiments on cells cotransfected with plasmids encoding MAN1-GFP and lamin A-RFP or RFP (Figure 6A) and calculated t1/2 values as described above for cells expressing emerin-GFP and lamin A-RFP or RFP (Figure 6B). Similar results were seen. The t1/2 values for MAN1-GFP fluorescence were significantly different between wild-type MEFs and Lmna −/− MEFs. The t1/2 values for MAN1-GFP fluorescence in Lmna −/− MEFs expressing MAN1-GFP and lamin A-RFP were similar to those in wild-type MEFs and significantly different from those in Lmna −/− MEFs not expressing lamin A-RFP. The t1/2 values in cells expressing MAN1-GFP and RFP were similar to those in Lmna −/− MEFs with no expressed lamin A-RFP. In conclusion, for both emerin-GFP and MAN1-GFP, there is a statistically significant difference between t1/2 in wild-type MEFs and Lmna −/− MEFs and this difference is abolished when lamin A-RFP is expressed in the Lmna −/− cells.
FIGURE 6.
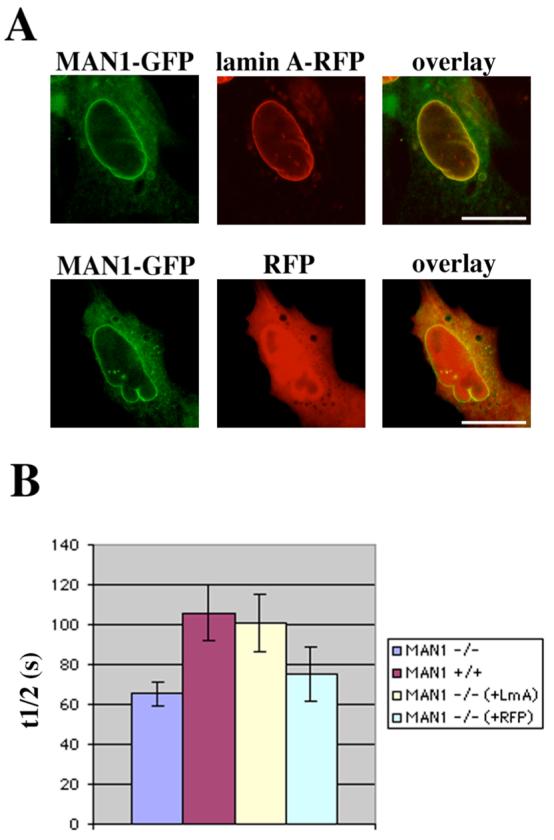
The mobility of MAN1-GFP in the nuclear envelope of MEFs lacking endogenous A-type lamins expressing MAN1-GFP and lamin A-RFP is similar to the mobility in wild-type MEFs. (A) Panels show laser scanning confocal microscopy images of representative MEFs lacking endogenous A-type lamins cotransfected with plasmids encoding MAN1-GFP and lamin A-RFP (upper panels) or MAN1-GFP and RFP (lower panels). Bars: 10 μm. (B) Diagram showing the time required after bleaching (t1/2) for fluorescence from MAN1-GFP in regions of the nuclear envelope bleached as in Figure 3 to recover to the median between the prebleach value and the value immediately after bleaching. Columns show t1/2 in MEFs lacking A-type lamins (−/−, blue), wild-type MEFs (+/+, red) and MEFs lacking endogenous A-type lamins expressing either lamin A-RFP (−/− (+LmA), yellow) or RFP (−/− (+RFP), green). Error bars indicate SEM, n = 28 for MEFs lacking A-type lamins and wild-type MEFs, n = 25 for MEFs expressing lamin A-RFP and n = 11 for MEFs expressing RFP. T-test showed significant differences in t1/2 from +/+ cells and −/− cells (p = 0.010) and from −/− cells and −/− (+LmA) cells (p = 0.022). There were no significant differences between t1/2 from +/+ cells and −/− (+Lma) cells (p = 0.81) or −/− cells and −/− (+RFP) cells (p = 0.45).
DISCUSSION
Both emerin and MAN1 have been shown to bind to A-type lamins (41, 42, 44, 45) and emerin mislocalizes to the ER in Lmna −/− MEFs (49). We have shown that emerin-GFP and MAN1-GFP are significantly more mobile in the nuclear envelope of MEFs lacking A-type lamins than in wild-type fibroblasts. This provides additional support for the “diffusion and retention” model, which has been proposed for the targeting of integral membrane proteins to the inner nuclear membrane (7-12). The results also support the hypothesis that A-type lamins are partly responsible for anchoring emerin and MAN1 in the inner nuclear membrane after their post-translational diffusion from the ER membranes. Both emerin and MAN1 also bind to several other proteins, among them B-type lamins (41, 45) and the chromatin protein barrier-to-integration factor (45, 60). Interactions with such proteins in cells lacking A-type lamins can explain why emerin and MAN1 are still more mobile in the ER than in inner nuclear membrane of Lmna −/− MEFs. Retention by interaction with barrier-to-integration factor does, however, seem less likely as this protein diffuses more rapidly at the nuclear envelope than emerin and MAN1 (61). We did not detect any difference in the mobility of LBR in the nuclear envelope between cells with or without A-type lamins. This is consistent with findings that LBR has very weak affinity for A-type lamins (34) and is likely retained in the inner nuclear membrane by binding other partners, such as B-type lamins (34, 38), heterochromatin proteins (39, 40) and DNA (38, 43).
Mutations in A-type lamins, emerin, MAN1 and LBR cause human diseases. Mutations in LMNA, encoding lamins A and C, cause a wide range of human disorders affecting several different tissues (62). Examples are limb-girdle muscular dystrophy and autosomal dominant Emery-Dreifuss muscular dystrophy, both affecting cardiac and skeletal muscle, lipodystrophy syndromes sometimes combined with abnormalities in skin and bone, peripheral neuropathy and premature aging syndromes. Mutations in emerin, in most cases leading to a loss of the protein, cause X-linked Emery-Dreifuss muscular dystrophy (63). MAN1 mutations cause osteopoikilosis, Buschke-Ollendorff syndrome and non-sporadic melorheostosis, diseases affecting mainly bone and skin (64). Heterozygous mutations in the LBR gene cause Pelger-Huët anomaly, a benign alteration in neutrophil nuclear morphology (65), while homozygous mutations cause fatal HEM/Greenberg skeletal dysplasia (66). The pathogenic mechanisms of most of these diseases are not understood. FRAP studies on transfected cells have shown that some lamin A mutants that cause disease are more mobile in the nuclear envelope than wild-type lamin A (67, 68). Emerin is also partly mislocalized to the ER in some cells transfected with plasmids encoding certain lamin A and C mutants (69-71). However, emerin appears to be exclusively localized to the nuclear envelope in cells from human subjects heterozygous for dominant A-type lamin mutations examined by immunofluorescence microscopy (72-74). Emerin is also localized exclusively to the nuclear envelope in mice, both heterozygous and homozygous, for the LmnaH222P/H222P mutation, which in humans cause autosomal dominant Emery-Dreifuss muscular dystrophy (75).
Subtle changes in the localization or dynamics of emerin and MAN1, secondary to mutations in A-type lamins and not detected by light microscopy, could affect the functions of these proteins. The function of emerin is poorly understood but it interacts with several nuclear proteins, including transcription factors, and has been suggested to anchor these to the inner nuclear membrane (76). Emerin also appears to be involved in transcriptional regulation of mechanosensitive genes in response to strain (77). Smad proteins are intracellular mediators of transforming growth factor β, bone morphogenic proteins and activin signaling (78). MAN1 binds to Smads and antagonizes these signaling pathways (56, 64, 79-81), potentially by sequestering the Smad proteins at the inner nuclear envelope and competing with other Smad-binding proteins for formation of transcription activator complexes. Alterations in A-type lamins may therefore affect the mobility of MAN1 in the inner nuclear membrane, as suggested by our studies, in turn affecting Smad sequestration.
ACKNOWLEDGEMENT
We thank Dr. Eugene Marcantonio for the RFP-C1 vector and Dr. Kunxin Luo and Dr. Harald Herrmann for antibodies.
Footnotes
C.Ö. was supported by a Muscular Dystrophy Association Research Development Grant (MDA3859) and H.J.W. by grants from the Muscular Dystrophy Association (MDA3711) and National Institutes of Health (AR048997).
Abbreviations: ER, endoplasmic reticulum; FITC, fluorescein isothiocyanate; FRAP, fluorescence recovery after photobleaching; GFP, green fluorescent protein; MEF, mouse embryonic fibroblast; PBS, phosphate-buffered saline; RFP, red fluorescent protein; TRITC, tetramethyl rhodamine isothiocyanate
REFERENCES
- 1.Blobel G. Intracellular protein topogenesis. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blobel G. Protein targeting (Nobel lecture) Chembiochem. 2000;1:86–102. doi: 10.1002/1439-7633(20000818)1:2<86::AID-CBIC86>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Palade GE. Intracellular aspects of the process of protein transport. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 4.Pelham HR, Rothman JE. The debate about transport in the Golgi--two sides of the same coin? Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 5.van Vliet C, Thomas EC, Merino-Trigo A, Teasdale RD, Gleeson PA. Intracellular sorting and transport of proteins. Prog. Biophys. Mol. Biol. 2003;83:1–45. doi: 10.1016/s0079-6107(03)00019-1. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Boulan E, Müsch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim. Biophys. Acta. 2005;1744:455–464. doi: 10.1016/j.bbamcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Soullam B, Worman HJ. The amino-terminal domain of the lamin B receptor is a nuclear envelope targeting signal. J. Cell Biol. 1993;120:1093–1100. doi: 10.1083/jcb.120.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J. Cell Biol. 1995;130:15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Östlund C, Ellenberg J, Hallberg E, Lippincott-Schwartz J, Worman HJ. Intracellular trafficking of emerin, the Emery-Dreifuss muscular dystrophy protein. J. Cell Sci. 1999;112:1709–1719. doi: 10.1242/jcs.112.11.1709. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Lin F, Worman HJ. Intracellular trafficking of MAN1, an integral protein of the nuclear envelope inner membrane. J. Cell Sci. 2002;115:1361–1372. doi: 10.1242/jcs.115.7.1361. [DOI] [PubMed] [Google Scholar]
- 12.Ohba T, Schirmer EC, Nishimoto T, Gerace L. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J. Cell Biol. 2004;167:1051–1062. doi: 10.1083/jcb.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amar-Costesec A, Wibo M, Thines-Sempoux D, Beaufay H, Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. II. Preparation and composition of the microsomal fraction. J. Cell Biol. 1974;61:201–212. doi: 10.1083/jcb.61.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathak RK, Luskey KL, Anderson RG. Biogenesis of the crystalloid endoplasmic reticulum in UT-1 cells: evidence that newly formed endoplasmic reticulum emerges from the nuclear envelope. J. Cell Biol. 1986;102:2158–2168. doi: 10.1083/jcb.102.6.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starr DA, Han M. ANChors away: an actin based mechanism of nuclear positioning. J. Cell Sci. 2003;116:211–216. doi: 10.1242/jcs.00248. [DOI] [PubMed] [Google Scholar]
- 16.Gerace L, Ottaviano Y, Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J. Cell Biol. 1982;95:826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallberg E, Wozniak RW, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J. Cell Biol. 1993;122:513–521. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wozniak RW, Blobel G, Rout MP. POM152 is an integral protein of the pore membrane domain of the yeast nuclear envelope. J. Cell Biol. 1994;125:31–42. doi: 10.1083/jcb.125.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wozniak RW, Blobel G. The single transmembrane segment of gp210 is sufficient for sorting to the pore membrane domain of the nuclear envelope. J. Cell Biol. 1992;119:1441–1449. doi: 10.1083/jcb.119.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Söderqvist H, Imreh G, Kihlmark M, Linnman C, Ringertz N, Hallberg E. Intracellular distribution of an integral nuclear pore membrane protein fused to green fluorescent protein--localization of a targeting domain. Eur. J. Biochem. 1997;250:808–813. doi: 10.1111/j.1432-1033.1997.00808.x. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa K, Panté N, Aebi U, Gerace L. Cloning of a cDNA for lamina-associated polypeptide 2 (LAP2) and identification of regions that specify targeting to the nuclear envelope. EMBO J. 1995;14:1626–1636. doi: 10.1002/j.1460-2075.1995.tb07151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 23.Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman AE, Maul G, Steinert PM, Yang HY, Goldman RD. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc. Natl. Acad. Sci. U.S.A. 1986;83:3839–3843. doi: 10.1073/pnas.83.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 26.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- 27.Machiels BM, Zorenc AHG, Endert JM, Kuijpers HJH, van Eys GJJM, Ramaekers FCS, Broers JLV. An alternative splicing product of the lamin A/C gene lacks exon 10. J. Biol. Chem. 1996;271:9249–9253. doi: 10.1074/jbc.271.16.9249. [DOI] [PubMed] [Google Scholar]
- 28.Biamonti G, Giacca M, Perini G, Contreas G, Zentilin L, Weighardt F, Guerra M, Della Valle G, Saccone S, Riva S, Falaschi A. The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol. Cell. Biol. 1992;12:3499–3506. doi: 10.1128/mcb.12.8.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin F, Worman HJ. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics. 1995;27:230–236. doi: 10.1006/geno.1995.1036. [DOI] [PubMed] [Google Scholar]
- 30.Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly, and interactions. J. Struct. Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- 31.Paddy MR, Belmont AS, Saumweber H, Agard DA, Sedat JW. Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell. 1990;62:89–106. doi: 10.1016/0092-8674(90)90243-8. [DOI] [PubMed] [Google Scholar]
- 32.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 33.Senior A, Gerace L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J. Cell Biol. 1988;107:2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worman HJ, Yuan J, Blobel G, Georgatos SD. A lamin B receptor in the nuclear envelope. Proc. Natl. Acad. Sci. U.S.A. 1988;85:8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worman HJ, Evans CD, Blobel G. The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J. Cell Biol. 1990;111:1535–1542. doi: 10.1083/jcb.111.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courvalin JC, Lassoued K, Worman HJ, Blobel G. Identification and characterization of autoantibodies against the nuclear envelope lamin B receptor from patients with primary biliary cirrhosis. J. Exp. Med. 1993;172:19035–19038. doi: 10.1084/jem.172.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foisner R, Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993;73:1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- 38.Ye Q, Worman HJ. Primary structure analysis and lamin B and DNA binding of human LBR, an integral protein of the nuclear envelope inner membrane. J. Biol. Chem. 1994;269:11306–11311. [PubMed] [Google Scholar]
- 39.Ye Q, Worman HJ. Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J. Biol. Chem. 1996;271:14653–14656. doi: 10.1074/jbc.271.25.14653. [DOI] [PubMed] [Google Scholar]
- 40.Ye Q, Callebaut I, Pezhman A, Courvalin JC, Worman HJ. Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J. Biol. Chem. 1997;272:14983–14989. doi: 10.1074/jbc.272.23.14983. [DOI] [PubMed] [Google Scholar]
- 41.Fairley EA, Kendrick-Jones J, Ellis JA. The Emery-Dreifuss muscular dystrophy phenotype arises from aberrant targeting and binding of emerin at the inner nuclear membrane. J. Cell Sci. 1999;112:2571–2582. doi: 10.1242/jcs.112.15.2571. [DOI] [PubMed] [Google Scholar]
- 42.Clements L, Manilal S, Love DR, Morris GE. Direct interaction between emerin and lamin A. Biochem. Biophys. Res. Commun. 2000;267:709–714. doi: 10.1006/bbrc.1999.2023. [DOI] [PubMed] [Google Scholar]
- 43.Duband-Goulet I, Courvalin JC. Inner nuclear membrane protein LBR preferentially interacts with DNA secondary structures and nucleosomal linker. Biochemistry. 2000;39:6483–6488. doi: 10.1021/bi992908b. [DOI] [PubMed] [Google Scholar]
- 44.Sakaki M, Koike H, Takahashi N, Sasagawa N, Tomioka S, Arahata K, Ishiura S. Interaction between emerin and nuclear lamins. J. Biochem. 2001;129:321–327. doi: 10.1093/oxfordjournals.jbchem.a002860. [DOI] [PubMed] [Google Scholar]
- 45.Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J. Biol. Chem. 2005;280:13863–13870. doi: 10.1074/jbc.M413020200. [DOI] [PubMed] [Google Scholar]
- 46.Manilal S, Man NT, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- 47.Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, Hayashi YK, Tsukahara T, Arahata K. Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat. Genet. 1996;12:254–259. doi: 10.1038/ng0396-254. [DOI] [PubMed] [Google Scholar]
- 48.Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J. Biol. Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999;147:913–919. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaughan OA, Alvarez-Reyes M, Bridger JM, Broers JLV, Ramaekers FCS, Wehnert M, Morris GE, Whitfield WGF, Hutchison CJ. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J. Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- 51.Muchir A, van Engelen BG, Lammens M, Mislow JM, McNally E, Schwartz K, Bonne G. Nuclear envelope alterations in fibroblasts from LMGD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp. Cell Res. 2003;291:352–362. doi: 10.1016/j.yexcr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Paulin-Levasseur M, Blake DL, Julien M, Rouleau L. The MAN antigens are non-lamin constituents of the nuclear lamina in vertebrate cells. Chromosoma. 1996;104:367–379. doi: 10.1007/BF00337226. [DOI] [PubMed] [Google Scholar]
- 53.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. [Google Scholar]
- 55.Cance WG, Chaudhary N, Worman HJ, Blobel G, Cordon-Cardo C. Expression of the nuclear lamins in normal and neoplastic human tissue. J. Exp. Clin. Cancer Res. 1992;11:233–246. [Google Scholar]
- 56.Pan D, Estévez-Salmerón LD, Stroschein SL, Zhu X, He J, Zhou S, Luo K. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J. Biol. Chem. 2005;280:15992–16001. doi: 10.1074/jbc.M411234200. [DOI] [PubMed] [Google Scholar]
- 57.Dreger CK, Konig AR, Spring H, Lichter P, Herrmann H. Investigation of nuclear architecture with a domain-presenting expression system. J. Struct. Biol. 2002;140:100–115. doi: 10.1016/s1047-8477(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 58.Phair RD, Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- 59.Harrington KS, Javed A, Drissi H, McNeil S, Lian JB, Stein JL, Van Wijnen AJ, Wang YL, Stein GS. Transcription factors RUNX1/AML1 and RUNX2/Cbfa1 dynamically associate with stationary subnuclear domains. J. Cell Sci. 2002;115:4167–4176. doi: 10.1242/jcs.00095. [DOI] [PubMed] [Google Scholar]
- 60.Lee KK, Haraguchi T, Lee RS, Koujin T, Hiraoka Y, Wilson KL. Distinct functional domains in emerin bind to lamin A and DNA-bridging protein BAF. J. Cell Sci. 2001;114:4567–4573. doi: 10.1242/jcs.114.24.4567. [DOI] [PubMed] [Google Scholar]
- 61.Shimi T, Koujin T, Segura-Totten M, Wilson KL, Haraguchi T, Hiraoka Y. Dynamic interaction between BAF and emerin revealed by FRAP, FLIP, and FRET analyses in living HeLa cells. J. Struct. Biol. 2004;147:31–41. doi: 10.1016/j.jsb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Muchir A, Worman HJ. The nuclear envelope and human disease. Physiology. 2004;19:309–314. doi: 10.1152/physiol.00022.2004. [DOI] [PubMed] [Google Scholar]
- 63.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 64.Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PCM, Costa T, Janssens K, Menten B, Van Roy N, Vermeulen SJT, Savarirayan R, Van Hul W, Vanhoenacker F, Huylebroeck D, De Paepe A, Naeyaert JM, Vandesompele J, Speleman F, Verschueren K, Coucke PJ, Mortier GR. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat. Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann K, Dreger CK, Olins AL, Olins DE, Shultz LD, Lucke B, Karl H, Kaps R, Muller D, Vayá A, Aznar J, Ware RE, Sotelo Cruz N, Lindner TH, Herrmann H, Reis A, Sperling K. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huët anomaly) Nat. Genet. 2002;31:410–414. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- 66.Waterham HR, Koster J, Mooyer P, van Noort G, Kelley RI, Wilcox WR, Wanders RJA, Hennekam RCM, Oosterwijk JC. Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3 beta-hydroxysterol delta 14-reductase deficiency due to mutations in the lamin B receptor gene. Am. J. Hum. Genet. 2003;72:1013–1017. doi: 10.1086/373938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilchrist S, Gilbert N, Perry P, Östlund C, Worman HJ, Bickmore WA. Altered protein dynamics of disease-associated lamin A mutants. BMC Cell Biol. 2004;5:46. doi: 10.1186/1471-2121-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broers JLV, Kuijpers HJH, Östlund C, Worman HJ, Endert J, Ramaekers FCS. Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization. Exp. Cell Res. 2005;304:582–592. doi: 10.1016/j.yexcr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 69.Raharjo WH, Enarson P, Sullivan T, Stewart C, Burke B. Nuclear envelope defects associated with LMNA mutations causing dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci. 2001;114:4447–4457. doi: 10.1242/jcs.114.24.4447. [DOI] [PubMed] [Google Scholar]
- 70.Östlund C, Bonne G, Schwartz K, Worman HJ. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J. Cell Sci. 2001;114:4435–4445. doi: 10.1242/jcs.114.24.4435. [DOI] [PubMed] [Google Scholar]
- 71.Holt I, Östlund C, Stewart CL, Man NT, Worman HJ, Morris GE. Effect of pathogenic mis-sense mutations in lamin A on its interaction with emerin in vivo. J. Cell Sci. 2003;116:3027–3035. doi: 10.1242/jcs.00599. [DOI] [PubMed] [Google Scholar]
- 72.Vigouroux C, Auclair M, Dubosclard E, Pouchelet M, Capeau J, Courvalin JC, Buendia B. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J. Cell Sci. 2001;114:4459–4468. doi: 10.1242/jcs.114.24.4459. [DOI] [PubMed] [Google Scholar]
- 73.Muchir A, Medioni J, Laluc M, Massart C, Arimura T, van der Kooi AJ, Desguerre I, Mayer M, Ferrer X, Briault S, Hirano M, Worman HJ, Mallet A, Wehnert M, Schwartz K, Bonne G. Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve. 2004;30:444–450. doi: 10.1002/mus.20122. [DOI] [PubMed] [Google Scholar]
- 74.Reichart B, Klafke R, Dreger C, Krüger E, Motsch I, Ewald A, Schäfer J, Reichmann H, Müller CR, Dabauvalle MC. Expression and localization of nuclear proteins in autosomal-dominant Emery-Dreifuss muscular dystrophy with LMNA R377H mutation. BMC Cell Biol. 2004;5:12. doi: 10.1186/1471-2121-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, Fromes Y, Toussaint M, Mura AM, Keller DI, Amthor H, Isnard R, Malissen M, Schwartz K, Bonne G. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 76.Bengtsson L, Wilson KL. Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr. Opin. Cell Biol. 2004;16:73–79. doi: 10.1016/j.ceb.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 79.Osada S, Ohmori SY, Taira M. XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development. 2003;130:1783–1794. doi: 10.1242/dev.00401. [DOI] [PubMed] [Google Scholar]
- 80.Raju GP, Dimova N, Klein PS, Huang HC. SANE, a novel LEM domain protein, regulates bone morphogenetic protein signaling through interaction with Smad1. J. Biol. Chem. 2003;278:428–437. doi: 10.1074/jbc.M210505200. [DOI] [PubMed] [Google Scholar]
- 81.Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum. Mol. Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]


