Abstract
Objective
According to the stress-toxicity hypothesis of depression, hippocampal volumes may diminish as the disease progresses. We sought to examine the changes in hippocampal and amygdala volumes at baseline and at 3 years after an acute depressive episode, and the impact of reduced hippocampal volumes on the outcome.
Methods
In a prospective, longitudinal study, we examined the hippocampus and amygdala of 30 inpatients with major depression from the Department of Psychiatry and Psychotherapy and 30 healthy participants from the community (control group) using high-resolution magnetic resonance images at baseline and after 3 years. Psychopathology was assessed at baseline, weekly during the inpatient phase and then after 1, 2 and 3 years.
Results
During the 3-year follow-up period, neither hippocampal nor amygdala volumes changed significantly among patients or participants in the control group. However, in the subgroup of patients who took antidepressants over the full 3 years, the left hippocampal volumes increased significantly. Patients with small hippocampal volumes and previous depressive episodes had a worse clinical outcome compared with patients with large hippocampal volumes and previous depressive episodes.
Conclusion
Overall, our results suggest that a relatively small hippocampal volume may be a vulnerability factor for a bad treatment response in major depression. Subtle changes in hippocampal volumes may be detectable during continuous antidepressant therapy. Such changes may be the result of neuroplastic processes.
Medical subject headings: hippocampus; amygdala; magnetic resonance imaging; depressive disorder, major
Abstract
Objectif
Selon l'hypothèse voulant que la dépression soit liée à la toxicité du stress, le volume de l'hippocampe pourrait diminuer à mesure que la maladie évolue. Nous avons examiné les variations de volume de l'hippocampe et des amygdales au départ et 3 ans après un épisode de dépression aiguë, et l'impact de la réduction du volume de l'hippocampe sur l'issue.
Méthodes
Au cours d'une étude longitudinale prospective, nous avons examiné l'hippocampe et les amygdales de 30 patients atteints d'une dépression majeure et hospitalisés au département de psychiatrie et de psychothérapie et de 30 participants en bonne santé de la communauté (groupe témoin). Nous avons utilisé l'imagerie par résonance magnétique à haute résolution au départ et après 3 ans. Nous avons évalué la psychopathologie au départ, une fois par semaine au cours de l'hospitalisation et ensuite après 1, 2 et 3 ans.
Résultats
Au cours de la période de suivi de 3 ans, ni le volume de l'hippocampe ni celui des amygdales n'ont changé considérablement chez les patients ou les participants du groupe témoin. Toutefois, dans le sous-groupe des patients qui ont pris des antidépresseurs au cours des 3 années, le volume de l'hippocampe gauche a augmenté considérablement. L'issue clinique a été moins bonne chez les patients dont le volume de l'hippocampe était faible et qui avaient déjà eu des épisodes de dépression, que chez les patients dont le volume de l'hippocampe était important et qui avaient déjà eu des épisodes de dépression.
Conclusion
Dans l'ensemble, nos résultats indiquent que le volume relativement faible de l'hippocampe peut constituer un facteur de vulnérabilité à une mauvaise réponse au traitement dans un cas de dépression majeure. Des changements subtils du volume de l'hippocampe peuvent être repérables au cours d'une thérapie continue aux antidépresseurs. Ces changements découlent peut-être de phénomènes neuroplastiques.
Introduction
Major depression is one of the most frequent human diseases, with a lifetime prevalence of 16% and a 12-month prevalence of 6.6%.1 About 40% of patients with depression do not respond to treatment with the first antidepressant prescribed, and 20% experience chronic depression. Therefore, neurobiological investigations are required to further improve our understanding of the biological mechanisms of poor response to treatment and poor clinical outcomes.
A dysfunction of neuronal plasticity or remodelling is presumed to be responsible for the pathophysiology of major depression.2 This hypothesis is supported by animal studies that demonstrated that stress and depressive-like states led to atrophy and loss of neurons in the adult hippocampus.3,4 These experimental studies found that prolonged stress decreased the numbers of apical dendritic branch points and the length of apical dendrites, particularly in the laminar CA3 region of the hippocampus. This effect was dependent on the use of glucocorticoids and emerged after 3 weeks of experimental corticosterone therapy.3,4 Moreover, antidepressants were found to suppress stress toxic effects on the hippocampus and increase hippocampal neurogenesis.5
This hypothesis is also supported by clinical studies that showed that recurrent depression was associated with reductions of the total volume of the hippocampus. Cross-sectional in vivo neuroimaging studies detected reduced hippocampal volumes in elderly patients6,7 and in younger patients8–10 with major depression. Two meta-analyses on cross-sectional studies confirmed that the volume of the hippocampus was consistently reduced in patients with major depression, especially in patients with recurrent depression.11,12 However, there were some negative findings. Cross-sectional studies found first indications of a relation between structural alterations and the course of the illness. One study found significant associations between chronic depression and reduced left hippocampal grey matter density measured by voxel-based analysis.13 A recent study using statistical parametric mapping demonstrated that the volume of the right hippocampus was reduced in elderly patients with depression, particularly in patients with a longer course of illness.14 Moreover, a significant negative correlation between smaller hippocampal volumes and longer cumulative duration of illness has been suggested.15
The amygdala is involved in the processing of emotion and in mood disorders. However, the results from volumetric cross-sectional studies of changes of amygdala volume have been inconsistent. It has been found that the amygdala was enlarged in patients with a first depressive episode,16 in young women with major depression17 and in patients with recurrent depression8 compared with healthy individuals of the same age. However, 2 studies failed to find altered amygdala volumes in patients with recurrent depression,10,18 and 1 study detected reductions of a subregion of the amygdala, the amygdala core nuclei.19
According to the stress toxicity hypothesis, hippocampal volumes are expected to diminish as a depressive disease progresses. To test this hypothesis, in a previous study we conducted a longitudinal, prospective follow-up investigation involving patients with major depression and healthy participants. After 1 year, hippocampal and amygdala volumes had not changed significantly from baseline among patients or participants in the control group. However, the subgroup of patients who were nonremitted at the follow-up investigation after 1 year showed significantly reduced left and right hippocampal volumes compared with remitted patients, both at follow-up and baseline.20
In the present study, we examined the same population at baseline and after 3 years using high resolution structural magnetic resonance imaging (MRI), and at baseline and after 1, 2 and 3 years using psychiatric examinations. We sought to determine whether depression resulted in a further reduction of hippocampal volumes or whether a smaller hippocampal volume predisposed an individual to the development of depression. Furthermore, we examined whether changes in volume were specific to the acute depressive episode and normalized during the treatment period, or whether they also persisted in patients who responded to treatment with an antidepressant.
Methods
Study population
Of the 78 patients who took part in our earlier study, we examined 30 inpatients of the Department of Psychiatry and Psychotherapy of the Ludwig-Maximilians-University in Munich, Germany, who were aged 18–65 years and who had received a diagnosis of depression after 3 years. We planned to examine patients and participants in the control group at baseline (hospital admission for patients with major depression), and after 1, 2 and 3 years using structural MRI. Data from our 1-year follow-up on these patients has been published previously.20 After 2 years, not enough patients agreed to be reinvestigated. However, it was possible to reach 30 of 78 patients in the baseline sample after 3 years.
We diagnosed psychiatric disorders according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition, (DSM-IV)21 criteria and the Structured Clinical Interview for DSM-IV (SCID),22 and the diagnoses were determined by a consensus of at least 2 psychiatrists. We documented clinical variables using the 21-item Hamilton Depression Rating Scale (HDRS).23
We defined full remission over the whole 3-year period as a score of 7 or less based on a 17-item (shortened from 21) HDRS. We assessed psychopathology and medication history at baseline, on a weekly basis during the inpatient phase and then after 1, 2 and 3 years.
We enrolled 30 healthy participants from the local community in the control group. These participants were matched with the patients for age (mean 43.6, standard deviation [SD] 13.1, range 22–64 yr), sex and handedness. We examined participants in the control group at baseline and after 3 years. Neither the participants in the control group nor their first-degree relatives had a history of neurologic or mental illness.
We excluded from our study all individuals who had a previous head injury with loss of consciousness, received earlier treatment with hydrocortisone, had a history of alcohol or substance abuse and who had neurologic diseases. We also excluded individuals with other mental illnesses, especially bipolar disorders and personality disorders. None of the patients had ever been treated with electroconvulsive therapy. We determined handedness using the Edinburgh inventory.24 We used a structured interview to assess medical history, trauma and other exclusion criteria.
We obtained written informed consent from patients and participants in the control group after they had been given a detailed description of the study. We designed the study in accordance with the ethical standards in the Declaration of Helsinki, and we received approval for the study from the local ethics committee from the Ludwig-Maximilians-University of Munich.
MRI procedures
To obtain MRI images we used a 1.5 T Magnetom Vision scanner (Siemens) with a coronal T2- and proton density-weighted dual-echo sequence (TR 3710 ms, TE 2290 ms, total acquisition time 9 min, number of acquisitions 1, FOV 230 mm, matrix 240 × 256, slice thickness 3 mm) and a 3D-MPRAGE sequence (TR 11.6 ms, TE 4.9 ms; total acquisition time 9 min, number of acquisitions 1, FOV 230 mm, matrix 512 × 512, slice thickness 1.5 mm). We used the Analyze (Mayo Foundation) commercial software package for further image processing, with size reduction from 16 to 8 bit and transformation to a uniform matrix of 256 × 256 on 192 slices of 1.0-mm thickness. We realigned all data sets and resampled them 3-dimensionally in the anterior commissure to posterior commissure (AC–PC) line according to the coordinates of Tailairach, using the Brain Research: Analysis of Images, Networks and Systems (BRAINS) software program developed by Andreasen and colleagues.25 The BRAINS program allowed the regions of interest (ROIs) to be controlled on sagittal and transverse sections simultaneously. It also allowed them to be segmented to enable calculation of the intracranial content and the grey and white matter volume (expressed in cubic millilitres) within each defined ROI. We used the same software and hardware throughout the 3-year study period.
Definition of the hippocampal and amygdala formation
A detailed description of the hippocampal and amygdala borders has been published previously.9,16 The description is illustrated in Figure 1. The evaluation staff (T.F., I.S.) was blind to participant allocation. We manually outlined the hippocampus and amygdala using a mouse-driven cursor.
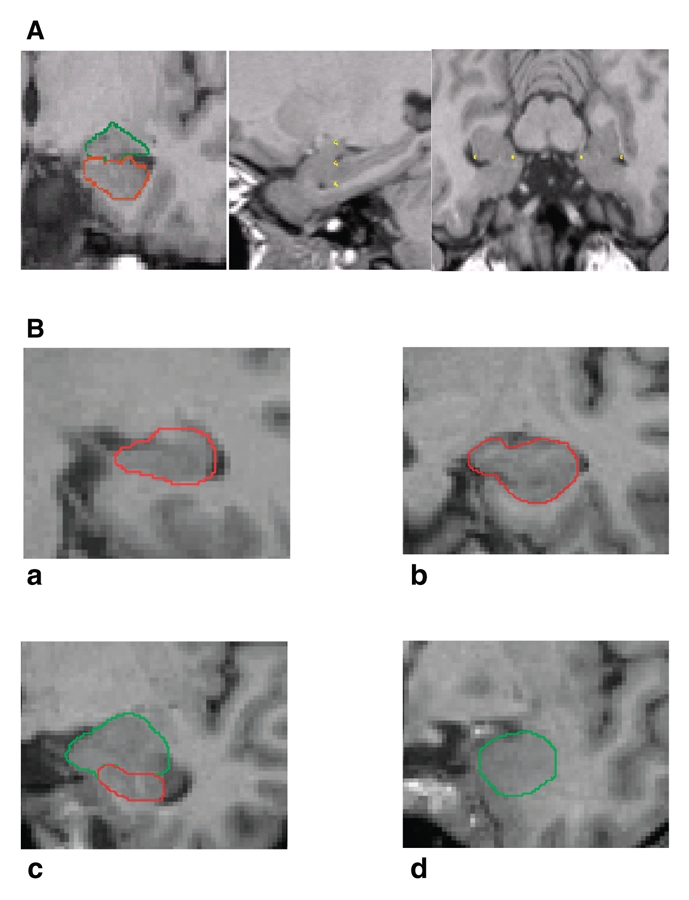
Fig. 1: Magnetic resonance imaging (MRI) slices. A: Triplanar view of the hippocampus and amygdala (coronal, sagittal, transverse views). B: Coronal MRI slices that run in an occipito-rostral direction show the hippocampal body (a), the shape of the hippocampus seen with the head directed vertically (b), the posterior-medial part of the amygdala (c) and a slice through the anterior-medial part of the amygdala (d).
To determine interrater reliability, we randomly selected 10 brains, and 2 raters (T.F., I.S) determined ROIs independently. The intraclass correlation for both the inter-rater reliability (hippocampus: rICC = 0.97, amygdala: rICC = 0.95) and the intrarater reliability (hippocampus: rICC = 0.96, amygdala: rICC = 0.91) was high.9,16
Statistical analyses
We considered all statistical tests to be significant at p < 0.05. We tested morphometric measurements in both groups for homogeneity of variance and for normality using the Kolmogorov–Smirnov test. Using age and total brain volume as covariates, we subjected hippocampal and amygdala volumes to an analysis of covariance (ANCOVA) to assess the effects of the interaction between within-subject factors in the left and right hemispheres of the brain and diagnosis (patients with depression v. participants in the control group).
We used the same ANCOVA design to compare the within-subject factors of patients with a first depressive episode with those of patients with recurrent depression (factor episode). We applied a multivariate analysis of variance (MANOVA), using the HDRS scores at 1, 2 and 3 years as time variables and baseline HDRS scores as a covariable, to compare participants with large versus small hippocampal or amygdala volumes and participants with a first depressive episode versus those with recurrent depression. We used the Fisher exact test to compare groups with respect to the proportions of relapses.
Controlling for the effect of age, we calculated partial correlation coefficients to investigate the relation between volumes and cumulative duration of illness.
Results
Study population
The demographic and clinical characteristics of the 30 patients and 30 participants in the control group are outlined in Table 1. According to participants' scores on the 17-item HDRS, we considered 17 patients to be in remission, and 13 patients were not remitted over the whole 3-year period. At baseline, 6 patients were taking serotonin reuptake inhibitors (2 sertraline, 3 citalopram, 1 paroxetine), 9 were taking tricyclic antidepressants (3 amitriptyline, 3 amitriptylinoxid, 3 doxepin), 12 were taking other new antidepressants (4 venlafaxine, 3 reboxetine, 5 mirtazapine), 2 were taking marprotiline and 1 patient was not being treated with an antidepressant. At the 3-year follow-up, 8 patients were taking serotonin reuptake inhibitors (2 sertraline, 4 citalopram, 1 paroxetine, 1 fluoxetine), 4 were taking tricyclic antidepressants (2 amitriptyline, 2 clomipramine), 9 were taking other new antidepressants (6 venlafaxine, 2 reboxetine, 1 mirtazapine), 1 was taking tranylcipromine, 3 were taking lithium and 5 patients were not being treated with an antidepressant.
Table 1
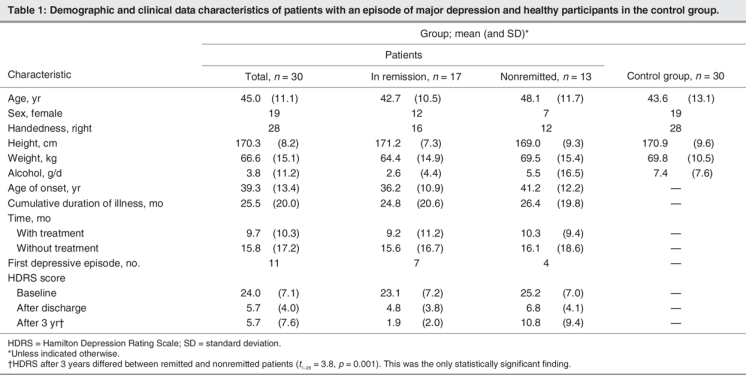
Morphometric data were normally distributed. We found no significant differences in age, sex, handedness, height, weight and total brain volume among patients and participants in the control group. Furthermore, we found no significant differences in these variables among patients remitted and nonremitted after 3 years. We found no significant difference in the proportions of patients with relatively small or large hippocampal volumes among patients with a first depressive episode and patients with recurrent depression (median split, χ21 = 2.6, p = 0.11). After 3 years, depression measured with the HDRS was more severe among nonremitted patients than among patients who were remitted at the 3-year follow-up (t1–28 = 3.8, p = 0.001).
Hippocampal volume
Hippocampal volumes at baseline and 3-year follow-up are shown in Table 2. Hippocampal volumes did not differ significantly between patients and participants in the control group (effect of diagnosis: F1–56 = 0.45, p = 0.51), and they did not change significantly from baseline to follow-up (effect of time: F1–56 = 1.5, p = 0.22). Furthermore, we found no significant interaction between time and diagnosis (F1–56 = 0.07, p = 0.80), which indicated that hippocampal volumes had not changed in patients or participants in the control group.
Table 2
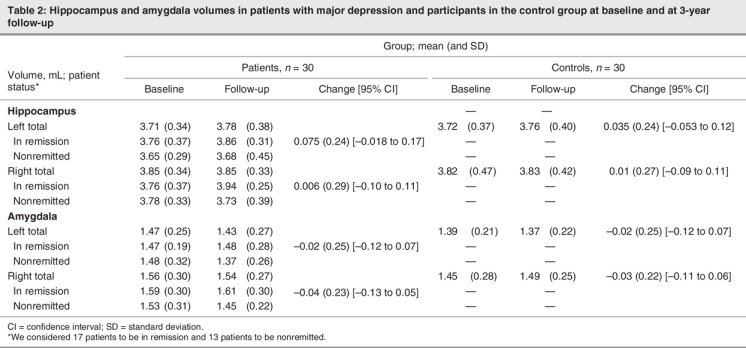
Amygdala volume
Amygdala volumes at baseline and follow-up are shown in Table 2. They did not differ significantly between patients and participants in the control group, we found no significant effect of diagnosis (F1–56 = 0.57, p = 0.45) or time (F1–56 = 0.016, p = 0.90), and there were no significant interactions between these factors.
Hippocampal volume and clinical remission
We found that hippocampal volume was not affected by remission status (F1–25 = 0.8, p = 0.37) and that there was no significant interaction between time and remission (F1–25 = 1.4, p = 0.25). There was a marginally significant interaction between remission and the factor episode (F1–25 = 4.1, p = 0.05).
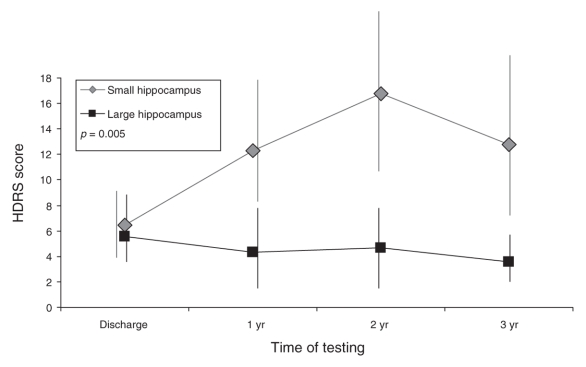
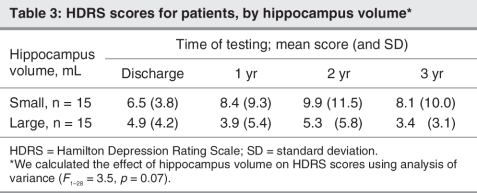
In the MANOVA design to test for predictive value of hippocampal volumes, we observed a significant interaction between larger versus smaller hippocampal volumes at baseline and the factor episode on HDRS scores at 1, 2 and 3 years (F1–25 = 5.4, p = 0.023). Patients with recurrent depression and relatively smaller hippocampal volumes experienced significantly worse outcomes, as measured by the HDRS scores at 1, 2 and 3 years, compared with those patients with recurrent depression and relatively larger hippocampal volumes (F1–16 = 10.5, p = 0.005) (Fig. 2). We observed a trend toward significant differences in HDRS scores in the total group of patients (Table 3).
Fig. 2: Hamilton Depression Rating Scale (HDRS) scores for patients with recurrent depression and with a relatively large hippocampal volume (n = 11), compared with those with a relatively small hippocampal volume (n = 8) (median split: 7.55 mL). Error bars for 1 standard deviation are included. We derived the p value shown in the figure by multivariate analysis of variance, and it refers to the difference between groups in the HDRS scores at 1, 2 and 3 years.
Table 3
Results of the Fisher exact test did not show significant differences in the number of relapses between patients with a larger hippocampus (n = 3 relapses) and those with a smaller hippocampus (n = 7 relapses) (Fisher test1 = 2.4, p = 0.24). Furthermore, there was no significant difference with respect to the number of patients remitted after 3 years among patients with a larger hippocampal volume (n = 1 with depression) and those with a smaller hippocampal volume (n = 5 with depression) (Fisher test1 = 3.3, p = 0.17). There was no effect in the number of patients being remitted over the full 3 time intervals between these groups (Fisher test1 = 1.4, p = 1.0).
Amygdala volume and remission
Amygdala volume was not affected by remission status (F1–26 = 0.16, p = 0.69), and there was no significant interaction between time and remission (F1–26 = 2.7, p = 0.11).
Patients with larger amygdala volumes (median split) at baseline did not have significantly lower HDRS scores at 1, 2 and 3 years (F1–25 = 0.74, p = 0.40) than patients with smaller hippocampal volumes at baseline. Furthermore, there was no significant interaction between amygdala volume and the factor episode (F1–25 = 1.1, p = 0.31).
Use of antidepressants
Left hippocampal volumes significantly increased during the 3 years of follow-up among patients who took their medication during this period. At baseline, the mean volume of the left hippocampus was 3.64 mL (SD 0.33 mL); at the 3-year follow-up, the mean volume was 3.81 mL (SD 0.37 mL), which was a mean change of 0.16 mL (SD 0.21 mL, 95% confidence interval [CI] = 0.051 to 0.27 mL, F1–15 = 7.5, p = 0.015). At baseline, the mean volume of the right hippocampus was 3.77 mL (SD 0.27 mL); at the 3-year follow-up, the mean volume was 3.84 mL (SD 0.30 mL), which was a mean change of 0.066 mL (SD 0.24 mL, 95% CI = –0.060 to 0.19, F1–15 = 0.65, p = 0.43). After performing a Bonferroni adjustment, this effect remained significant for the left hippocampus. There were no significant changes in hippocampal volume among patients who discontinued their use of antidepressants during follow-up (left hippocampus: F1–13 = 0.8, p = 0.39; right hippocampus: F1–13 = 0.65, p = 0.44).
Clinical variables
We found no significant correlation between age and hippocampal volumes among patients or participants in the control group. However, there was a significant negative correlation between age and the volume of the left amygdala among participants in the control group (r28 = –0.42, p = 0.022) and of the right amygdala among patients (r28 = –0.38, p = 0.041).
We found no significant correlation between age of onset and hippocampal volumes. Left hippocampal volumes at baseline significantly correlated with cumulative duration of illness (r = 0.39, p = 0.035) and duration of untreated depressive episodes (r = 0.40, p = 0.027).
The HDRS scores did not correlate significantly with hippocampal or amygdala volumes at baseline, discharge from hospital or the 3-year follow-up.
Discussion
Hippocampal and amygdala volumes did not significantly decrease over 3 years among patients or participants in the control group, and these volumes did not significantly differ in the study population. Thus our study cannot confirm that hippocampal volumes diminish during depressive episodes, as suggested in cross-sectional in vivo neuroimaging studies in elderly patients6,7 and in younger patients with major depression.8–10
Our main finding was that patients with recurrent depression who have a smaller hippocampal volume experienced a negative clinical outcome within the first 3 years after an acute depressive episode. Moreover, in a cross-sectional analysis Sheline and colleagues6 observed a correlation between smaller hippocampal volumes and a longer cumulative duration of illness. Thus hippocampal volumes and the course of a depressive episode may be related. Our findings supported the hypothesis that brain alterations such as reduced hippocampal volumes may predispose patients to the development of depression and a poor clinical outcome without full remission from depression. Thus stressful life events or other factors that influence neuronal development (e.g., pre-, peri-or postnatal infections; genetic vulnerability) may change hippocampal structures in a way that would render patients more vulnerable to the development of major depression.
Other studies support the hypothesis that a smaller hippocampus may predict psychiatric disease with more severe symptoms. Lyons and colleagues26 stated that paternal genetics, but not early stress, appeared to account for much of the variance in hippocampal size in squirrel monkeys. Experimental studies showed that monkeys with smaller hippocampal volumes responded with greater increases in adrenocorticotropic hormone levels after social manipulation.27 Interestingly, Gilbertson and colleagues28 found that smaller hippocampal volumes constituted a risk factor for the development of stress-related psychopathology because the severity of post-traumatic symptoms was negatively correlated with the hippocampal volume in both patients with post-traumatic stress disorders and the patients' unexposed identical twins.
We found that larger hippocampal volumes were associated with a good clinical response and with a low relapse rate over the 3 years. Relatively larger hippocampal volumes may prevent relapses in patients with recurrent depression. In line with this hypothesis, larger hippocampal volumes have been found to be associated with less executive dysfunctioning29 and memory impairment.30,31
Interestingly, this effect was seen in particular among patients with recurrent depression. It may be that patients with first depressive episodes are not defined well enough clinically because, compared with patients with unipolar recurrent depression, they may also have bipolar disorders with other neurobiological correlates.
Based on the stress-toxicity hypothesis of depression32 and on cross-sectional studies of the relation between duration of illness and hippocampal volumes,15 we might have expected a volume decline at least among patients who continued to experience depression during the 3 years of follow-up; however, we found no significant reduction of hippocampal volumes. These results indicated that there was no volume decline during depressive episodes and that total hippocampal and amygdala volumes were very stable over time. Only a small decline in the hippocampal volume with increasing age has been found in in vivo33–36 and postmortem studies37 that reported little or no hippocampal volume changes with increasing age. In particular, hippocampal volume decline was seen in patients with Alzheimer disease, which showed a progressive hippocampal decline owing to neurodegenerative processes.34 Therefore, the failure to find significant differences during a 3-year period among participants in a control group or among patients with major depression, in which most of the patients were remitted from depression, was not astonishing. It may be that subregions of the hippocampus, such as the gyrus dendatus or region CA3, are more sensitive to neuroplastic changes, as reported in studies of animals.2–4 Therefore, methods such as voxel-based morphometry or high-resolution MRI (e.g., 3 T or more), are required to detect changes in these subregions.
Recently, we found an association between the brain-derived neurotrophic factor polymorphism and hippocampal volumes, which was independent of a diagnosis of depression,38 suggesting that the volume of the hippocampus may be determined early during neuronal development. This finding, together with the main finding of our present study, suggested that the hippocampus may be resistant to disease effects, but that neuroplastic processes may cause subtle changes. Such small effects were seen among patients who had been taking antidepressants for a long time. We found that left hippocampal volumes increased significantly even after we performed a Bonferroni adjustment in those patients who took their antidepressants over the whole 3-year period, which indicated that the antidepressants had active effects (e.g., through neuroplastic processes), which have been suggested based on results of experimental studies.2,5 Our study supports the finding from a structural MRI study in 20 patients with post-traumatic stress disorder that the mean hippocampal volume increased by about 4.6% after a 36- to 48-week trial involving treatment with paroxetine.39 However, in patients with major depression, no significant change in hippocampal volumes was found after a mean of 7 months (SD 3 mo) of successful treatment with serotonin reuptake inhibitors, in particular with fluoxetine, compared with the pretreatment investigation.40 It may be that morphological changes are more likely to be seen after a longer time period, as in our study, than after a few months of treatment. However, we do not know of any evidence that antidepressants act unilaterally on neurogenesis or neuroplastic processes. Thus we have to regard our finding with caution. Future studies involving larger samples are necessary to investigate this question.
We found that amygdala volumes were not significantly altered among patients with a first depressive episode or with recurrent depression. This finding was in line with earlier studies, including our own investigations, which failed to find altered amygdala volumes in patients with recurrent depression10,18 and showed smaller19 or even larger amygdala volumes16 among patients with a first depressive episode. One explanation for these inconsistent findings may be that the amygdala can be less reliably measured than the hippocampus.
Limitations
Our study had a few limitations. All patients took antidepressants for at least 6 months after their depressive episodes, as recommended clinically. This may be one reason why we failed to find progressive changes in hippocampal volumes because, at this very early stage, the possible protective effects associated with the use of antidepressants might have been present in all patients. Another limitation was our relatively small sample; however, to date we are not aware of a larger follow-up investigation using structural MRI. In addition, we had to define large and small hippocampal volume groups post-hoc after baseline investigations; however, no normal values are available at the present time. Furthermore, hippocampal volumes that 1 or 2 units of standard deviation smaller than those of participants in the control group nearly did not exist in patients with major depression. Thus it is unlikely that hippocampal volume will be used as a diagnostic tool in the near future. It may be the case that mainly patients with good clinical outcomes participated in the follow-up investigation, whereas nonremitted patients with poor clinical outcomes did not. This study bias might explain the failure to find significantly reduced hippocampal volumes in the overall patient group.
In summary, the present data indicate that relatively smaller hippocampal and amygdala volumes might predispose patients with recurrent depression to a poor treatment response and to vulnerability for relapses.
Acknowledgments
This study was supported by the German Federal Research Ministry within the campaign “German Research Networks in Medicine” as part of the project “German Research Network on Depression” (main principle investigator: Prof. Hans-Jürgen Möller; subproject principle investigators: Eva Meisenzahl, Thomas Frodl). Data were presented at the German Congress of Psychiatry and Psychotherapy, November 2007. Ivana Smajstrlova and Tanja Palladino performed their doctoral theses within this study. We thank Jacqueline Klesing for English language review.
Footnotes
Contributors: Drs. Frodl and Meisenzahl designed the study. Drs. Frodl, Jäger, Smajstrlova, Born, Bottlender, Palladino, Reiser and Möller acquired the data. Dr. Frodl analyzed the data and wrote the article. All authors reviewed the article and gave final approval for publication.
Competing interests: None declared for Drs. Frodl, Jäger, Smajstrlova, Born, Bottlender, Palladino, Reiser and Meisenzahl. Dr. Möller is a member of the speaker bureaus of, has served as consultant or adviser to, and has received grant/research support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Organon, Pfizer and Sepracor. He is also on the speaker bureaus of Eisai and Sanofi Aventis, has served as consultant or adviser to Servier and Wyeth, and has received grant/research support from Eisai, Merck, Novartis, Sanofi Aventis, Servier and Wyeth.
Correspondence to: Dr. T. Frodl, Department of Psychiatry, Nussbaumstr. 7, 80336 Munich, Germany; fax 0049-89-5160-5343; Thomas.Frodl@med.uni-muenchen.de
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105. [DOI] [PubMed]
- 2.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 2002;17(Suppl 3):306-10. [DOI] [PubMed]
- 3.Reagan LP, McEwen BS. Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat 1997;13:149-67. [DOI] [PubMed]
- 4.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res 1990;531:225-31. [DOI] [PubMed]
- 5.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805-9. [DOI] [PubMed]
- 6.Sheline YI, Sanghavi M, Mintun MA, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19:5034-43. [DOI] [PMC free article] [PubMed]
- 7.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry 2000;48:301-9. [DOI] [PubMed]
- 8.Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry 2000;157:115-8. [DOI] [PubMed]
- 9.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002;159:1112-8. [DOI] [PubMed]
- 10.Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000;30:117-25. [DOI] [PubMed]
- 11.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004;161:598-607. [DOI] [PubMed]
- 12.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004;161:1957-66. [DOI] [PubMed]
- 13.Shah PJ, Ebmeier KP, Glabus MF, et al. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry 1998;172:527-32. [DOI] [PubMed]
- 14.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry 2002;159:1424-7. [DOI] [PubMed]
- 15.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry 2003;160:1516-8. [DOI] [PubMed]
- 16.Frodl T, Meisenzahl E, Zetzsche T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 2002;51:708-14. [DOI] [PubMed]
- 17.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med 2004;34:1059-64. [DOI] [PubMed]
- 18.Frodl T, Meisenzahl EM, Zetzsche T, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry 2003;53:338-44. [DOI] [PubMed]
- 19.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 1998;9:2023-8. [DOI] [PubMed]
- 20.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry 2004;65:492-9. [DOI] [PubMed]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 22.First MB, Spitzer RL, Gibbon M, et al. Structure Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Arlington (VA): American Psychiatric Publishing; 1997.
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 24.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97-113. [DOI] [PubMed]
- 25.Andreasen NC, Cohen G, Harris G, et al. Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992;4:125-33. [DOI] [PubMed]
- 26.Lyons DM, Yang C, Sawyer-Glover AM, et al. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry 2001;58:1145-51. [DOI] [PubMed]
- 27.Lyons DM, Parker KJ, Zeitzer JM, et al. Preliminary evidence that hippocampal volumes in monkeys predict stress levels of adrenocorticotropic hormone. Biol Psychiatry 2007;62:1171-4. [DOI] [PMC free article] [PubMed]
- 28.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7. [DOI] [PMC free article] [PubMed]
- 29.Frodl T, Schaub A, Banac S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 2006;31:316-23. [PMC free article] [PubMed]
- 30.O'Brien JT, Lloyd A, McKeith I, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 2004;161:2081-90. [DOI] [PubMed]
- 31.Von Gunten A, Ron MA. Hippocampal volume and subjective memory impairment in depressed patients. Eur Psychiatry 2004;19:438-40. [DOI] [PubMed]
- 32.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35. [DOI] [PubMed]
- 33.Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol 1995;16:241-51. [PMC free article] [PubMed]
- 34.Jack CR Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology 1998;51:993-9. [DOI] [PMC free article] [PubMed]
- 35.Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997;7:268-82. [DOI] [PubMed]
- 36.Sullivan EV, Pfefferbaum A, Swan GE, et al. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus 2001;11:754-62. [DOI] [PubMed]
- 37.Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging 1987;8:521-45. [DOI] [PubMed]
- 38.Frodl T, Schule C, Schmitt G, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry 2007;64:410-6. [DOI] [PubMed]
- 39.Vermetten E, Vythilingam M, Southwick SM, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 2003;54:693-702. [DOI] [PMC free article] [PubMed]
- 40.Vythilingam M, Vermetten E, Anderson GM, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry 2004;56:101-12. [DOI] [PubMed]