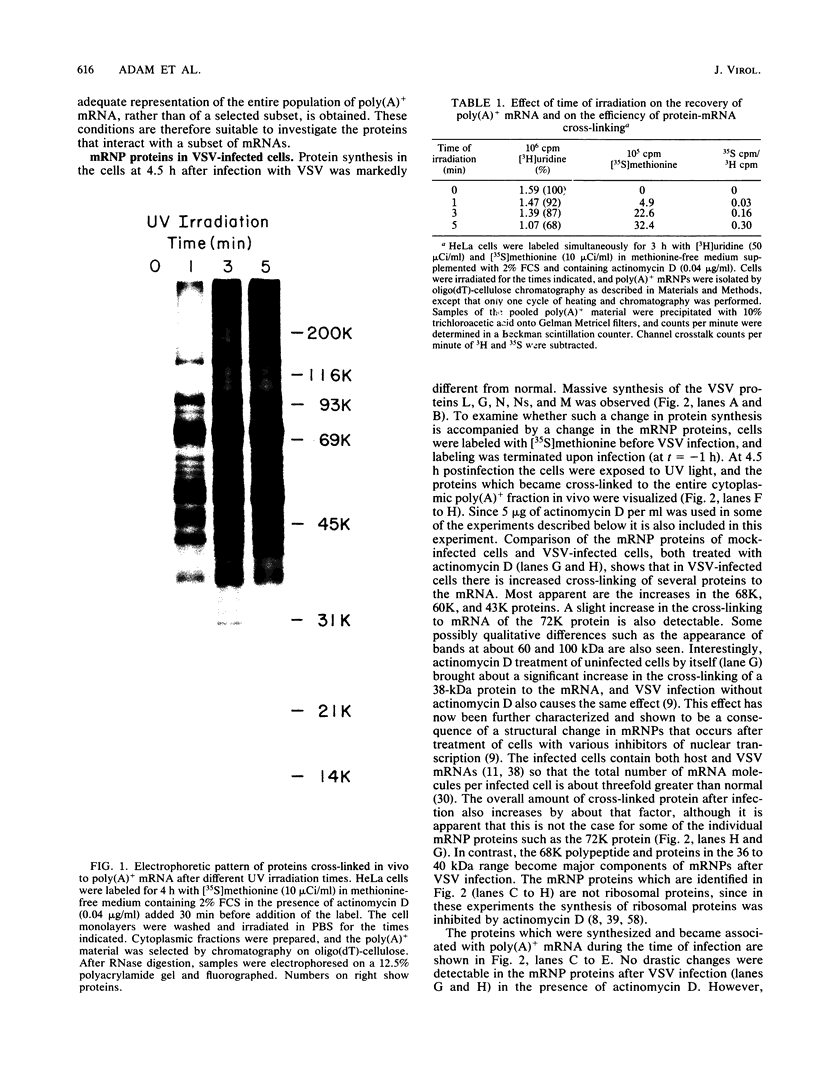
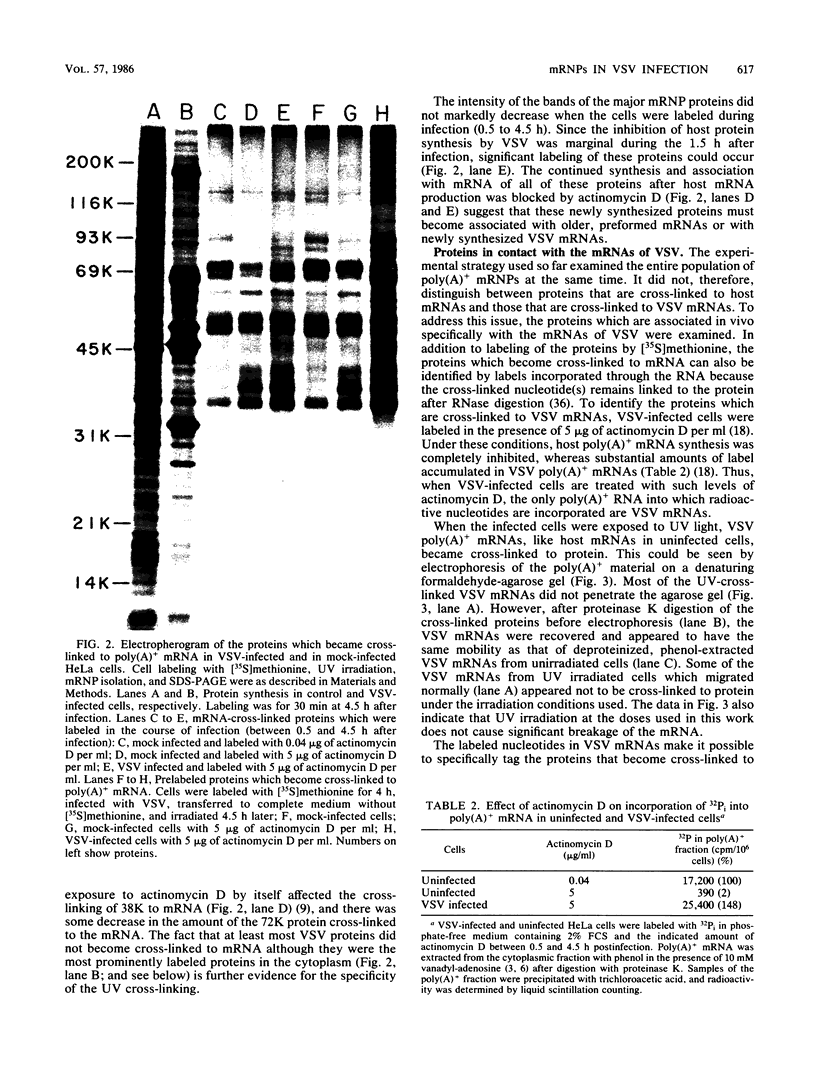
Abstract
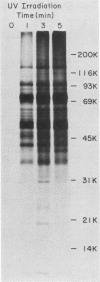
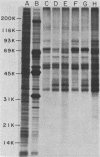
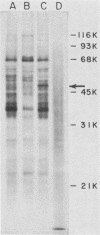
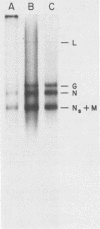
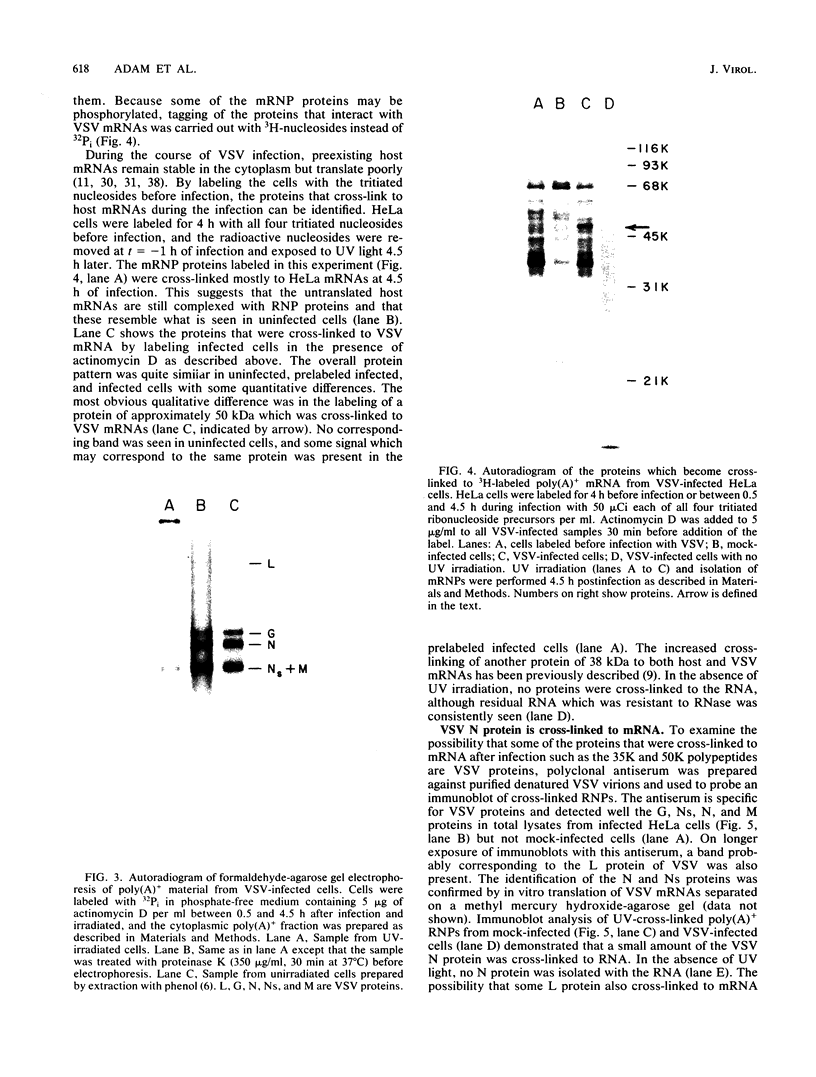
The interaction of mRNA with proteins in vesicular stomatitis virus (VSV)-infected cells was studied by photochemical cross-linking in intact cells. The major [35S]methionine-labeled proteins which became cross-linked by UV light to mRNA in uninfected and in VSV-infected HeLa cells were similar and had apparent mobilities in sodium dodecyl sulfate-polyacrylamide gel electrophoresis corresponding to 135, 93, 72, 68, 53, 50, 43, and 36 kilodaltons. The proteins which were cross-linked in vivo specifically to the five mRNAs of VSV were labeled through radioactive nucleotides incorporated only into VSV mRNAs under conditions (5 micrograms of actinomycin D per ml) in which only VSV mRNAs are labeled. The same major mRNP proteins that became cross-linked to host mRNAs also became cross-linked to VSV mRNAs, although several quantitative differences were detected. Photochemical cross-linking and immunoblotting of cross-linked mRNPs with VSV antiserum demonstrated that in addition to host proteins VSV mRNAs also became cross-linked to the VSV-encoded N protein. The poly(A) segment of both host and VSV mRNAs was associated in vivo selectively with the 72-kilodalton polypeptide. The major proteins of mRNA-ribonucleoprotein complexes are therefore ubiquitous and common to different mRNAs. Furthermore, since the major messenger ribonucleoproteins interact also with VSV mRNAs even though these mRNAs are transcribed in the cytoplasm, it appears that nuclear transcription and nucleocytoplasmic transport are not necessary for mRNA to interact with these proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer B. W., Kornberg R. D. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983 Mar;96(3):717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S. Interaction of HeLa cell proteins with RNA. J Mol Biol. 1970 Feb 14;47(3):263–273. doi: 10.1016/0022-2836(70)90301-3. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher P. D., Arnstein H. R. Efficient translation and polyribosome binding of 125I-labelled rabbit globin messenger ribonucleoprotein. FEBS Lett. 1983 Mar 7;153(1):119–124. doi: 10.1016/0014-5793(83)80130-6. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Monoclonal antibody characterization of the C proteins of heterogeneous nuclear ribonucleoprotein complexes in vertebrate cells. J Cell Biol. 1984 Dec;99(6):1997–1204. doi: 10.1083/jcb.99.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. C. On the regulation of the synthesis of ribosomal proteins in L-cells. J Mol Biol. 1971 Jan 14;55(1):129–134. doi: 10.1016/0022-2836(71)90288-9. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Choi Y. D., Adam S. A. Characterization of heterogeneous nuclear RNA-protein complexes in vivo with monoclonal antibodies. Mol Cell Biol. 1984 Jun;4(6):1104–1114. doi: 10.1128/mcb.4.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunigan D. D., Lucas-Lenard J. M. Two transcription products of the vesicular stomatitis virus genome may control L-cell protein synthesis. J Virol. 1983 Feb;45(2):618–626. doi: 10.1128/jvi.45.2.618-626.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Summers D. F. Adenylate-rich sequences in vesicular stomatitis virus messenger ribonucleic acid. J Virol. 1972 Oct;10(4):683–688. doi: 10.1128/jvi.10.4.683-688.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R., Carroll E., 3rd Reconstitution of functional mRNA-protein complexes in a rabbit reticulocyte cell-free translation system. Mol Cell Biol. 1985 Feb;5(2):342–351. doi: 10.1128/mcb.5.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Proteins crosslinked to messenger RNA by irradiating polyribosomes with ultraviolet light. Nucleic Acids Res. 1980 Dec 11;8(23):5685–5701. doi: 10.1093/nar/8.23.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. The polyribosomal mRNA--protein complex is a dynamic structure. Proc Natl Acad Sci U S A. 1981 May;78(5):2923–2926. doi: 10.1073/pnas.78.5.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M. J., Shafritz D. A. Identification and characterization of messenger ribonucleoprotein complexes from vesicular stomatitis virus-infected HeLa cells. Virology. 1977 Aug;81(1):1–16. doi: 10.1016/0042-6822(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Heimark R. L., Davie E. W. Isolation and characterization of bovine plasma prekallikrein (Fletcher factor). Biochemistry. 1979 Dec 11;18(25):5743–5750. doi: 10.1021/bi00592a035. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Pluskal M. G., Sarkar S. Thermal chromatography of eukaryotic messenger ribonucleoprotein particles on oligo (dT)-cellulose. Evidence for common mRNA-associated proteins in various cell types. FEBS Lett. 1979 Jan 1;97(1):84–90. doi: 10.1016/0014-5793(79)80058-7. [DOI] [PubMed] [Google Scholar]
- Jaye M. C., Godchaux W., 3rd, Lucas-Lenard J. Further studies on the inhibition of cellular protein synthesis by vesicular stomatitis virus. Virology. 1982 Jan 15;116(1):148–162. doi: 10.1016/0042-6822(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Characterization of polypeptides associated with messenger RNA and its polyadenylate segment in Ehrlich ascites messenger ribonucleoprotein. J Biol Chem. 1977 May 25;252(10):3525–3532. [PubMed] [Google Scholar]
- Jones C. L., Ehrenfeld E. The effect of poliovirus infection on the translation in vitro of VSV messenger ribonucleoprotein particles. Virology. 1983 Sep;129(2):415–430. doi: 10.1016/0042-6822(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Poly (A)-rich ribonucleoprotein complexes from HeLa cell messenger RNA. J Biol Chem. 1976 Oct 10;251(19):5888–5894. [PubMed] [Google Scholar]
- Kumar A., Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975 Aug 15;96(3):353–365. doi: 10.1016/0022-2836(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Setyono B., Spindler E., Köhler K. Comparison of proteins bound to the different functional classes of messenger RNA. Biochim Biophys Acta. 1976 Apr 2;425(4):373–383. doi: 10.1016/0005-2787(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Vesicular stomatitis virus mRNA and inhibition of translation of cellular mRNA--is there a P function in vesicular stomatitis virus? J Virol. 1981 May;38(2):504–517. doi: 10.1128/jvi.38.2.504-517.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvaldi J., Sekellick M. J., Marcus P. I., Lucas-Lenard J. Inhibition of mouse L cell protein synthesis by ultraviolet-irradiated vesicular stomatitis virus requires viral transcription. Virology. 1978 Jan;84(1):127–133. doi: 10.1016/0042-6822(78)90224-6. [DOI] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Nuclear ribonucleoprotein particles probed in living cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2208–2212. doi: 10.1073/pnas.78.4.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Abraham G., Adler R., Banerjee A. K. Methylated and blocked 5' termini in vesicular stomatitis virus in vivo mRNAs. Cell. 1975 May;5(1):59–67. doi: 10.1016/0092-8674(75)90092-6. [DOI] [PubMed] [Google Scholar]
- Möller K., Brimacombe R. Specific cross-linking of proteins S7 and L4 to ribosomal RNA, by UV irradiation of Escherichia coli ribosomal subunits. Mol Gen Genet. 1975 Dec 9;141(4):343–355. doi: 10.1007/BF00331455. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Alterations in the protein synthetic apparatus of Friend erythroleukemia cells infected with vesicular stomatitis virus or herpes simplex virus. J Virol. 1978 Jan;25(1):422–426. doi: 10.1128/jvi.25.1.422-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Rosen C. A., Ennis H. L., Cohen P. S. Translational control of vesicular stomatitis virus protein synthesis: isolation of an mRNA-sequestering particle. J Virol. 1982 Dec;44(3):932–938. doi: 10.1128/jvi.44.3.932-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Siekierka J., Ennis H. L., Cohen P. S. Inhibition of protein synthesis in vesicular stomatitis virus infected Chinese hamster ovary cells: role of virus mRNA-ribonucleoprotein particle. Biochemistry. 1984 May 22;23(11):2407–2411. doi: 10.1021/bi00306a014. [DOI] [PubMed] [Google Scholar]
- Ruzdijic S., Bag J., Sells B. H. Cross-linked proteins associated with a specific mRNA in the cytoplasm of HeLa cells. Eur J Biochem. 1984 Jul 16;142(2):239–245. doi: 10.1111/j.1432-1033.1984.tb08277.x. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., O'Banion M. K., Poirot M. K., Reichmann M. E. Effect of intracellular vesicular stomatitis virus mRNA concentration on the inhibition of host cell protein synthesis. J Virol. 1983 Jan;45(1):206–214. doi: 10.1128/jvi.45.1.206-214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H., Darnell J. E. The association of protein with the polyadenylic acid of HeLa cell messenger RNA: evidence for a "transport" role of a 75,000 molecular weight polypeptide. J Mol Biol. 1976 Jul 15;104(4):833–851. doi: 10.1016/0022-2836(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Soria M., Huang A. S. Association of polyadenylic acid with messenger RNA of vesicular stomatitis virus. J Mol Biol. 1973 Jul 5;77(3):449–455. doi: 10.1016/0022-2836(73)90450-6. [DOI] [PubMed] [Google Scholar]
- Stamatos N. M., Gomatos P. J. Binding to selected regions of reovirus mRNAs by a nonstructural reovirus protein. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3457–3461. doi: 10.1073/pnas.79.11.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. R., Wagner R. R. Inhibition of translation in lysates of mouse L cells infected with vesicular stomatitis virus: presence of a defective ribosome-associated factor. Biochemistry. 1983 Mar 29;22(7):1540–1546. doi: 10.1021/bi00276a004. [DOI] [PubMed] [Google Scholar]
- Van Eekelen C. A., Mariman E. C., Reinders R. J., Van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with host cell proteins. Eur J Biochem. 1981 Oct;119(3):461–467. doi: 10.1111/j.1432-1033.1981.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Boettiger D., Murphy H. M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977 Feb;76(2):808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., Riemen T., van Venrooij W. J. Specificity in the interaction of hnRNA and mRNA with proteins as revealed by in vivo cross linking. FEBS Lett. 1981 Aug 3;130(2):223–226. doi: 10.1016/0014-5793(81)81125-8. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Riemen T., van Eekelen C. A. Host proteins are associated with adenovirus specific mRNA in the cytoplasm. FEBS Lett. 1982 Aug 16;145(1):62–71. doi: 10.1016/0014-5793(82)81207-6. [DOI] [PubMed] [Google Scholar]