Abstract
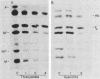
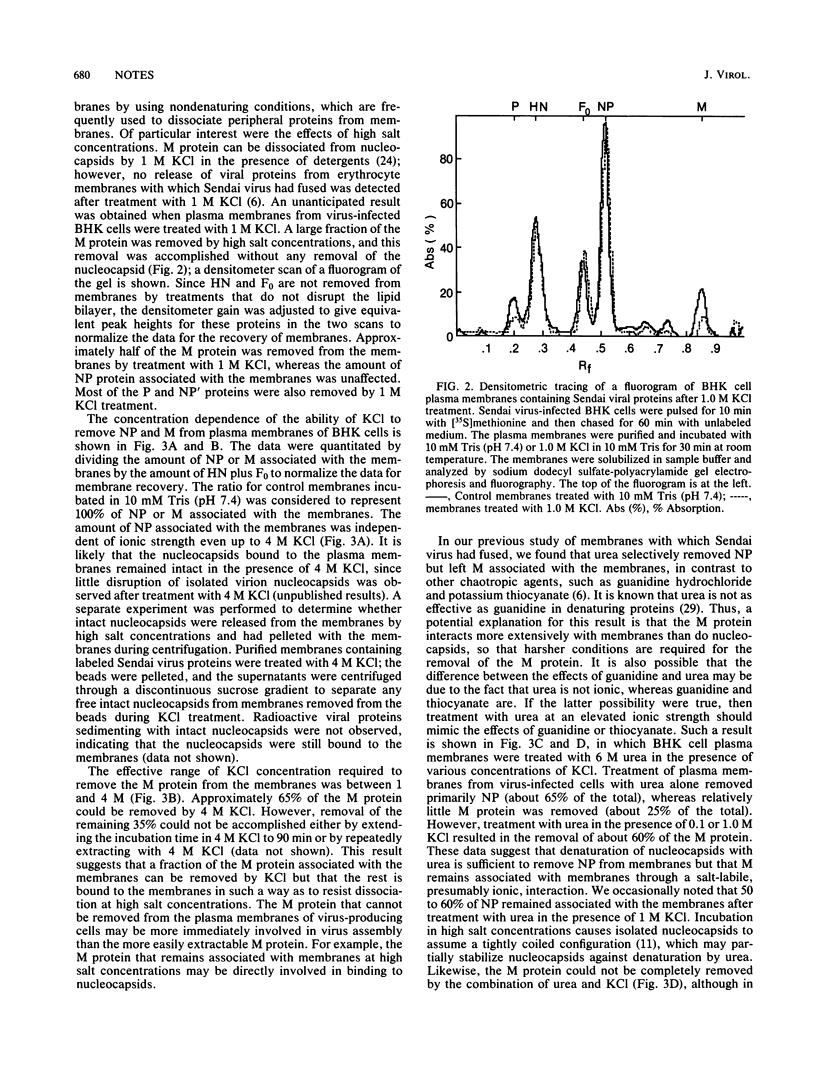
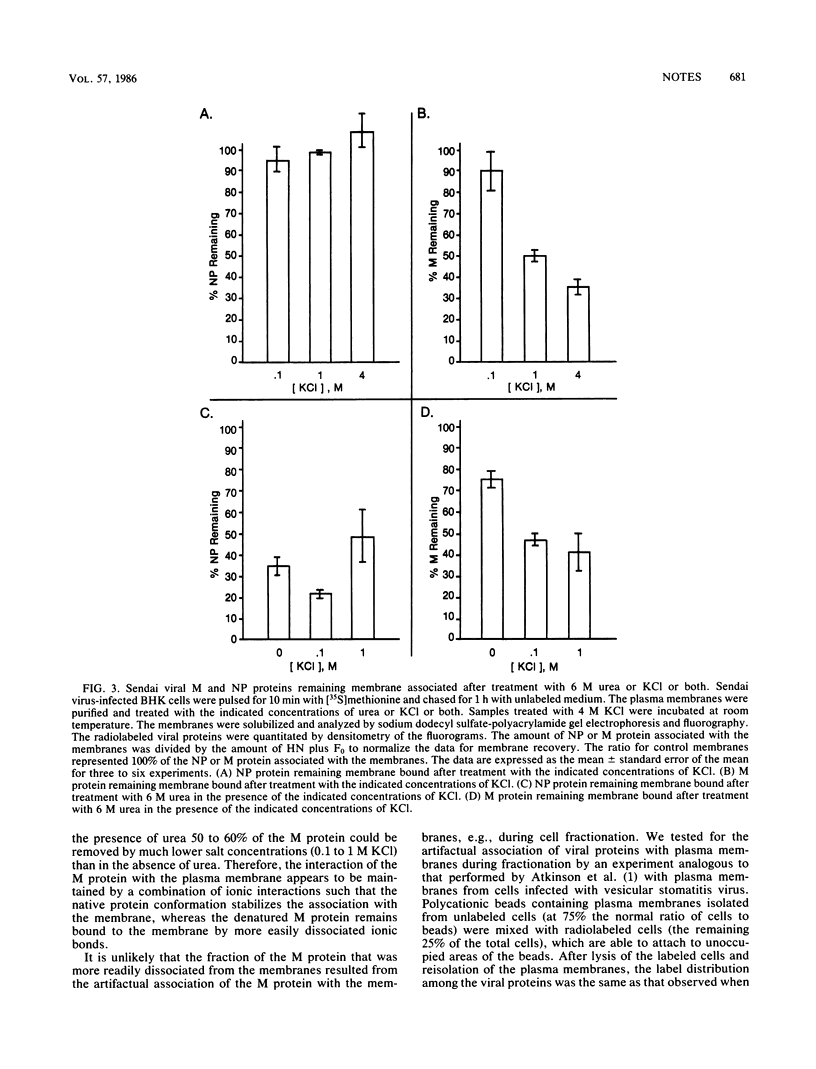
The interaction of Sendai viral proteins with the membranes of infected cells during budding of progeny virions was studied. BHK cells infected with Sendai virus were labeled with [35S]methionine, and the plasma membranes were purified on polycationic polyacrylamide beads. The isolated membranes were incubated with various agents which perturb protein structure to dissociate viral proteins from the membranes. Incubation of membranes with thiocyanate and guanidine removed both the M and nucleocapsid proteins. Urea (6 M) removed the nucleocapsid proteins but removed M protein only in the presence of 0.1 or 1.0 M KCl. In contrast, high salt concentrations alone eluted only the M protein, leaving the nucleocapsid proteins completely membrane bound. About 65% of the M protein was eluted in the presence of 4 M KCl. The remaining membrane-associated M protein was resistant to further extraction by 4 M KCl. Thus, M protein forms two types of interaction with the membrane, one of them being a more extensive association with the membrane than the other.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Moyer S. A., Summers D. F. Assembly of vesicular stomatitis virus glycoprotein and matrix protein into HeLa cell plasma membranes. J Mol Biol. 1976 Apr 15;102(3):613–631. doi: 10.1016/0022-2836(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Bowen H. A., Lyles D. S. Kinetics of incorporation of Sendai virus proteins into host plasma membranes and virions. Virology. 1982 Aug;121(1):1–11. doi: 10.1016/0042-6822(82)90113-1. [DOI] [PubMed] [Google Scholar]
- Bowen H. A., Lyles D. S. Structure of Sendai viral proteins in plasma membranes of virus-infected cells. J Virol. 1981 Mar;37(3):1079–1082. doi: 10.1128/jvi.37.3.1079-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D. J., Kharitonenkov I. G., Zakomirdin J. A., Grigoriev V. B., Klimenko S. M., Davis J. F. Incorporation of influenza virus M-protein into liposomes. J Virol. 1980 Nov;36(2):586–590. doi: 10.1128/jvi.36.2.586-590.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büechi M., Bächi T. Microscopy of internal structures of Sendai virus associated with the cytoplasmic surface of host membranes. Virology. 1982 Jul 30;120(2):349–359. doi: 10.1016/0042-6822(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Caldwell S. E., Lyles D. S. Interaction of Sendai virus proteins with the cytoplasmic surface of erythrocyte membranes following viral envelope fusion. J Biol Chem. 1981 May 25;256(10):4838–4842. [PubMed] [Google Scholar]
- Cohen C. M., Kalish D. I., Jacobson B. S., Branton D. Membrane isolation on polylysine-coated beads. Plasma membrane from HeLa cells. J Cell Biol. 1977 Oct;75(1):119–134. doi: 10.1083/jcb.75.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Ottaviano Y., Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982 Dec;95(3):826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A., Frangione B. Insertion of influenza M protein into the viral lipid bilayer and localization of site of insertion. J Virol. 1981 Oct;40(1):323–328. doi: 10.1128/jvi.40.1.323-328.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Scheid A., Choppin P. W. Conformation of the helical nucleocapsids of paramyxoviruses and vesicular stomatitis virus: reversible coiling and uncoiling induced by changes in salt concentration. Proc Natl Acad Sci U S A. 1980 May;77(5):2631–2635. doi: 10.1073/pnas.77.5.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Hsu C. H., Murti K. G. Intracellular metabolism of sendai virus nucleocapside. Virology. 1978 Nov;91(1):86–94. doi: 10.1016/0042-6822(78)90357-4. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lively M. O., Walsh K. A. Hen oviduct signal peptidase is an integral membrane protein. J Biol Chem. 1983 Aug 10;258(15):9488–9495. [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella D. A., Lenard J. Interactions of wild-type and mutant M protein of vesicular stomatitis virus with viral nucleocapsid and envelope in intact virions. Evidence from [125I]iodonaphthyl azide labeling and specific cross-linking. Biochemistry. 1981 Nov 24;20(24):6872–6877. doi: 10.1021/bi00527a020. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Lackland H., Choppin P. W. Isolation and characterization of the nonglycosylated membrane protein and a nucleocapsid complex from the paramyxovirus SV5. Virology. 1975 Oct;67(2):365–374. doi: 10.1016/0042-6822(75)90438-9. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y., Maeno K., Iinuma M., Hamaguchi M., Matsumoto T., Nagayoshi S., Hoshino M. Studies on the role of M protein in virus assembly using a ts mutant of HVJ (Sendai virus). Virology. 1979 Jan 15;92(1):139–154. doi: 10.1016/0042-6822(79)90220-4. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]