Abstract
Mechanistic and empirical modelling are compared in context of dimeric receptors. In particular, the supposed advantages of the two-state dimer model for fitting of binding data with respect to classical approaches such as the two-independent sites model are investigated. The two models are revisited from both the mechanistic and empirical point of views. The problem of overparameterized models and the benefits of the concurrent use of mechanistic and empirical models for mechanism analysis are discussed. The pros and cons of mathematical models are examined with special emphasis given to the interpretation of the connection between the shapes of the curves and receptor cooperativity. It is shown that a given pharmacological phenotype (curve shape) can be obtained from different receptor genotypes (as, for instance, non-interconvertible monomeric receptor species, receptor-G protein interactions and dimeric receptors), though values of the Hill coefficient greater than one are indicative of receptor oligomerization. The existence of a relationship between the recently defined dimer cooperativity index and the more familiar Hill coefficient is proven.
Keywords: empirical models, dimer cooperativity index, Hill coefficient, mathematical modelling, mechanistic models, model fitting, receptor dimerization
Introduction
The so-called two-state dimer model (Franco et al., 2005, 2006) has been reviewed in two recent articles, with the authors putting a particular emphasis on the advantages of this model for fitting of binding data as compared to classical approaches such as the two independent sites model (Casadó et al., 2007; Franco et al., 2008). In addition, a new parameter reflecting the molecular communication within the dimer, the cooperativity index, was defined.
The two-state dimer model was developed (Franco et al., 2005, 2006) with the aim of accurately describing the binding and function of G-protein-coupled receptors (GPCRs). GPCRs are of great relevance in pharmacological and therapeutic research, as they represent nearly half of the current targets in the drug discovery pipelines (Overington et al., 2006). Although most of the experimental evidence accumulated over the last decade suggests that GPCRs exist and function as dimers or higher-order oligomers (see Gurevich and Gurevich, 2008; Milligan, 2008; Szidonya et al., 2008 for review), there is an open debate on the monomeric/dimeric nature of GPCRs (James et al., 2006; Bouvier et al., 2007). In particular, the issue on whether there is a requirement for the receptor to be dimeric for G-protein activation is a key topic of investigation (White et al., 2007; Whorton et al., 2007). Mathematical models can help to elucidate the complexity of the receptor dynamics, and, accordingly, accurate fitting to the experimental data points is of prime importance.
Here, the two-state dimer model and the two independent sites model are revisited from both the mechanistic and empirical points of view. The pros and cons of both approaches are discussed, and the existence of a relationship between the cooperativity index and the more familiar Hill coefficient is proved.
Empirical and mechanistic models
Curve fitting of experimental data points by mathematical models is a common way to extract relevant information from biological systems (Kenakin, 1997). Mathematical models can be classified either as mechanistic or empirical, depending on whether the equation used derives from an explicitly written chemical process or not. As the equation parameters pose a biophysical meaning only in the mechanistic models, it is in these models that the main features of the mechanism involved are captured by the fitting. In contrast, but not as a minor feature, the empirical approach is just limited to finding the most appropriate function and obtaining the parameter values that best characterize the shape of the curve (Giraldo et al., 2002). In an ideal world, mechanistic models would be the preferred models, as they can lead to new knowledge of the biological process; yet the high number of parameters they may contain and the likely correlation between them often precludes their use in standard curve-fitting procedures.
Receptor oligomerization and curve modelling
There are several mechanistic proposals in the literature that include receptor oligomerization to account for the shape of binding and response curves (see Colquhoun, 1973; Wells, 1992; Christopoulos and Kenakin, 2002; Springael et al., 2007 for review). Some illustrative examples follow: (1) An application of a model, originally thought for multi-subunit enzymes (Monod et al., 1965), to the acetylcholine receptor (Karlin, 1967), allowed the author to account for responses with Hill coefficient greater than one. (2) Wreggett and Wells (1995) performed an investigation of the binding properties of purified cardiac muscarinic receptors from porcine atria. The variation of the Hill coefficient for some muscarinic ligands, with values lower and greater than unity, together with the presence of a disparity of curve shapes (biphasic and bell-shaped curves were found), led the authors to suggest the presence of a multivalent receptor (at least tetravalent), in which data could be described in terms of cooperative interactions (Wreggett and Wells, 1995). (3) A subsequent study of the same receptors but from Syrian hamster-washed membranes yielded data that were mechanistically described in terms of a model comprising cooperative and non-cooperative forms of the receptor (Chidiac et al., 1997). For the cooperative form, at least trivalent or divalent states were necessary for consideration depending on whether native or alkylated membranes were examined. (4) Armstrong and Strange (2001) studied the binding of two radioligands ([3H]spiperone and [3H]raclopride) to D2 dopamine receptors expressed in Chinese hamster ovary cells both in the presence and absence of sodium ions. Data were interpreted in terms of a model where the receptor exists as a dimer, and, in the absence of sodium ions, raclopride exerts negative cooperativity across the dimer both for its own binding and for the binding of spiperone. (5) The cross-talk between protomers within a dimer and the resulting cooperativity properties of the bound ligands were the subject of an article in which the model proposed for a dimeric receptor consisted of two oscillating states: one that enables binding sites to cross-talk and another that does not (Durroux, 2005). (6) An explanation of negative binding cooperativity in terms of receptor dimerization was also used in the investigation of glycoprotein hormone receptors (Urizar et al., 2005). (7) Positive and negative binding cooperativity were also observed for vasopressin and oxytocin receptors (Albizu et al., 2006). As positive cooperative binding cannot be explained without considering receptor as multivalent, the authors proposed a dimeric arrangement for these receptors.
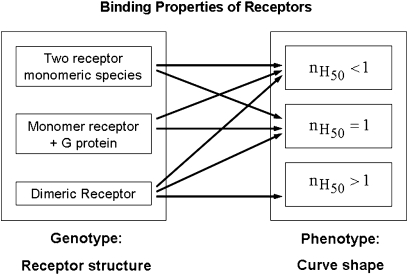
From the above, we see that the cross talk between the protomers within a dimeric receptor can result in positive, negative or absence cooperativity for ligand binding, which is reflected by the shape of the curves and can be quantified by the Hill coefficient at the midpoint (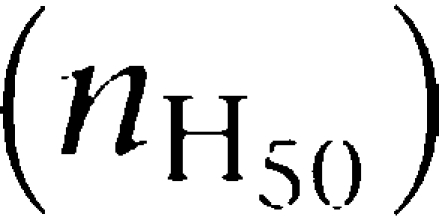 ), with values higher, lower or equal to one, respectively. Importantly, although saturation binding curves with
), with values higher, lower or equal to one, respectively. Importantly, although saturation binding curves with  cannot be described without considering the receptors as multivalent complexes (Mattera et al., 1985; Christopoulos and Kenakin, 2002; Albizu et al., 2006; Franco et al., 2006),
cannot be described without considering the receptors as multivalent complexes (Mattera et al., 1985; Christopoulos and Kenakin, 2002; Albizu et al., 2006; Franco et al., 2006),  can result not only from an oligomeric receptor, but also from either distinct pools of non-interconverting receptor species or a monomeric receptor that recognize accessory cellular proteins (for example, G-proteins) whose concentrations are limited, so they fall as they bind to the receptor (Lee et al., 1986; Green et al., 1997; Colquhoun, 1998). It thus appears that different mechanistic models can predict similar behaviours, in many cases not being possible a unique interpretation of a single curve, and, consequently, being necessary to perform some complementary experiments (for instance, binding experiments in the presence and absence of Gpp(NH)p to examine the influence of G-protein interaction) to exclude wrong explanations nevertheless compatible with individual data sets (Wells, 1992) (Figure 1).
can result not only from an oligomeric receptor, but also from either distinct pools of non-interconverting receptor species or a monomeric receptor that recognize accessory cellular proteins (for example, G-proteins) whose concentrations are limited, so they fall as they bind to the receptor (Lee et al., 1986; Green et al., 1997; Colquhoun, 1998). It thus appears that different mechanistic models can predict similar behaviours, in many cases not being possible a unique interpretation of a single curve, and, consequently, being necessary to perform some complementary experiments (for instance, binding experiments in the presence and absence of Gpp(NH)p to examine the influence of G-protein interaction) to exclude wrong explanations nevertheless compatible with individual data sets (Wells, 1992) (Figure 1).
Figure 1.
Relationship between receptor structure (genotype) and curve shape (phenotype) for receptor oligomerization in receptor-ligand binding studies. A dimeric receptor has been supposed for one of the genotypes, although, more generally, an oligomeric receptor or a multivalent receptor can be assumed. Hill coefficients at the midpoint  , both lower and equal to one, can be obtained from either of the genotypes (non-interconvertible monomeric receptor species; receptor-G protein interaction, where the concentration of the G protein is limited; and a dimeric receptor); however, values greater than one are indicative of receptor oligomerization.
, both lower and equal to one, can be obtained from either of the genotypes (non-interconvertible monomeric receptor species; receptor-G protein interaction, where the concentration of the G protein is limited; and a dimeric receptor); however, values greater than one are indicative of receptor oligomerization.
The two-state dimer model
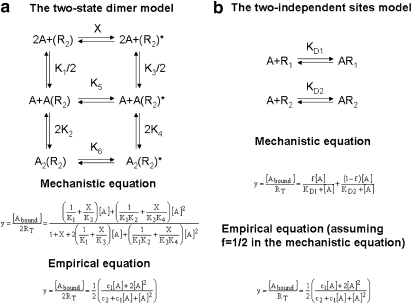
Assuming that GPCRs form dimers or higher-order oligomers, the so-called two-state dimer model (Franco et al., 2005, 2006) was proposed with the aim of explaining the observed cooperativity effects of these receptors under the simplest formulation. The two-state dimer model considers receptors as dimers in two possible states, an inactive (RR) state and an active (RR)* state, where the protomers within each state are undistinguishable (see Figure 2a). As in the two-state model (for a review, see Leff, 1995) developed earlier for monomeric receptors and for which the two-state dimer model is an extension, the active receptor species in the absence of ligand is a necessary element to account for basal response (Lefkowitz et al., 1993; Samama et al., 1993). In presence of a ligand, the free, the singly and the doubly bound receptor concentrations are governed by five equilibrium constants (Figure 2a, mechanistic equation). Equation 1 shows the relationship between the amount of bound ligand and the ligand concentration.
 |
where [Abound] and [A] are the concentrations of bound and free ligands, respectively, RT is the total concentration of receptor dimers, and KD1 and KD2 are particular combinations of the chemical equilibrium constants of the model. It should be noted that KD1 and KD2 are not true but apparent constants, quantifying the binding of the first and second ligand molecules, respectively, to the overall (active and inactive) populations of receptor species. Accordingly, Equation 1 can be considered as a hybrid between the mechanistic and the empirical approaches (see Colquhoun and Farrant, 1993; Colquhoun, 1998, 2007; Giraldo et al., 2007) for a discussion on the characterization of affinity constants when active receptor conformations are present).
Figure 2.
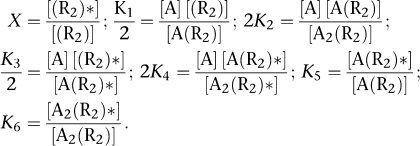 |
 |
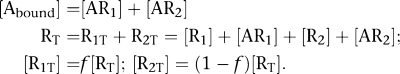 |
Equation 1 gives a maximum asymptote of 2RT, which is the total concentration of binding sites, or Bmax. To allow for a proper comparison between this model and others describing the relationship between bound and free receptor ligand, Equation 1 is divided by Bmax leading to Equation 2, where y ranges between 0 and 1, and the c1 and c2 parameters are defined as KD2 and KD1KD2 products, respectively.
 |
It is worth noting that, as written, Equation 2 is an empirical equation and that the c1 and c2 parameters determine the location and shape of the curve but lack physical meaning.
The two independent sites model
The two independent sites model considers the receptor system as comprising two sites with no interaction between them, as would be observed, for instance, for two non-interconvertible monomeric receptor species (see Figure 2b). Equation 3 displays the variation of bound ligand with free ligand concentration for this model.
In Equation 3, y ranges between 0 and 1, RT being the total receptor concentration, f and (1−f), the fractions of receptor species, and KD1 and KD2 the corresponding ligand-receptor dissociation equilibrium constants. Fixing f to 1/2 and rearranging terms yield Equation 4.
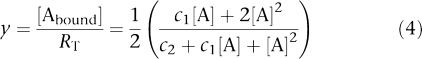 |
where c1=KD1+KD2 and c2=KD1KD2.
The cooperativity property under the two-state dimer model and the two independent sites model
When comparing Equations 2 and 4, we can distinguish between empirical (c parameters) and mechanistic (K constants) approaches. In terms of the empirical parameters, Equations 2 and 4 are identical; fitting data with the two independent sites model, with f fixed to one half, or with the two-states dimer model gives the same accuracy. However, there is a region of the pharmacological space that cannot be accommodated by the two independent sites model when the mechanistic equilibrium constants are used, namely, the positive cooperativity condition (see below). Thus, although both approaches are the same from an empirical point of view, differences appear when a mechanistic analysis is followed. On the other hand, allowing variability in the f parameter will increase the flexibility of the two independent sites model, allowing for a better fitting in situations where f is far from 1/2.
It is known that, mechanistically, a dimeric receptor system can display cooperativity. From a curve-fitting perspective, apparent cooperativity in an experimental data set can be empirically determined from the relationship between the c12 and 4c2 values. Setting c12=4c2 reduces Equations 2 and 4 to a rectangular hyperbola with a Hill coefficient of one, indicating the absence of cooperativity. Accordingly, positive and negative cooperativity are assigned to c12<4c2 and c12>4c2, respectively (Franco et al., 2006). Incorporation of the K constants of Equation 1 into the c parameters allowed the authors (Casadó et al., 2007; Franco et al., 2008) to quantify the extension of cooperativity within their two-states dimer model, proposing a new pharmacologic parameter, namely the ‘dimer cooperativity index' (DC) (Equation 5). Thus, absence, positive, and negative cooperativity were defined as those experimental conditions making DC=0 (4KD1=KD2), DC>0 (4KD1>KD2), and DC<0 (4KD1<KD2), respectively.
 |
By making use of the definition of the Hill coefficient at the midpoint  for a given y(x) function (Giraldo, 2003), the relationship between DC and the Hill coefficient for the absence of cooperativity (Casadó et al., 2007) can be extended to the other two cooperativity conditions.
for a given y(x) function (Giraldo, 2003), the relationship between DC and the Hill coefficient for the absence of cooperativity (Casadó et al., 2007) can be extended to the other two cooperativity conditions.
 |
where x=log A; x50, the midpoint; a, the maximum asymptote; ln, the natural logarithm; and d/dx, the derivative operator. Incorporation of the y function into Equation 6, as expressed in Equation 2, leads to Equation 7.
 |
Replacing the c parameter values in Equation 7 by the apparent K constants of the two-state dimer receptor model (c1=KD2 and c2=KD1KD2) and regrouping yields the mathematical connection between the cooperativity index and the classical Hill coefficient.
 |
or, equivalently,
 |
It can be seen that, whereas  ranges between 0 and 2, DC varies between −∞ and +∞. Then, absence of cooperativity is defined as
ranges between 0 and 2, DC varies between −∞ and +∞. Then, absence of cooperativity is defined as 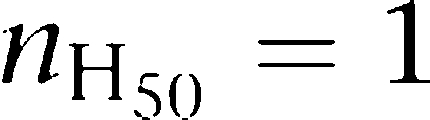 or DC=0, positive cooperativity as
or DC=0, positive cooperativity as  or DC>0, and negative cooperativity as
or DC>0, and negative cooperativity as  or DC<0.
or DC<0.
In the two independent sites model, the relationship between the values of c12 and 4c2 and the sign of cooperativity remains the same. However, mechanistically speaking, a restriction is found. Thus, although there are no contradictions between absence of apparent cooperativity (c12=4c2 implies KD1=KD2) and apparent negative cooperativity (c12>4c2 implies KD1≠KD2), for the condition of apparent positive cooperativity (c12<4c2) there is a mathematically impossible outcome ((KD1−KD2)2<0). Consequently, when using Equation 7, it can be seen that the two independent sites model cannot produce curves with 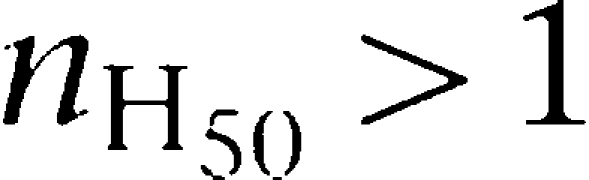 by using any combination of the mechanistic K constants.
by using any combination of the mechanistic K constants.
To allow for apparent positive cooperativity within the two independent sites model, the nH parameter must be introduced manually, thus leading to Equation 10.
 |
In as much as Equation 10 does not derive from a mechanistic model, it should be taken as purely empirical. A word of caution is needed, however, when pretending to interpret mechanistically an empirical model, as meaningless conclusions could be reached.
The issue of data fitting
The question arises on which is, in general, the best fitting approach: mechanistic or empirical? Mechanistic models are the proper formulations for the analysis of pharmacologic systems under physico-chemical principles. However, the many parameters that these models often include preclude classical fitting by gradient nonlinear procedures. Different strategies are possible to overcome this problem; two studies were chosen as examples. (1) The interaction of the nicotinic acetylcholine receptor from Torpedo marmorata with [3H]acetylcholine and the fluorescent agonist NBD-5-acylcholine was studied by equilibrium binding and kinetic experiments (Prinz and Maelicke, 1992). The model included two binding sites per receptor molecule, a pre-existing equilibrium between two states of the nAChR, and a ligand-induced transition between receptor states (note that this scheme is basically the same as that of the two-state dimer model). In addition, an extra doubly occupied receptor state to account for ion transmission was incorporated. The more complete model contained 16 rate constants, bringing the total to 21 parameters when fluorescence quantum yields were considered. After a careful strategy of parameter reduction, the number of parameters was reduced to 8, but even this was a high number to be determined reliably by classical fitting procedures of a single kinetic experiment. Instead, a simultaneous fit to a total of 128 sets, including binding, rapid filter kinetics and fluorescence kinetics, was used. (2) Recently, a mathematical model has been proposed for the constitutively dimeric metabotropic glutamate receptors, integrating a triple state (open-open, closed-open and closed-closed) for the extracellular (venus flytrap) domain, where orthosteric ligands bind, and a double state (inactive, active) for the heptahelical domain responsible for G-protein activation (Rovira et al., 2008). The model included nine parameters for binding and 12 for function. To validate the model, a published study (Kniazeff et al., 2004), including functional concentration-response curves for both wild-type and mutated receptors, was reanalysed. To avoid the problem of the fitting being trapped in a particular local minimum, the authors used a stochastic evolutionary algorithm (Roche et al., 2006). One hundred independent runs were performed for both wild-type and mutated receptor curves allowing statistical comparisons between parameters and a rational interpretation of the parameters that significantly changed after mutation. In addition, the model allowed a mechanistic distinction between two types of cooperativity for the cross talk between the protomers of the venus flytrap domain: one associated with the successive binding to inactive open-open states (binding cooperativity) and the other to the induction of closure of one of the venus flytrap subunits from the partner protomer (induction cooperativity). This conceptual distinction for the cooperativity property allowed a mechanistic explanation for the apparent negative binding cooperativity (Suzuki et al., 2004) and positive functional cooperativity (Kniazeff et al., 2004) found for mGluR agonists.
What about empirical models? Empirical models are the right choice if one is interested only in the geometric characterization of the shape of the curve or else the mechanistic analysis becomes an impossible task. Another feature that can make an empirical model extremely useful is the possibility of questioning the conclusions drawn by a mechanistic model. Thus, interesting issues may appear when one finds that a particular empirical model fits better than a mechanistic equation. Let us suppose, for example, that an experimental biphasic curve is obtained and that we are sure that dimerization, and not two populations of non-interconvertible monomeric receptors, is present. If, Equation 3 would fit data better than Equation 2, this would imply that in the two-state dimer model some aspects of the biological system would be missing. A similar proposal might be suggested if, for an experimental curve bearing apparent positive cooperativity, Equation 10 provides a better fitting than Equation 2. If this is the case, then the fitting by Equation 10 would be preferred when one is more interested in finding the best adjustment rather than in biologically meaningful parameters. Yet, and this is a secondary but not an irrelevant issue, the significant increase in the fitting accuracy of the empirical models, such as Equations 3 and 10 as compared to Equation 2, might indicate the presence of various receptor oligomerization states in the system.
To illustrate the discussion, the above-mentioned models were fitted to a set of data points selected from the literature, which displayed a typical biphasic curve (Motulsky and Christopoulos, 2004). Here, the original response variable was assigned to the ligand-bound concentration and analysed accordingly. Figure 3 shows the theoretical curves obtained from the models, and Table 1 shows the fitting comparison between them by the extra-sum of squares F-test; this is a standard statistical procedure commonly used for model comparison and is available in most data analysis programs. As mentioned above, and assuming that data points correspond to a real situation, the improved accuracy provided by the addition of the f parameter and the nH1 and nH2 Hill coefficients in the two independent sites model, as compared to the two-state dimer receptor model, would suggest that some aspects of the complexity of receptor dimerization are missing in the two-state dimer receptor model. The question as to whether more than one oligomerization state is coexisting in the system would require further work and, most likely, a more complex mechanistic model to account for it.
Figure 3.
Collection of data points following a biphasic curve extracted from Motulsky and Christopoulos (2004). The curve has been chosen solely because of its shape, and the meaning of the original ordinate has been changed here to concentration of bound ligand. Equation parameters and comparison between models are listed in Table 1. Blue line: theoretical curve from models A, B or C. Red line: theoretical curve from model D. Green line: theoretical curve from model E.
Table 1.
Curve fitting of data points displayed on Figure 3
| Model | dfa | SSEb | Parameter estimates |
|---|---|---|---|
| A. Two-state dimer model | 18 | 0.1287 | KD1=10−7.6; KD2=10−4.9 |
 |
|||
| B. Empirically used two-state dimer and two independent sites models | 18 | 0.1287 | c1=10−4.9; c2=10−12.4 |
 |
|||
| C. Two independent sites model with f fixed to ½ | 18 | 0.1287 | KD1=10−7.6; KD2=10−4.9 |
 |
|||
| D. Two independent sites model* | 17 | 0.0544 | KD1=10−8.1; KD2=10−5.1; f=0.33 |
 |
|||
| E. Two independent sites model with the empirical inclusion of Hill coefficients* # | 15 | 0.0186 | KD1=10−8.0; KD2=10−5.1; f=0.38; nH1=1.16; nH2=2.25 |
 |
Parameter estimates of a set of models, and statistical comparisons of resulting fittings.
Comparisons between models by the extra-sum of squares F-test (Motulsky and Christopoulos, 2004): *P<0.05 with either of Models A, B or C; #P<0.05 with Model D.
Degrees of freedom.
Sum of squares of the error.
It is worth noting that the feature that a particular empirical model (constructed by including extra parameters in a mechanistic one) fits data better than the mechanistic model from which it is derived, does not prove that the latter is wrong, but sets a warning message that some pieces of the puzzle could have been omitted in the former mechanistic formulation. In this line, the extra complexity derived by new evidences suggesting direct interactions between receptors belonging to different GPCR classes as, for example, between α2A-adrenergic and μ-opioid receptors (Vilardaga et al., 2008) or between 5-HT2A and metabotropic glutamate receptors (González-Maeso et al., 2008) becomes a challenge for further investigations on mechanistic modelling approaches.
Concluding remarks
Receptor dimerization, the cross-talk between protomers and the ensuing cooperativity property are current topics in pharmacologic research. The shapes of binding and function curves reflect the molecular interactions between the components of the signal transduction machinery, often being the only information available for the experimenter. Mathematical models can be helpful for assessing the receptor-ligand interactions involved in these processes and the quantification of the magnitude and sign of receptor cooperativity. The latter issue was the main subject of the present article; several mathematical models were compared, and the equivalence between the Hill coefficient and the dimer cooperativity index was shown.
Mathematical models can be classified as either mechanistic or empirical. Mechanistic models represent the real system by a set of equilibrium/kinetic constants that precisely characterize the mechanism of binding or function of the receptor. Because of their biophysical nature, mechanistic models are the ideal formulations for the analysis of experimental curve data points. Regretfully, the numerous parameters often included by these models preclude their use by classical curve-fitting procedures such as gradient nonlinear regression. Stochastic approaches can be the right choice when several local minima are present, as these techniques, in contrast to the widely used regression procedures, explore the complete parameter space, avoiding the problem of the fitting being trapped in a particular local minimum. However, if there are too many parameters for the amount of available data, the problem of parameter identifiability can be solved only by either getting more experimental data or using an empirical model that would contain the same amount of information as the available data. Empirical models employ the minimum number of parameters for the determination of the shape of the curve and, accordingly, do not present difficulties for standard curve fitting. In general, empirical models lack physical basis and are limited to obtaining the common geometric descriptors (midpoint location and slope, asymptotes, and so on) of the curves. Yet if the empirical model is a simplification of a mechanistic model, then some physical principles would be reflected in its formulation. Interestingly, empirical and mechanistic models can be used concurrently in ligand-binding data fitting allowing for complementary information to be obtained, with the analysis of accuracy of fitting being an indication for further investigation on the complexity of receptor dynamics.
Acknowledgments
This study was supported in part by Ministerio de Educación y Ciencia (SAF2007-65913) and Fundació La Marató de TV3 (Ref. 070530). JG is grateful to Arthur Christopoulos and Antonio Guzmán for a critical reading of the paper and to Carmen Castro for technical assistance. The author is also grateful to the anonymous referees for their helpful comments.
Abbreviations
- GPCR
G-protein-coupled receptor
Conflict of interest
The author states no conflict of interest.
References
- Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, et al. Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol. 2006;70:1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: evidence from ligand binding. J Biol Chem. 2001;276:22621–22629. doi: 10.1074/jbc.M006936200. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Heveker N, Jockers R, Marullo S, Milligan G. BRET analysis of GPCR oligomerization: newer does not mean better. Nat Methods. 2007;4:3–4. doi: 10.1038/nmeth0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadó V, Cortés A, Ciruela F, Mallol J, Ferré S, Lluis C, et al. Old and new ways to calculate the affinity of agonists and antagonists interacting with G-protein-coupled monomeric and dimeric receptors: the receptor-dimer cooperativity index. Pharmacol Ther. 2007;116:343–354. doi: 10.1016/j.pharmthera.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Chidiac P, Green MA, Pawagi AB, Wells JW. Cardiac muscarinic receptors. Cooperativity as the basis for multiple states of affinity. Biochemistry. 1997;36:7361–7379. doi: 10.1021/bi961939t. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Colquhoun D.The relationship between classical and cooperative models for drug action A Symposium on Drug Receptors 1973University Park Press: Baltimore; 149–182.Rang HP (ed). [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Why the Schild method is better than Schild realised. Trends Pharmacol Sci. 2007;28:608–614. doi: 10.1016/j.tips.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Farrant M. The binding issue. Nature. 1993;366:510–511. doi: 10.1038/366510b0. [DOI] [PubMed] [Google Scholar]
- Durroux T. Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol Sci. 2005;26:376–384. doi: 10.1016/j.tips.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Franco R, Casado V, Cortes A, Mallol J, Ciruela F, Ferre S, et al. G-protein-coupled receptor heteromers: function and ligand pharmacology. Br J Pharmacol. 2008;153:590–598. doi: 10.1038/sj.bjp.0707571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Casadó V, Mallol J, Ferrada C, Ferré S, Fuxe K, et al. The two-state dimer receptor model: a general model for receptor dimers. Mol Pharmacol. 2006;69:1905–1912. doi: 10.1124/mol.105.020685. [DOI] [PubMed] [Google Scholar]
- Franco R, Casadó V, Mallol J, Ferré S, Fuxe K, Cortés A, et al. Dimer-based model for heptaspanning membrane receptors. Trends Biochem Sci. 2005;30:360–366. doi: 10.1016/j.tibs.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Giraldo J. Empirical models and Hill coefficients. Trends Pharmacol Sci. 2003;24:63–65. doi: 10.1016/S0165-6147(02)00048-2. [DOI] [PubMed] [Google Scholar]
- Giraldo J, Serra J, Roche D, Rovira X. Assessing receptor affinity for inverse agonists: Schild and Cheng-Prusoff methods revisited. Curr Drug Targets. 2007;8:197–202. doi: 10.2174/138945007779315687. [DOI] [PubMed] [Google Scholar]
- Giraldo J, Vivas NM, Vila E, Badia A. Assessing the (a)symmetry of concentration-effect curves: empirical versus mechanistic models. Pharmacol Ther. 2002;95:21–45. doi: 10.1016/s0163-7258(02)00223-1. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MA, Chidiac P, Wells JW. Cardiac muscarinic receptors. Relationship between the G protein and multiple states of affinity. Biochemistry. 1997;36:7380–7394. doi: 10.1021/bi961940s. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends Pharmacol Sci. 2008;29:234–240. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods. 2006;3:1001–1006. doi: 10.1038/nmeth978. [DOI] [PubMed] [Google Scholar]
- Karlin A. On the application of ‘a plausible model' of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967;16:306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-Receptor Interaction 1997Lippincott-Raven Publishers: Philadelphia; 3rd edn. [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Lee TW, Sole MJ, Wells JW. Assessment of a ternary model for the binding of agonists to neurohumoral receptors. Biochemistry. 1986;25:7009–7020. doi: 10.1021/bi00370a038. [DOI] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- Mattera R, Pitts BJ, Entman ML, Birnbaumer L. Guanine nucleotide regulation of a mammalian myocardial muscarinic receptor system. Evidence for homo- and heterotropic cooperativity in ligand binding analyzed by computer-assisted curve fitting. J Biol Chem. 1985;260:7410–7421. [PubMed] [Google Scholar]
- Milligan G. A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol. 2008;153:S216–S229. doi: 10.1038/sj.bjp.0707490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. Oxford University Press: New York; 2004. [Google Scholar]
- Overington JP, Al Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Prinz H, Maelicke A. Ligand binding to the membrane-bound acetylcholine receptor from Torpedo marmorata: a complete mathematical analysis. Biochemistry. 1992;31:6728–6738. doi: 10.1021/bi00144a012. [DOI] [PubMed] [Google Scholar]
- Roche D, Serra J, Rovira X, Giraldo J.A genetic algorithm for curve fitting: a possible choice for unsatisfactory nonlinear regressions Proceedings of the British Pharmacological Society 2006. at
- Rovira X, Roche D, Serra J, Kniazeff J, Pin JP, Giraldo J. Modeling the binding and function of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2008;325:443–456. doi: 10.1124/jpet.107.133967. [DOI] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric properties of G protein-coupled receptor oligomers. Pharmacol Ther. 2007;115:410–418. doi: 10.1016/j.pharmthera.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Moriyoshi E, Tsuchiya D, Jingami H. Negative cooperativity of glutamate binding in the dimeric metabotropic glutamate receptor subtype 1. J Biol Chem. 2004;279:35526–35534. doi: 10.1074/jbc.M404831200. [DOI] [PubMed] [Google Scholar]
- Szidonya L, Cserzo M, Hunyady L. Dimerization and oligomerization of G-protein-coupled receptors: debated structures with established and emerging functions. J Endocrinol. 2008;196:435–453. doi: 10.1677/JOE-07-0573. [DOI] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, et al. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha(2A)-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- Wells JW.Analysis and interpretation of binding at equilibrium Receptor-Ligand Interactions. A Practical Approach 1992Oxford University Press: Oxford; 289–395.Hulme EC (ed). [Google Scholar]
- White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, et al. Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc Natl Acad Sci USA. 2007;104:12199–12204. doi: 10.1073/pnas.0705312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreggett KA, Wells JW. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J Biol Chem. 1995;270:22488–22499. doi: 10.1074/jbc.270.38.22488. [DOI] [PubMed] [Google Scholar]