Abstract
Background and purpose:
The internal anal sphincter has been shown to contract in response to α1-adrenoceptor stimulation and therefore α1-adrenoceptor agonists may be useful in treating faecal incontinence. This study characterizes the α1-adrenoceptor subtype responsible for mediating contraction of the internal anal sphincter of the pig.
Experimental approach:
The potency of agonists and the affinities of several receptor subtype selective antagonists were determined on smooth muscle strips for the pig internal anal sphincter. Cumulative concentration–response curves were performed using phenylephrine and noradrenaline.
Key results:
The potency of the α1A-adrenoceptor selective agonist A61603 (pEC50=7.79±0.04) was 158-fold greater than that for noradrenaline (pEC50=5.59±0.02). Phenylephrine (pEC50=5.99±0.05) was 2.5-fold more potent than noradrenaline. The α1D-adrenoceptor selective antagonist BMY7378 caused rightward shifts of the concentration–response curves to phenylephrine and noradrenaline, yielding low affinity estimates of 6.59±0.15 and 6.33±0.13, respectively. Relatively high affinity estimates were obtained for the α1A-adrenoceptor selective antagonists, RS100329 (9.01±0.14 and 9.06±0.22 with phenylephrine and noradrenaline, respectively) and 5-methylurapidil (8.51±0.10 and 8.31±0.10, respectively). Prazosin antagonized responses of the sphincter to phenylephrine and noradrenaline, yielding mean affinity estimates of 8.58±0.10 and 8.15±0.08, respectively. The Schild slope for prazosin with phenylephrine was equal to unity (1.01±0.24), however the Schild slope using noradrenaline was significantly less than unity (0.50±0.11, P<0.05).
Conclusion and implications:
The results suggest that contraction of circular smooth muscle from the pig internal anal sphincter is mediated via a population of adrenoceptors with the pharmacological characteristics of the α1A/L-adrenoceptor, most probably the α1L-adrenoceptor form of this receptor.
Keywords: internal anal sphincter, α-adrenoceptor, receptor classification, pig incontinence
Introduction
Faecal incontinence is a common problem that is defined as the uncontrolled passing of faecal material. It is estimated to affect more than 10% of the general population and generates a huge economic burden (Kalantar et al., 2002). For the year 1996, treatment costs for faecal incontinence in women with obstetric injuries were estimated at US$17 166 per patient before follow-up charges (Mellgren et al., 1999). Another study estimated the yearly cost of incontinence in nursing homes to be US$9771 per patient (Smith, 1995). The treatments for faecal incontinence have varying rates of success but most are associated with unwanted side effects.
The internal anal sphincter has an important role in maintaining continence, as it contributes up to 85% of resting anal tension (Lestar et al., 1989), yet none of the current treatments work by increasing tone of this muscle. The internal anal sphincter exhibits spontaneous tonic contraction and a variety of factors influence this resting tone including noradrenaline, acetylcholine, nitric oxide, vasoactive intestinal polypeptide and carbon monoxide (Rattan, 2005). Noradrenaline has a major effect on the sphincter but has both contractile and inhibitory effects; activation of β-adrenoceptors causing relaxation and α-adrenoceptor stimulation causing contraction. The contractile response normally predominates over the inhibitory response (Shibamoto et al., 1994) and is due to the activation of α1-adrenoceptors located on the smooth muscle (Yamato and Rattan, 1990).
Three subtypes of α1-adrenoceptors have been cloned and pharmacologically characterized (α1A, α1B and α1D) and another receptor with a low affinity for prazosin has been proposed (the α1L-adrenoceptor) (Langer, 1999; Alexander et al., 2008). It has been suggested that the α1L-adrenoceptor may represent a distinct conformational state of the α1A-adrenoceptor (Ford et al., 1997). It is not known which subtype mediates contraction in the internal anal sphincter, but this knowledge would clearly be of benefit to those involved in developing drugs for the treatment of faecal incontinence. Receptor subtype selective drugs have been used to identify the adrenoceptor subtype responsible for mediating contraction of the internal anal sphincter of the pig, a tissue whose gastrointestinal physiology and enteric nervous system are known to be similar to that of human (Aggestrup et al., 1986; Sand et al., 1997; Brown and Timmermans, 2004).
An internal anal sphincter selective α1-adrenoceptor agonist would increase resting anal tone and could be useful in the treatment of faecal incontinence. Some clinical trials using the general α1-adrenoceptor agonist (phenylephrine) have shown improvement in faecal incontinence by a mechanism involving an increase in maximum resting anal pressure (Carapeti et al., 1999, 2000a, 2000b; Cheetham et al., 2001; Badvie and Andreyev, 2005). However, the studies were short-term investigations and the risk of side effects has prevented wider use. The specific α1-adrenoceptor subtype responsible for mediating contraction of the internal anal sphincter is an important drug target for the treatment of faecal incontinence. Therefore, the aim of this study was to identify the α1-adrenoceptor subtype (Alexander et al., 2008) present in this tissue.
Methods
Female pig internal anal sphincter muscle samples were obtained from a local abattoir. The tissues were immediately placed in Krebs-bicarbonate solution (composition in mmol L−1: NaCl 118.4, NaHCO3 24.9, KCl 4.7, CaCl2 1.9, MgSO4 1.15, KH2PO4 1.15, glucose 11.7) at 4 °C. The mucosa and submucosa were removed and the circular muscle of the internal anal sphincter, approximately 2.5 cm from the anal opening, was cut into strips (15 mm × 3 mm).
Internal anal sphincter muscle strips were set up in 25 mL tissue baths containing Krebs-bicarbonate solution at 37 °C and gassed with 5% CO2 in oxygen. The muscle strips were attached to isometric force transducers connected to a MacLab recording system (ADI Instruments Ltd, Chalgrove, Oxfordshire, UK) and the developed tension was measured using ‘CHART' software. Tissues were mounted under 1 g resting tension and allowed to equilibrate for 30 min during which time they were washed with fresh Krebs-bicarbonate solution every 15 min.
Cumulative concentration-response curves were performed with the α1A-adrenoceptor subtype selective agonist A61603, the general α1-adrenoceptor agonist phenylephrine and the endogenous agonist noradrenaline. Further cumulative concentration–response curves to either noradrenaline or phenylephrine were obtained in the absence and presence of α1-adrenoceptor antagonists. Control experiments were performed in the absence of antagonist, whereas separate strips were used for each concentration of one of the following α1-adrenoceptor antagonists, BMY7378 (1–30 μM), prazosin (1–100 nM), 5-methyl-urapidil (3–100 nM), RS100329 (3–100 nM) or RS17053 (1–10 μM). Only one concentration–response curve was obtained on each tissue. Tissues were allowed to equilibrate with the antagonist for 30 min. All experiments using noradrenaline were performed in the presence of corticosterone (10 μM) and cocaine (10 μM) to inhibit amine uptake and propranolol (1 μM) to antagonize β-adrenoceptors.
To investigate the possible role of 5HT receptors, responses to noradrenaline were obtained in the presence of methiothepin (1 μM), ketanserin (1 μM) and ondansentron (1 μM) antagonists of 5HT1, 5HT2 and 5HT3 receptors, respectively. To investigate the involvement of α2-adrenoceptors, responses to noradrenaline were also obtained in the absence and presence of the α2-adrenoceptor antagonist idazoxan (1 μM). The receptor nomenclature used here conforms with the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008).
Data analysis
The contractile responses to both phenylephrine and noradrenaline were expressed in grams and as a percentage of the maximum response for each concentration–response curve (expressed as mean±s.e.mean). Individual EC50 values (concentration producing 50% maximal response) were calculated by nonlinear regression fitting to sigmoidal dose-response curves (variable slope) by GraphPad Prism software (based on a four-parameter logistic equation). Mean −logEC50 (pEC50) values (±s.e.mean) were also calculated. Where several concentrations of antagonist shifted the concentration–response curves, the shifts were used to construct Schild plots (Arunlakshana and Schild, 1959). Individual affinity estimates (apparent pKB values) were determined from the equation:
where CR is the concentration ratio calculated as the ratio of the EC50 values obtained in the absence and presence of a concentration [B] of the antagonist. Statistical significance was determined by Student's t-test or ANOVA (where more than two groups are compared). Probability values of <0.05 were considered statistically significant.
Drugs used
(−)-Phenylephrine hydrochloride, (±)-noradrenaline hydrochloride, corticosterone 21-acetate, (±)-propranolol hydrochloride, 5MU, prazosin hydrochloride, ketanserin tartrate salt, ondansetron hydrochloride dihydrate, methiothepin mesylate salt and idazoxan hydrochloride were obtained from Sigma (St Louis, MO, USA). 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4,5]decane-7,9-dione (BMY7378) dihydrochloride, 5-methyl-3-[3-[4-[2-(2,2,2,-trifluroethoxy)phenyl]-1-piperazinyl] propyl]-2,4-(1H.3H)-pyrimidinedione hydrochloride (RS100329) and N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α,α-dimethyl-1H-indole-3-ethanamide hydrochloride (RS17053) were obtained from Tocris (Ellisville, MO, USA). N-[5-(4,5-dihydro-1H-imidazol-2yl)-2hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl]methane-sulphonamide (A61603) was obtained from Abbott Laboratories (Chicago, IL, USA). Corticosterone was dissolved in 70% ethanol, whereas RS17053 was dissolved in 100% dimethyl sulphoxide (Sigma) and diluted in 5 mM phosphoric acid. Prazosin was dissolved in distilled water with a drop of glacial acetic acid and diluted in distilled water. All other drugs were dissolved in distilled water and diluted in Krebs-bicarbonate solution.
Results
Responses to agonist
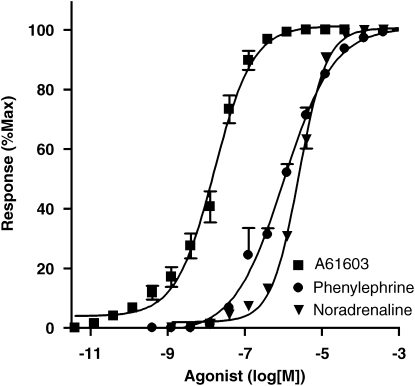
All three α-adrenoceptor agonists (A61603, noradrenaline and phenylephrine) produced concentration-dependent contractions of the pig internal anal sphincter (Figures 1 and 2). The α1A-adrenoceptor selective agonist, A61603, was the most potent agonist being 158-fold (P=<0.0001) more potent than noradrenaline, whereas phenylephrine was 2.5-fold (P=0.0016) more potent than noradrenaline (Table 1). All three agonists produced similar maximum responses (noradrenaline=15.22±2.52 g; phenylephrine 15.45±4.28 g; A61603 12.81±0.46 g).
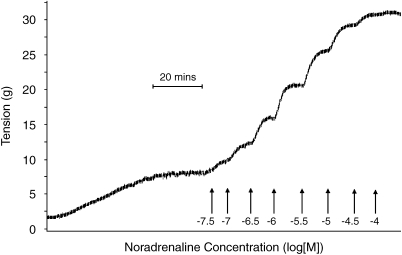
Figure 1.
Typical trace of a cumulative concentration–response curve to noradrenaline. Each addition of noradrenaline occurs when the response has reached a plateau. Note the initial generation of spontaneous tone.
Figure 2.
Mean (±s.e.mean) cumulative concentration–response curves for the α1-adrenoceptor agonists A61603 (α1A-selective), phenylephrine and noradrenaline in the pig internal anal sphincter. Responses are expressed as a percentage of the maximum response for individual curves.
Table 1.
Mean potency (pEC50) and potency ratio (ratio of EC50 values) compared to noradrenaline
| Agonist | Mean pEC50 | Potency ratio | n |
|---|---|---|---|
| Noradrenaline | 5.59±0.02 | 1.00 | 8 |
| Phenylephrine | 5.99±0.05 | 2.49 | 5 |
| A61603 | 7.79±0.04 | 158.46 | 7 |
Data represented as mean±s.e.mean.
Antagonism of responses to phenylephrine
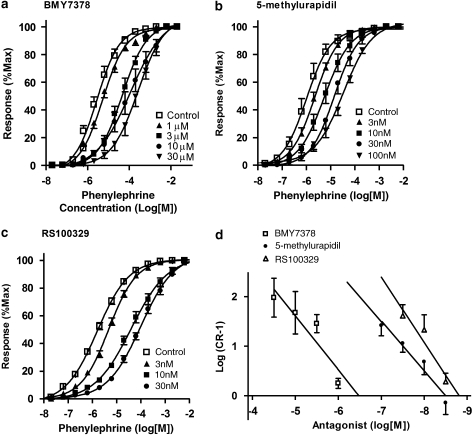
BMY7378 (1–30 μM), RS100329 (3–100 nM) and 5MU (3–100 nM) all produced rightward shifts of the concentration–response curves of the pig internal anal sphincter to phenylephrine (Figure 3). However, the mean affinity (pKB) estimate for BMY7378 (α1D-selective) was low (6.59±0.15), whereas those for RS100329 and 5MU (both α1A-selective) were relatively higher (Table 2). The resulting Schild plots (Figure 3) for all three antagonists had slopes similar to unity (1.08±0.32, 1.33±0.44 and 1.01±0.16 for BMY7378, RS100329 and 5MU, respectively). Maximum responses were not significantly reduced by the antagonists and even the highest concentrations of BMY7378 (control=9.36±1.92 g, 30 μM=12.22±2.63 g), RS100329 (control=14.61±3.45 g, 30 nM=12.15±2.09 g) and 5MU (control=22.89±4.95 g, 100 nM=20.88±5.83 g) failed to significantly alter maximum contractile responses to phenylephrine.
Figure 3.
Mean concentration–response curves to phenylephrine in the absence and presence of (a) BMY7378 (1–30 μM), (b) 5-methylurapidil (3–100 nM) and (c) RS100329 (3–30 nM). Responses are expressed as a percentage of the maximum response for individual curves and the antagonist-induced shifts used to construct the Schild plot illustrated in (d).
Table 2.
Mean±s.e.mean pKB values (affinity estimates), pA2 (intercept on the Schild plot) and Schild slope values±s.e.mean for both phenylephrine and noradrenaline
|
Phenylephrine |
Noradrenaline |
|||||||
|---|---|---|---|---|---|---|---|---|
| pKB | n | pA2 | Slope | pKB | n | pA2 | Slope | |
| BMY7378 | 6.59±0.15 | 21 | 6.49 | 1.08±0.32 | 6.33±0.13 | 17 | 6.32 | 1.11±0.33 |
| 5-MU | 8.51±0.10 | 24 | 8.50 | 1.01±0.16 | 8.31±0.10 | 24 | 8.27 | 1.21±0.28 |
| Prazosin | 8.58±0.10 | 35 | 8.53 | 1.01±0.24 | 8.15±0.08 | 19 | 8.32 | 0.50±0.11 |
| RS100329 | 9.01±0.14 | 23 | 8.81 | 1.33±0.44 | 9.06±0.22 | 16 | 9.18 | 0.80±0.10 |
| RS17053 | <7.0 | 12 | NA | NA | <7.0 | 9 | NA | NA |
Abbreviation: NA, not applicable.
When using phenylephrine as the agonist, prazosin (3–100 nM) produced rightward shifts of the agonist concentration–response curves, yielding a Schild plot with a slope similar to unity (1.01±0.24) and giving an affinity estimate of 8.58±0.10. Prazosin did not affect maximum responses to phenylephrine (control=10.21±2.67 g, n=9; 100 nM prazosin=11.51±1.96 g, n=7).
In contrast, the antagonist RS17053 at concentrations below 10 μM (1–3 μM) failed to shift the concentration–response curve to phenylephrine. However, in the presence of a higher concentration of this antagonist (10 μM) a small shift was observed, yielding an affinity estimate of 5.50±0.25 (n=5).
Antagonism of responses to noradrenaline
BMY7378, RS100329 and 5MU all produced rightward shifts of the concentration–response curves of the pig internal anal sphincter to noradrenaline. The mean affinity estimate for BMY7378 (α1D-selective) was low, whereas those for RS100329 and 5MU (both α1A-selective) were again relatively high (Table 2). The resulting Schild plots for all three antagonists had slopes similar to unity. Maximum responses were not significantly reduced by the antagonists, even at the highest concentrations used: BMY7378 (control=5.82±1.84 g, 30 μM=12.22±2.63 g), RS100329 (control=3.93±1.67 g, 30 nM=3.39±0.61 g) and 5MU (control=3.03±0.84 g, 30 nM=5.26±1.78 g).
As with phenylephrine, the antagonist RS17053 at 10 μM caused a small shift of the concentration–response curve to noradrenaline, yielding an affinity estimate of 5.42±0.77.
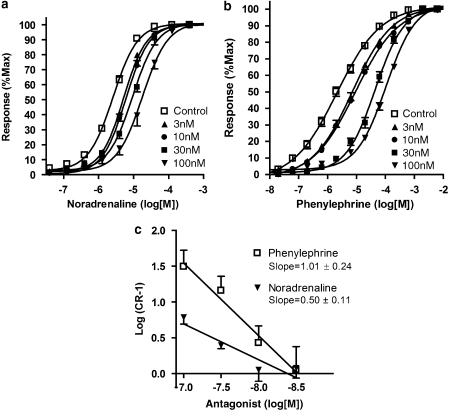
When using noradrenaline as the agonist, prazosin (3–100 nM) elicited rightward shifts of the concentration–response curves, producing a Schild plot with a slope significantly less than unity (0.50±0.11 P<0.05) (Figure 4) and giving an affinity estimate of 8.15±0.08. Prazosin did not significantly reduce maximum responses even at the highest concentration of 100 nM (control=15.22±2.52 g, 100 nM prazosin=22.87±1.52 g).
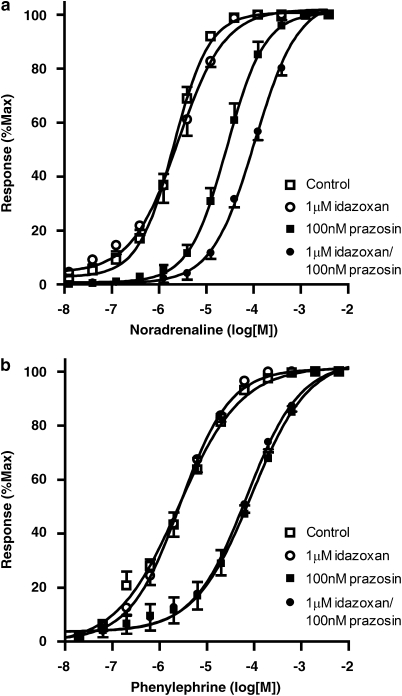
Figure 4.
Mean concentration–response curves to (a) noradrenaline and (b) phenylephrine in the absence and presence of prazosin (3–100 nM). (c) Schild plot for the antagonism of responses to noradrenaline and phenylephrine by prazosin (3–100 nM). The Schild slope using noradrenaline is significantly less than that derived from the data with phenylephrine, which has a slope of unity.
To investigate the possibility that the low Schild slope for prazosin when using noradrenaline as the agonist might be due to an action at α2-adrenoceptors or 5HT receptors, the effect of antagonists at these receptors was examined. A cocktail of 5HT receptor antagonists (1 μM ketanserin, ondansetron and methiothepin) had no effect on responses to noradrenaline in the presence of prazosin, the pEC50 value being 5.91±0.01 in the presence of the 5HT antagonists compared to a control value of 6.16±0.01. However, the α2-adrenoceptor antagonist idazoxan (1 μM) did cause a significant shift (P=0.0005) of the concentration–response curve to noradrenaline when the experiment was performed in the presence of prazosin, with the pEC50 value being 3.97±0.03 compared to the control value of 4.57±0.04. This shift was not seen in the absence of prazosin with the pEC50 values in the absence (5.69±0.03) and presence (5.59±0.03) of idazoxan being similar (Figure 5).
Figure 5.
Antagonism of (a) noradrenaline and (b) phenylephrine concentration–response curves by idazoxan in the presence and absence of 100 nM prazosin. Idazoxan causes a significant shift of the noradrenaline concentration–response curve only in the presence of prazosin.
Discussion and conclusions
The receptor subtype mediating contraction of the internal anal sphincter is important because a sphincter-selective agonist could prove useful in treating faecal incontinence. Three α1-adrenoceptor subtypes have been cloned and named as the α1A-, α1B- and α1D-adrenoceptor subtypes (Alexander et al., 2008). These subtypes are thought to correlate with the pharmacologically defined, functional α1-adrenoceptor subtypes (Zhong and Minneman, 1999).
Preliminary studies found that the mucosal layer interfered with the responses of the smooth muscle strips and for this reason this layer was removed before the tissues were set up. Few α1-adrenoceptor agonists are selective enough to be useful in the characterization of these receptor subtypes. However, A61603 is unusual in having a marked selectivity for the α1A-adrenoceptors over the α1B- and α1D-adrenoceptors. A61603 has been found to be 130–300-fold more potent than either noradrenaline or phenylephrine in tissues where responses are mediated by the α1A-adrenoceptor (Knepper et al., 1995). A61603 and phenylephrine were 158-fold and 2.5-fold more potent than noradrenaline, respectively and all three agonists gave similar maximum responses. This suggests that the α1A-adrenoceptor subtype mediates contraction of the internal anal sphincter. The α1A-adrenoceptor is thought to exist in two different pharmacological states (α1A and α1L) but A61603 does not discriminate between the two forms, having a high potency on tissues such as the rat small mesenteric artery, which possesses the low-affinity state of the α1A-adrenoceptor (that is the α1L-adrenoceptor) (Stam et al., 1999).
The agonist data therefore suggest that it is the α1A- or α1L-adrenoceptor that mediates contraction of the circular smooth muscle of the pig internal anal sphincter. This conclusion was further strengthened by the results of the antagonist studies. The first antagonist to be investigated was BMY7378, which is selective for the α1D-adrenoceptor subtype and has a 100- and 250-fold selectivity over the α1A- and α1B-adrenoceptors, respectively. The affinity of BMY7378 at cloned human α1D-adrenoceptors and at functional α1D-adrenoceptors of the rat aorta is 8.9 (Goetz et al., 1995; Buckner et al., 1996). In the pig internal anal sphincter, the affinity estimates for BMY7378 were very low for both noradrenaline and phenylephrine. The apparent pKB values for BMY7378 for both agonists were 6.59 and 6.33, which is >100-fold less than the reported affinity for this antagonist at α1D-adrenoceptors. This rules out the involvement of α1D-adrenoceptors in mediating contraction of the pig internal anal sphincter.
Two other α1-adrenoceptor antagonists had high affinities for the α1-adrenoceptors of the internal anal sphincter. 5MU is often used to identify α1A-adrenoceptors, as it has 40- and 8-fold selectivity over the α1B- and α1D-adrenoceptors, respectively (Hanft and Gross, 1989). In the pig internal anal sphincter, the apparent affinities for 5MU using phenylephrine and noradrenaline were 8.51 and 8.31, respectively, which are within the range for responses mediated by the α1A-adrenoceptor subtype (Table 3). Similarly, RS100329, an α1A-adrenoceptor selective antagonist is at least 50-fold more selective for the α1A-adrenoceptor over the α1B- and α1D-adrenoceptor subtypes (Williams et al., 1999). In the pig internal anal sphincter the apparent affinities for RS100329 using phenylephrine and noradrenaline were high (9.01 and 9.06, respectively) consistent with the α1A-adrenoceptor subtype mediating contraction.
Table 3.
Affinity estimates of antagonists on the circular smooth muscle of the pig internal anal sphincter.
| Antagonist |
Affinity at published α1-adrenoceptor subtypes |
Affinity at pig internal anal sphincter with |
|||
|---|---|---|---|---|---|
| Phenylephrine | Noradrenaline | ||||
| α1A | α1B | α1D | Mean pKB | Mean pKB | |
| BMY7378 | 6.5±0.2 | 6.9±0.2 | 8.9±0.5 | 6.59±0.15 | 6.33±0.13 |
| 5-MU | 8.8±0.1 | 7.2±0.1 | 7.9±0.1 | 8.51±0.10 | 8.31±0.10 |
| Prazosin | 9.3±0.2 | 9.6±0.1 | 9.6±0.1 | 8.58±0.10 | 8.15±0.08 |
| RS100329 | 9.6±0.1 | 7.8±0.2 | 7.9±0.1 | 9.01±0.14 | 9.06±0.22 |
| RS17053 | 9.4±0.1 | 7.6±0.3 | 7.4±0.3 | <7.0 | <7.0 |
Published affinity ranges from functional and binding studies (Michel and Insel, 1994; Burt et al., 1995; Goetz et al., 1995; Kenny et al., 1995; Buckner et al., 1996; Marshall et al., 1996; Ford et al., 1997; Noble et al., 1997; Langer, 1999; Williams et al., 1999; Mackenzie et al., 2000).
Both the agonist and antagonist data suggest that the responses of the pig internal anal sphincter are mediated by the α1A-adrenoceptor or one of the forms (α1A or α1L) of this receptor. To investigate the involvement of the α1L-arenoceptor in the mediation of smooth muscle contraction in the internal anal sphincter, experiments were performed with the two antagonists, prazosin and RS17053. RS17053 discriminates between α1A- and α1L-adrenoceptors. This antagonist has high affinity at cloned and native α1A-adrenoceptors (pKB=9.1–9.9) (Ford et al., 1996). However, RS17053 was found to be >300-fold less potent (pKB=7.3) at α1L-adrenoceptors of the human prostate. On pig internal anal sphincter, RS17053 at concentrations below 10 μM failed to significantly shift the concentration–response curve to either phenylephrine or noradrenaline with 10 μM giving an affinity of <7.0 in both cases, which suggests the involvement of the α1L-adrenoceptor. This conclusion is also supported by the data obtained with another drug, prazosin, which also discriminates between α1A- and α1L-adrenoceptors.
Prazosin has a high affinity (approximately 9.6) for all three of the cloned human α1-adrenoceptor subtypes (Michel et al., 1995). However, a number of receptors classified as α1A-adrenoceptors have a low affinity for prazosin (Muramatsu et al., 1990; Stam et al., 1999). Four concentrations of prazosin were used to determine an affinity estimate of 8.54 with phenylephrine. This affinity value is too low for responses to be mediated by the α1A-adrenoceptor and suggests the involvement of the α1L-adrenoceptor. A similar result was found using noradrenaline as the agonist; however, the resulting Schild slope was significantly less than unity (0.50) suggesting the involvement of more than one receptor in mediating the response. This possibility was investigated using a 5HT-receptor cocktail containing (ketanserin 1 μM, ondansetron 1 μM and methiothepin 1 μM) and an α2-adrenoceptor antagonist, idazoxan (1 μM). The 5HT antagonist cocktail did not significantly shift the concentration–response curve to noradrenaline, indicating that noradrenaline does not stimulate 5HT receptors in the internal anal sphincter. In contrast, the α2-adrenoceptor antagonist, idazoxan caused a significant rightward shift of the concentration–response curve in the presence of α1-adrenoceptor blockade, which suggests the involvement of α2-adrenoceptors at higher concentrations of noradrenaline. This effect was not observed at lower concentrations of noradrenaline, as no shift was seen in the absence of the α1-adrenoceptor antagonist. When using phenylephrine, this effect was absent in the absence and presence of the α1-adrenoceptor antagonist, suggesting that phenylephrine does not stimulate α2-adrenoceptors at high or low concentrations.
The present study, using circular smooth muscle from the pig internal anal sphincter, is the first to use agonist potencies and selective antagonist affinities to characterize the receptor subtype responsible for mediating contraction. From the data it can be concluded that a receptor with α1A/L-adrenoceptor pharmacology mediates contraction of the circular smooth muscle of the pig internal anal sphincter and may play an important role in maintaining faecal continence.
Abbreviations
- 5MU
5-methylurapidil
Conflicts of interest
The authors state no conflict of interest.
References
- Aggestrup S, Uddman R, Jensen SL, Hakanson R, Sundler F, Schaffalitzky de Muckadell O, et al. Regulatory peptides in lower esophageal sphincter of pig and man. Dig Dis Sci. 1986;31:1370–1375. doi: 10.1007/BF01299816. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 (Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. B J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badvie S, Andreyev H. Topical phenylephrine in the treatment of radiation-induced faecal incontinence. Clin Oncol (R Coll Radiol) 2005;17:122–126. doi: 10.1016/j.clon.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Brown D, Timmermans J. Lesson from the porcine enteric nervous system. Neurogastroenterol Motil. 2004;16:50–54. doi: 10.1111/j.1743-3150.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- Buckner SA, Oheim KM, Morse PA, Knepper SM, Hancock AA. Alpha 1-adrenoceptor-induced contractility in rat aorta is mediated by the alpha 1D subtype. Eur J Pharmacol. 1996;267:241–248. doi: 10.1016/0014-2999(95)00755-5. [DOI] [PubMed] [Google Scholar]
- Burt RP, Chapple CR, Marshall I. Evidence for a functional alpha 1A- (alpha 1C-) adrenoceptor mediating contraction of the rat epididymal vas deferens and an alpha 1B-adrenocepor mediating contraction of the rat spleen. Br J Pharmacol. 1995;115:467–475. doi: 10.1111/j.1476-5381.1995.tb16356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapeti E, Kamm M, Evans B, Phillips R. Topical phenylephrine increases anal sphincter resting pressure. Br J Surg. 1999;86:267–270. doi: 10.1046/j.1365-2168.1999.01021.x. [DOI] [PubMed] [Google Scholar]
- Carapeti EA, Kamm MA, Nicholls RJ, Phillips RK. Randomized, controlled trial of topical phenylephrine for fecal incontinence in patients after ileoanal pouch construction. Dis Colon Rectum. 2000a;43:1059–1063. doi: 10.1007/BF02236550. [DOI] [PubMed] [Google Scholar]
- Carapeti EA, Kamm MA, Phillips RK. Randomized controlled trial of topical phenylephrine in the treatment of faecal incontinence. Br J Surg. 2000b;87:38–42. doi: 10.1046/j.1365-2168.2000.01306.x. [DOI] [PubMed] [Google Scholar]
- Cheetham MJ, Kamm MA, Phillips RK. Topical phenylephrine increases anal canal resting pressure in patients with faecal incontinence. Gut. 2001;48:356–359. doi: 10.1136/gut.48.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AP, Arredondo NF, Blue DR, Jr, Bonhaus DW, Jasper J, Kava MS, et al. RS 17053 (N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α,α-dimethyl-1H-indole-3-ethanamine hydrochloride, a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol Pharmacol. 1996;49:209. [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleitropism of the human recombinant alpha1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL. BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur J Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- Hanft G, Gross G. Subclassification of alpha 1-adrenoceptor recognition sites by urapidil derivatives and other selective antagonists. Br J Pharmacol. 1989;97:691–700. doi: 10.1111/j.1476-5381.1989.tb12005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar J, Howell S, Talley J. Prevalence of faecal incontinence and associated risk factors. An underdiagnosed problem in the Australian community? Medical Journal of Australia. 2002;176:54–57. [PubMed] [Google Scholar]
- Kenny BA, Chalmers DH, Philpott PC, Naylor AM. Characterization of an alpha 1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper SM, Buckner SA, Brune ME, Debernardis JF, Meyer MD, Hancock AA. A-61630, a potent alpha 1-adrenergic receptor agonist, selective for the alpha 1A receptor subtype. J Pharmacol Exp Ther. 1995;274:97–103. [PubMed] [Google Scholar]
- Langer SZ. History and nomenclature of alpha1-adrenoceptors. Eur Urol. 1999;36 (Suppl. 1):2–6. [PubMed] [Google Scholar]
- Lestar B, Pennickx F, Kerremans R. The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Colorectal Dis. 1989;4:118–122. doi: 10.1007/BF01646870. [DOI] [PubMed] [Google Scholar]
- Mackenzie JF, Daly CJ, Pediani JD, McGrath JC. Quantitative imaging in live human cells reveals intracellular alpha(1)-adrenoceptor ligand-binding sites. J Pharmacol Exp Ther. 2000;294:434–443. [PubMed] [Google Scholar]
- Marshall I, Burt RP, Green GM, Hussain MB, Chapple CR. Different subtypes of α1A-adrenoceptor mediating contraction of rat epididymal vas deferens, rat hepatic portal vein and human prostate distinguished by the antagonist RS 17053. Br J Pharmacol. 1996;119:407–415. doi: 10.1111/j.1476-5381.1996.tb16001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren A, Jensen LL, Zetterstrom JP, Wong WD, Hofmeister JH, Lowry AC. Long-term cost of faecal incontinence secondary to obstetric injuries. Dis Colon Rectum. 1999;42:857–865. doi: 10.1007/BF02237089. [DOI] [PubMed] [Google Scholar]
- Michel MC, Insel PA. Comparison of cloned and pharmacologically defined rat tissue alpha 1-adrenoceptor subtypes. Naunyn-Schmiedeberg's Arch Pharmacol. 1994;352:1–10. doi: 10.1007/BF00241087. [DOI] [PubMed] [Google Scholar]
- Michel MC, Kenny B, Schwinn DA. Classification of alpha 1-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:1–10. doi: 10.1007/BF00169183. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Kigoshi S, Oshita M. Two distinct alpha 1-adrenoceptor subtypes involved in noradrenaline contraction of the rabbit thoracic aorta. Br J Pharmacol. 1990;101:662–666. doi: 10.1111/j.1476-5381.1990.tb14137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble AJ, Chess-Williams R, Couldwell C, Furukawa K, Uchyiuma T, Korstanje C, et al. The effects of tamsulosin, a high affinity antagonist at functional alpha 1A- and alpha 1D-adrenoceptor subtypes. Br J Pharmacol. 1997;120:231–238. doi: 10.1038/sj.bjp.0700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil. 2005;17 (Suppl. 1):50–59. doi: 10.1111/j.1365-2982.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- Sand J, Arvola P, Jantti V, Oja S, Singaram C, Baer G, et al. The inhibitory role of nitric oxide in the control of porcine and human sphincter of Oddi activity. Gut. 1997;41:375–380. doi: 10.1136/gut.41.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto T, Chakder S, Rattan S. Role of hypogastric nerve activity in opossum internal anal sphincter function: influence of surgical and chemical denervation. J Pharmacol Exp Ther. 1994;271:277–284. [PubMed] [Google Scholar]
- Smith DA.The current state of continence care and treatment 1995. IDEA 95 Conference papers; INDA 25–27, Philadelphia, PA
- Stam WB, Van der Graaf PH, Saxena PR. Analysis of alpha 1L-adrenoceptor pharmacology in rat small mesenteric artery. Br J Pharmacol. 1999;127:661–670. doi: 10.1038/sj.bjp.0702598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, Gever JR, et al. In vitro alpha 1-adrenoceptor pharmacology of RO70-0004 and RS-100329, novel alpha 1A-adrenoceptor selective antagonists. Br J Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato S, Rattan S. Role of alpha adrenoceptors in opossum internal anal sphincter. J Clin Invest. 1990;86:424–429. doi: 10.1172/JCI114728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol. 1999;375:261–276. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]