Abstract
Background and purpose:
CNS 7056 is a new, rapidly metabolized benzodiazepine. The effects of escalating doses of CNS 7056 on sedation, and respiratory and cardiovascular function, were examined in conscious, chronically instrumented sheep for the first time.
Experimental approach:
Three sheep were given doses of CNS 7056 (0, 0.037, 0.074, 0.18, 0.37, 0.74, 2.21, 4.41 and 8.82 mg kg−1) as 2 min intravenous infusions in order on consecutive days. A range of physiological variables, including the EEG, were measured.
Key results:
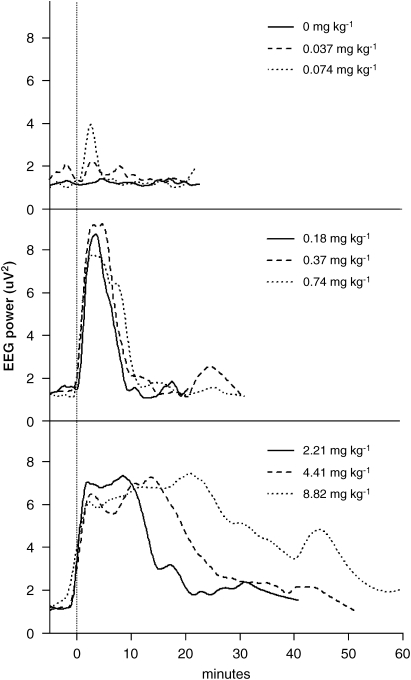
Sheep became transiently drowsy for the lowest (0.037 and 0.074 mg kg−1) doses of CNS 7056, whereas the highest (8.82 mg kg−1) dose produced profound loss of consciousness (LOC) for over 30 min. The EEG alpha power correlated well (r=0.91) with duration of LOC and had a high signal-to-noise ratio. CNS 7056 reduced respiratory rate (maximum 33%) and dose-dependently increased arterial carbon dioxide tension (maximum 12%). There was a transient, dose-related reduction in arterial oxygen tension (maximum 34%), but haemoglobin desaturation was minimal (maximum 4%). CNS 7056 produced a dose-related transient drop in mean arterial blood pressure (maximum 12%) but cardiac output was unchanged.
Conclusions and implications:
Doses of 0.37–2.21 mg kg−1 of CNS 7056 produced sedation for 9–25 min without excessive respiratory or cardiovascular depression, and would be suitable for pharmacokinetic studies. The power in the alpha band of the EEG can be used as a continuous measure of the sedative effects of CNS 7056.
Keywords: CNS 7056, sheep, sedation, cardiovascular effects, respiratory effects
Introduction
CNS 7056 is an esterase-metabolized sedative currently undergoing development. Esterase-metabolized drugs (such as the opioid remifentanil and the β-adrenoceptor antagonist esmolol) are increasingly popular in anaesthesia because they have a high clearance that allows rapid titration of drug effects when used through intravenous infusion. There is currently no clinically available esterase-metabolized sedative.
CNS 7056 was shown to have a high and specific affinity for all subtypes of the benzodiazepine GABAA receptor (Kilpatrick et al., 2007), as shown by studies using patch clamping and cells transfected with subtypes of the GABAA receptor. In rats, CNS 7056 caused inhibition of neuronal firing and, at a dose of 25 mg kg−1, caused loss of righting reflex for approximately 10 min (Kilpatrick et al., 2007). Therefore, CNS 7056 may have a potential role as a rapidly cleared benzodiazepine with a shorter duration of action than that of similar sedative agents. A short duration of action may be useful for some clinical procedures.
To further understand the properties of CNS 7056 in vivo, the sedative, respiratory and cardiovascular effects of a range of doses of CNS 7056 were studied using chronically instrumented sheep. This experimental preparation allows serial studies of the effects of drugs on the EEG, the cardiovascular system and the respiratory system and has been used previously to study the cerebral uptake and effects of a number of anaesthetics, sedatives and analgesics (Ludbrook and Upton, 1997; Upton and Ludbrook, 1997, 1999).
The aim was to study escalating doses of CNS 7056 in sheep to
determine the dose range of CNS 7056 that produced sedation in this species;
define the quality and duration of the sedation, and to find a derived parameter from the EEG that reflected the time course of sedation;
define the dose-dependent effects of CNS 7056 on the cardiovascular and respiratory systems to gain insight into which pharmacological effects may limit its clinical usefulness.
A longitudinal, within sheep, study design was chosen to provide maximum information, while minimizing the use of experimental compound. It was found that CNS 7056 produced sedation in sheep and did not produce dangerous respiratory or cardiovascular depression.
Methods
Ethical approval
Studies were approved by the Animal Ethics Committee of the Institute of Medical and Veterinary Science and complied with the National Health and Medical Research Council of Australia Code of Conduct for the Care and Use of Animals.
General methods for sheep preparation
Three female Merino sheep of 1–3 years age with an average weight of 46 kg (33, 54 and 51 kg) were bought from Windarra Farm of the Institute of Medical and Veterinary Science, South Australia. They were housed in floor pens when not undergoing acute experimentation. The sheep were acclimatized to animal house conditions and human contact for at least 2 days before surgery and had free access to food and water.
Sheep were prepared surgically with instrumentation for blood sampling and physiological measurements. Under isoflurane anaesthesia and sterile surgical conditions, each sheep was prepared with two aortic catheters (for blood sampling and arterial blood pressure), one Swan–Ganz pulmonary artery thermodilution catheter (for blood sampling and cardiac output measurement), one sagittal sinus catheter (to sample effluent blood from the brain) and three catheters outside the right atrium (for central venous pressure, cardiac output injectate and drug administration). A Doppler flow probe was secured through a trephine hole above the dorsal sagittal sinus (for measuring an index of cerebral blood flow (Upton et al., 1994; Doolette et al., 1999). For EEG measurements, two silver electrodes were placed under the skull but above the dura on either side of the trephine hole (over the left and right cerebral cortices), and a reference electrode was placed under the scalp.
The sheep recovered from anaesthesia, and postoperative pain was managed using a single dose of intramuscular xylazine (0.05 mg kg−1) as indicated. The sheep were standing and eating, 2–3 h following surgery.
Studies commenced 2 days after surgery. Sheep were taught to walk from their pen into a mobile experimental crate and, for each study day, the sheep in its crate was moved into a quiet experimental room isolated from other sheep. The sheep were placed in a weight-bearing sling inside the crate that supported the sheep with the onset of sedation. Sheep breathed room air with unassisted ventilation during the studies.
Experimental studies
Each sheep was given, in order, doses of 0, 0.05, 0.1, 0.25, 0.5, 1, 3, 6 and 12 mg kg−1 of CNS 7056 besylate infused intravenously over 2 min on consecutive days, but with no studies done on a weekend. These doses equate to 0, 0.037, 0.074, 0.18, 0.37, 0.74, 2.21, 4.41 and 8.82 mg kg−1 of CNS 7056 base. All doses subsequently refer to the base. The designated target of CNS 7056 is the GABAA transmitter-gated ion channel receptor, using the nomenclature of the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008). The metabolite CNS 7054 (Figure 1) has insignificant affinity for the GABAA receptor (Kilpatrick et al., 2007).
Figure 1.
Structure of CNS 7056 and its metabolite CNS 7054.
The drug in powder form was dissolved in 30 mL of 0.9% saline immediately prior to administration. The resulting dose solution was sterilized by passage through a 0.2 μm filter. All experimental times are referenced to the start of the drug infusion. The following rules were applied to the conduct of the study:
The dose escalation series in any given sheep would not continue if the last dose produced significant respiratory depression (rate of less than six breaths per min or oxygen saturation of less than 95%).
The dose escalation series in any given sheep would not continue if the last dose produced significant cardiovascular depression (lowest mean arterial pressure less than 50 mm Hg or heart rate less than 40 b.p.m.).
A maximum dose of 8.82 mg kg−1 was used as dictated by compound availability.
A dose that produced minimal effects in the first sheep would not be repeated in subsequent sheep.
In all studies, the physiological measurements described below were made for 5 min before the start of the drug infusion, and after the administration of the drug until EEG readings had returned to baseline for at least 10 min.
General measurement methods
Sedative effects
The EEG was recorded using a Datex-Ohmeda S/5 monitor. The entropy module of this monitor was used to record the state entropy (SE) and response entropy (RE) at 5-s intervals. These are two parameters derived from the EEG that are validated indices of sedative and anaesthetic depth (Vakkuri et al., 2004). The algorithms for these entropies are published (Viertio-Oja et al., 2004). The SE ranges from a value of 91 (fully awake) to 0 (deep anaesthesia, isoelectric EEG). For the RE, these values are 100 and 0, respectively.
The S/5 monitor was also used to record the raw EEG waveform as sampling rate of 400 Hz. The raw EEG was processed to calculate a number of additional derived EEG parameters also previously used to quantitate the sedative effects of drugs. This analysis sought to rank the various EEG parameters by their ability to reflect the depth of sedation caused by CNS 7056 in this experimental preparation. The raw EEG data for each study were analysed by dividing the EEG waveform into 8 s epochs. Each epoch was examined manually for artefacts due to animal movement or baseline drift sending the signal off-scale. Epochs with artefacts were not subject to further analysis. Epochs without artefacts were filtered with a digital band-pass filter to exclude frequencies less than 3 Hz and greater than 30 Hz. The Shannon entropy of the EEG and the singular value decomposition entropy of the EEG were derived from the raw waveform (Muncaster et al., 2003; Schwilden, 2006). Parameters were also derived from the power spectrum of each epoch using the fast fourier transform (Schwilden, 2006): These included spectral entropy of the EEG, spectral edge for 95% of power of the EEG (SE95), spectral edge for 50% of power of the EEG (SE50), average power in the delta power band of the EEG (delta, 3–5 Hz), the theta band (theta, 5–8 Hz), the alpha band (alpha, 8–13 Hz), the beta band (beta, 13–30 Hz) and total power (total, 3–30 Hz). The ratio of powers in the alpha and beta bands of the EEG (RatioAB), the total and beta bands of the EEG (RatioTB) and the total and alpha bands of the EEG (RatioTA) were also calculated.
The values of the parameters for each 8-s epoch were fitted with a loess-smoothing function to define the time course of each calculated parameter. The ability of each derived EEG parameter to respond to the sedative effects of CNS 7056 was tested by calculating the signal-to-noise ratio of the parameter for the 2.21 mg kg−1 doses of CNS 7056. The noise period was taken as the baseline EEG, and the signal period as 1–3 min after the dose. Signal EEG processing was performed using scripts written for the ‘R' data analysis software, Version 2.4.1 (R Development Core Team, 2005).
Images of the head and front of the sheep were recorded during the studies using a digital video camera and stored on DVD for later analysis. The videos were reviewed by a single observer. Loss of consciousness (LOC) was taken as loss of the ability of the sheep to hold its head up. The start of LOC was taken to be when the head was at its nadir. During LOC, the head was generally motionless except for manual handling and respiratory movement. On recovery, it was common for the sheep to suddenly lift their heads, which was taken as the end of the period of LOC.
Respiratory effects
The inlet tube of a capnograph (Datex Cardiocap) was placed at a nostril of each sheep to sample inspired and expired air. The analogue output of the capnograph (percent CO2) was collected digitally at a sample rate of 50 Hz. The resultant data file was analysed off-line using an R script that counted the time between five consecutive breaths to calculate the respiratory rate at 1-min intervals. Prior to each study, the capnograph was tested by comparison with a reference gas (5.19% CO2, balance air).
Arterial blood and sagittal sinus gas samples were taken at 0 (baseline), 2, 5, 10 and 20 min after the start of the drug infusion. The samples were sealed in air-tight heparinized syringes and stored in ice for later analysis using an Osmetech OPTI blood gas analyser.
Cardiovascular effects
Calibrated blood pressure transducers were connected to an arterial and a central venous catheter of each sheep to measure mean arterial blood pressure (MAP) and central venous blood pressure, respectively. The analogue pressure waveforms were recorded digitally at a sampling frequency of 50 Hz. An index of cerebral blood flow was recorded from the output of the ultrasonic Doppler crystal on the sagittal sinus (Upton et al., 1994; Doolette et al., 1999), also at a sampling frequency of 50 Hz. Cardiac output was measured using the thermodilution method and the Swan–Ganz thermistor-tipped pulmonary artery catheter. Measurements were made in triplicate with injections of 10 mL of ice-cold saline at 0 (baseline), 2, 5 and 10 min, and each 10 min thereafter. The cardiac output at a particular time was taken as the mean of the three readings. Core (pulmonary artery) body temperature was recorded concurrently with cardiac output.
The time courses of MAP, central venous blood pressure and cerebral blood flow at 1-min intervals were derived by fitting a loess function to the data, after manually removing artefacts due to disconnections or movement. A separate analysis recorded the lowest MAP in the 10-min period after the start of the drug infusion.
Statistical analysis
The fundamental approach used was that developed by Venables and Ripley (2002). To examine statistically significant changes at each dose, data with multiple measurements in the baseline period (EEG, blood pressures, respiratory rate, heart rate and cerebral blood flow) were analysed for each dose group using a linear mixed effect model. ‘Sheep' was the random grouping factor and the model compared the observed values of the variable in the baseline period (−5 to 0 min) and the values of the variable in the 2-min period following the drug administration that centred about the peak drug effect (that is, 1–3 min). Data that were measured only once in the baseline period (cardiac output and blood gas measurements) were analysed using a similar approach, but with a comparison of the variable for time=0 min (baseline) and the single post-drug time point representing the peak drug effect (drug).
To examine systematic changes in a variable with increasing dose, the values of the variable reflecting the maximum change from baseline were plotted against log dose. Using log dose was found to produce a linear relationship, which was quantified using linear mixed effect models (random factor=‘Sheep'). The dose=0 values were necessarily excluded from the log transform. A significant positive slope implied that the variable increased with increasing dose, whereas significant negative slope implied that the variable decreased with increasing dose.
The significance level was P=0.05. All data were analysed using the ‘R' data analysis software, Version 2.4.1 (R Development Core Team, 2005). All statistical variables were reported with a standard error to indicate the statistical power of the analysis.
Materials
CNS 7056 besylate (molecular weight 597.5, chemical purity 97.6% and chiral purity 98.3%) was supplied by CeNeS Limited, Cambridge, UK. The structures of CNS 7056 and its carboxylic acid metabolite CNS 7054 are shown in Figure 1.
Results
All sheep reached the maximum dose (8.82 mg kg−1) of the dose escalation series without exceeding the cutoff criterion for respiratory or cardiovascular depression. The 0.037 mg kg−1 dose was not given to sheep 2 and 3 after sheep 1 showed minimal sedative effect for this dose.
Sedative effects of CNS 7056
In all three sheep, no sedation was observed when the vehicle was administered. Sheep became transiently drowsy for the lowest (0.037 and 0.074 mg kg−1) doses of CNS 7056, whereas the highest (8.82 mg kg−1) dose produced profound LOC for over 30 min.
The video analysis showed that head posture was a viable measure of LOC, particularly for the higher doses where there was a clear transition between the conscious and unconscious states. The period of LOC was characterized by minimal extraneous movement of the animals. The times of LOC and recovery of consciousness (ROC) as assessed by the video analysis for the dose escalation study are summarized in Table 1.
Table 1.
Duration of loss of consciousness following CNS 7056
| Dose (mg kg−1) | Sheep |
Through SE and RE traces |
Through EEG alpha |
Through video |
|||
|---|---|---|---|---|---|---|---|
| LOC (min) | ROC (min) | LOC (min) | ROC (min) | LOC (min) | ROC (min) | ||
| 0 | 1 | NC | NC | NC | NC | NC | NC |
| 0 | 2 | NC | NC | NC | NC | NC | NC |
| 0 | 3 | NC | NC | NC | NC | NC | NC |
| Average | NA | NA | NA | NA | NA | NA | |
| 0.037 | 1 | NC | NC | NC | NC | 1.00 | 2.68 |
| Average | NA | NA | NA | NA | 1.00 | 2.68 | |
| 0.074 | 1 | 3.50 | 3.67 | 0.68 | 4.75 | 2.13 | 3.37 |
| 0.074 | 2 | 3.63 | 3.80 | 0.69 | 4.51 | NC | NC |
| 0.074 | 3 | 2.02 | 5.35 | 0.89 | 5.09 | 1.78 | 5.02 |
| Average | 3.05 | 4.27 | 0.76 | 4.78 | 1.96 | 4.20 | |
| 0.18 | 1 | 2.08 | 8.15 | 0.44 | 12.35 | 0.63 | 8.42 |
| 0.18 | 2 | 1.83 | 5.75 | NA | NA | 3.83 | 5.32 |
| 0.18 | 3 | 1.67 | 7.67 | 0.44 | 7.98 | 1.50 | 6.87 |
| Average | 1.86 | 7.19 | 0.44 | 10.17 | 1.99 | 6.87 | |
| 0.37 | 1 | 1.82 | 11.57 | −0.15 | 13.93 | 0.93 | 13.47 |
| 0.37 | 2 | 1.80 | 5.47 | 0.28 | 6.21 | 2.92 | 5.05 |
| 0.37 | 3 | 1.63 | 9.80 | 0.36 | 9.64 | 1.67 | 8.93 |
| Average | 1.75 | 8.94 | 0.16 | 9.93 | 1.84 | 9.15 | |
| 0.74 | 1 | 1.67 | 16.67 | 0.26 | 20.38 | 0.77 | 16.48 |
| 0.74 | 2 | 3.08 | 7.67 | −0.06 | 10.47 | 2.08 | 7.07 |
| 0.74 | 3 | 1.68 | 11.35 | 0.18 | 11.99 | 0.83 | 10.12 |
| Average | 2.14 | 11.89 | 0.13 | 14.28 | 1.23 | 11.22 | |
| 2.21 | 1 | 1.52 | 24.27 | −0.26 | 34.57 | 0.43 | 24.08 |
| 2.21 | 2 | 1.45 | 13.53 | −0.06 | 25.63 | 1.40 | 13.42 |
| 2.21 | 3 | 1.28 | 13.12 | −0.01 | 13.21 | 0.65 | 10.40 |
| Average | 1.42 | 16.97 | −0.11 | 24.47 | 0.83 | 15.97 | |
| 4.41 | 1 | 1.20 | 34.12 | 0.01 | 39.84 | 0.42 | 33.65 |
| 4.41 | 2 | 1.55 | 22.88 | −0.16 | 40.12 | 0.73 | 20.82 |
| 4.41 | 3 | 1.08 | 21.33 | 0.12 | 20.99 | 0.55 | 20.22 |
| Average | 1.28 | 26.11 | −0.01 | 33.65 | 0.57 | 24.90 | |
| 8.82 | 1 | 1.41 | 41.18 | 0.09 | 54.86 | 0.58 | 40.22 |
| 8.82 | 2 | 1.45 | 27.62 | 0.18 | 40.68 | 0.72 | 25.10 |
| 8.82 | 3 | 0.28 | 46.61 | NA | NA | 0.68 | 31.50 |
| Average | 1.05 | 38.47 | 0.14 | 47.77 | 0.66 | 32.27 | |
Abbreviations: LOC, time of loss of consciousness; NA, not available; NC, no change detected; ROC, time of recovery of consciousness.
The small negative onset times for the alpha power are an artefact of the loess smoothing function applied to the data.
The SE and RE entropy readings were generally displayed in real time by the S/5 monitor during the baseline period, but the readings were at times blank during the period of drug effect. The values returned once baseline conditions were re-established following the return of consciousness. When the readings were blank, the monitor returned an internal error code (−32 767), indicating there was no valid data. It was hypothesized that the use of internal rather than external electrodes produced EEG voltages too large for the internal SE and RE algorithms to process. However, it was noted that the presence or absence of the SE and RE readings correlated closely with the onset and offset of consciousness, and the times for this to occur were documented. The LOC and ROC times as assessed by the SE/RE trace of the EEG are also summarized in Table 1.
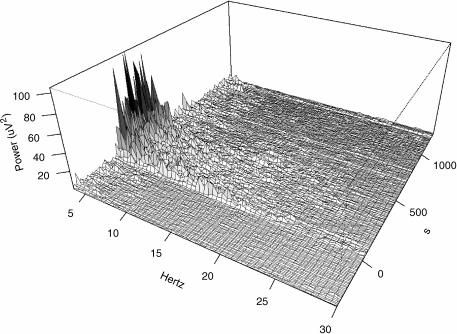
The raw EEG could not be collected for the 0.18 mg kg−1 dose of sheep 2, and the 8.82 mg kg−1 dose of sheep 3 due to computer configuration errors. An example of the effect of CNS 7056 on the EEG power spectrum is shown in Figure 2. The drug effects were clear and unambiguous, with large increases in spectral power in the lower and mid-range frequencies during the period of sedation. The various calculated EEG parameters are shown ranked by their signal-to-noise ratio in Table 2. The average power in the alpha band was least affected by noise, had stable baseline readings and showed a graded response consistent with the observed sedative effects (Figure 3). It was concluded that the alpha power of the EEG was therefore a suitable dynamic measure for the sedative effect of CNS 7056 in future pharmacokinetic–pharmacodynamic studies.
Figure 2.
Effect of CNS 7056 on the EEG power spectrum. Data are shown for one sheep. CNS 7056 (0.74 mg kg−1) was infused over 2 min, starting at zero seconds. There was 5 min (300 s) of pre-drug baseline readings. Note the large increases in spectral power in the lower frequencies during the period of sedation. Consciousness had returned in this animal at approximately 10 min (600 s), where after the EEG had also returned to baseline.
Table 2.
Signal-to-noise ratio (SNR) for various parameters derived from the EEG
| Parameters | Description | SNR |
|---|---|---|
| Alpha | Average power in the alpha band (8–13 Hz) | 6.00 |
| Theta | Average power in the theta band (5–8 Hz) | 3.84 |
| Delta | Average power in the delta band (3–5 Hz) | 3.72 |
| Total | Average power in all bands (3–30 Hz) | 3.19 |
| RatioAB | Ratio of power in alpha band over beta band | 2.81 |
| Beta | Average power in the beta band (13–30 Hz) | 1.95 |
| RatioTB | Ratio of power in total band over beta band | 0.62 |
| EntSpec | Spectral entropy | 0.04 |
| SE95 | Spectral edge for 95% of power | 0.02 |
| EntSh | Shannon entropy | 0.00 |
| EntSVD | Singular value decomposition entropy | −0.09 |
| SE50 | Spectral edge for 50% of power | −0.24 |
| RatioTA | Ratio of power in total band over alpha band | −0.36 |
The mean signal-to-noise ratios for each parameter were calculated from baseline EEG data (noise, from −5 to 0 min) and the EEG data after 2.21 mg kg−1 of CNS 7056 (signal, from 1 to 3 min). A good description of the effects of CNS 7056 on the EEG requires a high SNR.
Figure 3.
The effect of increasing doses of CNS 7056 on EEG alpha power. The data for each dose group were pooled and fitted with a loess smoothing function. The vertical lines are the start of the 2-min drug infusions. The increase in alpha power before the start of the infusion is an artefact of the loess smoothing function applied to the data.
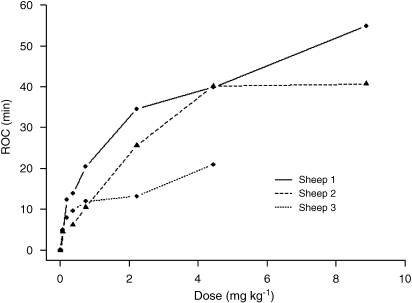
The alpha power increased above the 95% tolerance intervals of the baseline readings following all doses of 0.074 mg kg−1 and above. The time courses of the alpha EEG power for each dose are summarized in Figure 3. There were minimal changes in alpha power for the vehicle control, but increasing the dose of CNS 7056 increased the period for which the alpha power was above baseline. This dose–response relationship is summarized in Figure 4, which shows the times of ROC for the alpha power. There were inter-sheep differences in response, but with each sheep being internally consistent. It is also apparent in Figure 3 that increasing the dose of CNS 7056 did not produce corresponding increases in the maximum EEG alpha power. The LOC and ROC times derived from the alpha power are summarized in Table 1.
Figure 4.
The effect of CNS 7056 dose on the time of recovery of consciousness (ROC) through EEG alpha power. Each point is one dose in one sheep.
In summary, three different methods (SE/RE, alpha power and video analysis) were used to quantify the onset (LOC) and offset (ROC) times of the sedative effects of CNS 7056 (Table 1). When averaged across sheep, these measures were in good agreement, although the alpha power in general took longer to return to baseline than the other measures, perhaps indicating that it could detect more subtle changes in the EEG and sedation than is evident by the other methods. The recovery times by the three methods were highly correlated, with the correlation coefficient of video vs SE/RE being 0.94, and that of video vs alpha power being 0.91.
Respiratory effects
The baseline respiratory rate in the sheep was variable, as expected for a species that uses ventilation for thermoregulation. Respiratory rate was therefore expressed as a percentage of baseline values. CNS 7056 produced reductions in respiratory rate of the order of 20–30% that were not dose related (Table 3). The reductions were significant for all doses except 8.82 mg kg−1 (Table 4). The paradoxical respiratory response to the 8.82 mg kg−1 doses may reflect compensatory mechanisms not evoked with the lower doses.
Table 3.
Dose–response relationships
| Variables | Symbol | Slope | Standard error of slope | P-value for slope term |
|---|---|---|---|---|
| Respiratory rate | RR | −0.72 | 1.01 | 0.482 |
| Arterial CO2 | pCO2 | 0.65 | 0.30 | 0.046 |
| Arterial pO2 | paO2 | −6.17 | 1.15 | 0.000 |
| Arterial oxygen saturation | O2Sat | −0.86 | 0.22 | 0.001 |
| Arterial pH | pHa | −0.01 | 0.00 | 0.040 |
| Sagittal sinus pO2 | psO2 | 0.44 | 0.20 | 0.037 |
| Mean arterial blood pressure | MAP | −1.30 | 0.60 | 0.045 |
| Central venous blood pressure | CVP | −0.20 | 0.17 | 0.248 |
| Heart rate | HR | 6.28 | 1.83 | 0.003 |
| Cerebral blood flow | CBF | −3.21 | 1.06 | 0.007 |
| Cardiac output | CO | −0.14 | 0.09 | 0.152 |
| Core body temperature | T | 0.10 | 0.06 | 0.088 |
Data were pooled across all non-zero doses. The largest change in the variable following the drug was fitted to log-dose using a linear mixed effect model with ‘sheep' as a random variable. A slope of zero indicates no dose–response relationship. A positive slope indicates a variable increased with dose, and vice versa. P-values less than 0.05 are shown in bold.
Table 4.
Summary of the effect of CNS 7056 on respiratory variables
| Dose (mg kg−1) |
Baseline |
Change with drug |
P-value | % change | ||
|---|---|---|---|---|---|---|
| RR | s.e. | Delta RR | s.e. | |||
| 0 | 79.3 | 28.7 | −5.9 | 5.3 | 0.28 | −8 |
| 0.074 | 50.3 | 10.8 | −11.3 | 3.9 | 0.01 | −22 |
| 0.18 | 52.2 | 10.9 | −16.3 | 3.9 | 0.00 | −31 |
| 0.37 | 42.6 | 6.8 | −7.2 | 2.1 | 0.00 | −17 |
| 0.74 | 54.2 | 12.8 | −18.0 | 4.5 | 0.00 | −33 |
| 2.21 | 58.6 | 2.1 | −19.0 | 2.3 | 0.00 | −32 |
| 4.41 | 51.4 | 2.6 | −13.8 | 4.2 | 0.00 | −27 |
| 8.82 | 35.6 | 8.1 | 1.3 | 2.7 | 0.63 | 4 |
|
Dose (mg kg−1) |
pCO2 |
s.e. |
Delta pCO2 |
s.e. |
P-value |
% change |
| 0 | 33.4 | 1.2 | 1.1 | 0.5 | 0.16 | 3 |
| 0.074 | 34.6 | 0.6 | 2.0 | 0.5 | 0.05 | 6 |
| 0.18 | 33.9 | 1.4 | 3.9 | 1.2 | 0.08 | 11 |
| 0.37 | 36.2 | 1.4 | 1.0 | 1.5 | 0.55 | 3 |
| 0.74 | 33.1 | 2.4 | 3.9 | 0.7 | 0.03 | 12 |
| 2.21 | 33.3 | 2.0 | 4.0 | 1.3 | 0.19 | 12 |
| 4.41 | 34.8 | 1.9 | 3.9 | 1.0 | 0.06 | 11 |
| 8.82 | 32.9 | 1.3 | 3.4 | 1.9 | 0.31 | 11 |
|
Dose (mg kg−1) |
paO2 |
s.e. |
Delta paO2 |
s.e. |
P-value |
% change |
| 0 | 99.8 | 1.9 | −3.9 | 1.0 | 0.06 | −4 |
| 0.074 | 101.4 | 2.7 | −11.1 | 3.8 | 0.10 | −11 |
| 0.18 | 106.3 | 5.0 | −21.1 | 7.1 | 0.10 | −20 |
| 0.37 | 105.1 | 7.9 | −13.0 | 4.0 | 0.08 | −12 |
| 0.74 | 106.5 | 7.1 | −24.8 | 6.7 | 0.07 | −23 |
| 2.21 | 102.9 | 7.5 | −34.7 | 5.9 | 0.11 | −34 |
| 4.41 | 92.5 | 6.8 | −20.9 | 7.6 | 0.11 | −23 |
| 8.82 | 87.5 | 8.8 | −18.8 | 12.4 | 0.37 | −21 |
|
Dose (mg kg−1) |
O2Sat |
s.e. |
Delta O2Sat |
s.e. |
P-value |
% change |
| 0 | 102.3 | 0.1 | 0.1 | 0.1 | 0.42 | 0 |
| 0.074 | 102.2 | 0.1 | −0.3 | 0.2 | 0.26 | 0 |
| 0.18 | 102.4 | 0.4 | −1.2 | 0.6 | 0.18 | −1 |
| 0.37 | 102.1 | 0.3 | −0.6 | 0.3 | 0.22 | −1 |
| 0.74 | 102.5 | 0.6 | −1.8 | 0.7 | 0.12 | −2 |
| 2.21 | 102.3 | 2.0 | −3.9 | 2.4 | 0.35 | −4 |
| 4.41 | 102.3 | 1.5 | −3.1 | 2.0 | 0.26 | −3 |
| 8.82 | 101.6 | 1.5 | −2.6 | 2.2 | 0.44 | −3 |
Data are shown for respiratory rate (RR, breath per min), arterial carbon dioxide tension (pCO2, mm Hg), arterial oxygen tension (pO2, mm Hg) and arterial oxygen saturation (O2Sat, %). The value of the variable in the baseline period (0 min), and the change in the variable (delta) with the drug at 2 min by a linear mixed effect model at each dose. The percent change in the variable is also shown. P-values less than 0.05 are shown in bold.
The reductions in respiratory rate observed with most doses of CNS 7056 were associated with transient increases in arterial carbon dioxide tension, but the overall increase was small and was not statistically significant at any dose level other than 0.74 mg kg−1 (Table 4). Nevertheless, there was a significant positive dose–response relationship for the effect of increasing doses of CNS 7056 on arterial carbon dioxide tension (Table 3). The slight hypercarbia produced by CNS 7056 was associated with slight dose-related acidosis (Table 3). Arterial oxygen tension decreased from baseline for each dose group (Table 4), which was statistically significant when pooled across doses (Table 3). The observed decrease in oxygen tension was exacerbated by the fact that the sheep were breathing room air. Transient dose-related arterial haemoglobin desaturation was also evident (Table 3), but was not of sufficient extent or duration to endanger the animals.
Cardiovascular effects
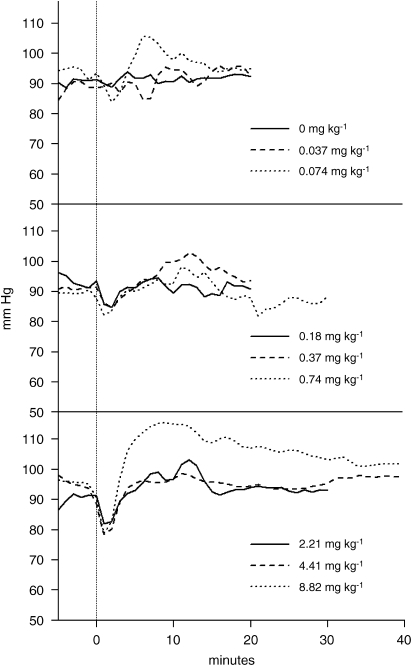
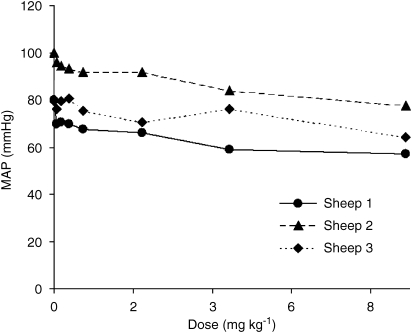
CNS 7056 produced a dose-related (Figure 5 and Table 3) transient drop in MAP, which was statistically significant at 2 min for all doses (Table 5). However, the magnitude of the drop was small, being less than 12%. The 8.82 mg kg−1 dose was associated with a ‘rebound' increase in MAP (Figure 5), consistent with the activation of compensatory sympathetic stimulation. As the lowest blood pressure achieved in any given situation is the most significant clinically, this was calculated separately and plotted (Figure 6). There was a clear inverse relationship with dose for all three sheep. The observed reductions in MAP are consistent with the dilation of blood vessels in response to CNS 7056.
Figure 5.
The effect of increasing doses of CNS 7056 on mean arterial blood pressure. Data are the mean in each dose group. The vertical lines are the start of the drug infusions.
Table 5.
Summary of the effect of CNS 7056 on cardiovascular variables
| Dose (mg kg−1) |
Baseline |
Change with drug |
P-value | % change | ||
|---|---|---|---|---|---|---|
| MAP | s.e. | Delta MAP | s.e. | |||
| 0 | 93.8 | 10.7 | −0.2 | 1.1 | 0.84 | 0 |
| 0.074 | 93.9 | 6.8 | −7.1 | 1.4 | 0.00 | −8 |
| 0.18 | 93.4 | 8.2 | −6.6 | 1.5 | 0.00 | −7 |
| 0.37 | 91.0 | 6.6 | −5.0 | 0.8 | 0.00 | −5 |
| 0.74 | 89.3 | 6.0 | −4.6 | 1.2 | 0.00 | −5 |
| 2.21 | 90.2 | 5.9 | −5.6 | 1.7 | 0.00 | −6 |
| 4.41 | 94.2 | 6.4 | −11.7 | 1.7 | 0.00 | −12 |
| 8.82 | 94.8 | 5.8 | −8.8 | 2.3 | 0.00 | −9 |
|
Dose (mg kg−1) |
CVP |
s.e. |
Delta CVP |
s.e. |
P-value |
% change |
| 0 | −2.7 | 0.7 | 0.6 | 0.2 | 0.00 | 24 |
| 0.074 | −4.1 | 0.8 | −0.6 | 0.3 | 0.05 | −14 |
| 0.18 | −3.5 | 1.5 | −1.2 | 0.2 | 0.00 | −34 |
| 0.37 | −5.9 | 1.0 | −0.7 | 0.2 | 0.00 | −12 |
| 0.74 | −6.3 | 1.7 | −1.1 | 0.2 | 0.00 | −17 |
| 2.21 | −5.3 | 0.7 | −1.2 | 0.2 | 0.00 | −23 |
| 4.41 | −4.4 | 1.2 | −1.6 | 0.2 | 0.00 | −37 |
| 8.82 | −6.9 | 1.2 | 0.4 | 0.2 | 0.07 | 5 |
|
Dose (mg kg−1) |
HR |
s.e. |
Delta HR |
s.e. |
P-value |
% change |
| 0 | 80.7 | 2.2 | −3.8 | 1.7 | 0.05 | −5 |
| 0.074 | 79.3 | 7.6 | 3.7 | 3.3 | 0.28 | 5 |
| 0.18 | 86.4 | 4.4 | 8.3 | 2.1 | 0.00 | 10 |
| 0.37 | 93.5 | 7.9 | 9.1 | 1.9 | 0.00 | 10 |
| 0.74 | 95.4 | 6.1 | 19.6 | 3.2 | 0.00 | 21 |
| 2.21 | 93.2 | 2.1 | 23.2 | 3.6 | 0.00 | 25 |
| 4.41 | 85.4 | 14.1 | 18.0 | 3.1 | 0.00 | 21 |
| 8.82 | 102.3 | 10.1 | 15.3 | 3.6 | 0.00 | 15 |
|
Dose (mg kg−1) |
CBF |
s.e. |
delta CBF |
s.e. |
P-value |
% change |
| 0 | 100.1 | 1.6 | −1.5 | 2.7 | 0.58 | −2 |
| 0.074 | 100.6 | 2.1 | 2.9 | 2.0 | 0.15 | 3 |
| 0.18 | 100.0 | 2.0 | −13.4 | 2.0 | 0.00 | −13 |
| 0.37 | 99.9 | 1.4 | −8.1 | 2.2 | 0.00 | −8 |
| 0.74 | 99.8 | 2.7 | −13.6 | 2.5 | 0.00 | −14 |
| 2.21 | 99.0 | 2.4 | −10.3 | 3.3 | 0.00 | −10 |
| 4.41 | 99.9 | 2.2 | −11.9 | 2.2 | 0.00 | −12 |
| 8.82 | 99.7 | 1.4 | −20.0 | 2.3 | 0.00 | −20 |
Data are shown for mean arterial blood pressure (MAP, mm Hg), central venous blood pressure (CVP, mm Hg), heart rate (HR, beats per min) and the index of cerebral blood flow (CBF, % baseline). The table shows the value of the variable in the baseline period (0 min), and the change in the variable (delta) with the drug at 2 min by a linear mixed effect model at each dose. The percent change in the variable is also shown. P-values less than 0.05 are shown in bold.
Figure 6.
The effect of the dose of CNS 7056 on the lowest recorded mean arterial blood pressure. Each point is one dose in one sheep.
All doses other than 0.074 and 8.82 mg kg−1 were associated with significant but small (up to 1.6 mm Hg) reductions in central venous blood pressure (Table 5) that were not dose-related (Table 3). This decrease could be interpreted as a drug-dependent reduction in sympathetic tone causing an increase in venous compliance. The paradoxical effect for the 8.82 mg kg−1 dose was presumably also due to the dose exceeding a threshold for sympathetic stimulation and cardiovascular compensation. The effect was absent for the 0.074 mg kg−1 dose because the sedative effect of this dose was too low. The 0 mg kg−1 dose showed an increase in central venous blood pressure.
Heart rate did not change for the 0 and 0.074 mg kg−1 dose groups, but all other doses were associated with significant increases in heart rate of up to 25% (Table 5) and the effect was dose-related (Table 3). The increased heart rate is consistent with a compensatory response to the reductions in MAP caused by CNS 7056.
Two measures of organ perfusion were recorded—cardiac output and cerebral blood flow. Cardiac output, reflecting the average perfusion of the organs, was unaffected by CNS 7056 (Table 3). The index of cerebral blood flow was significantly reduced for all doses of 0.18 mg kg−1 and higher (Table 5). The effect was dose-related (Table 3), with a maximum reduction of 20% for the highest doses. CNS 7056 caused a dose-related increase in sagittal sinus (cerebral venous) oxygen tension (Table 3), but the lower magnitude of the change relative to the change in cerebral blood flow suggests that brain oxygen consumption and cerebral blood flow were essentially coupled.
Core body temperature is a good indicator of the health status of a chronically instrumented animal. In sheep, the normal value is approximately between 39 and 40 °C, whereas a sick, septic animal has values over 41 °C. The temperature was in the normal range for all studies in this series and did not change significantly as the dose escalation series progressed (Table 3).
Discussion and conclusions
This study was the first time that CNS 7056 had been administered to sheep. The longitudinal study design allowed within-sheep comparisons and was, statistically, relatively powerful. Statistically significant changes could be detected with only three sheep, thereby minimizing the use of the experimental compound and animals. Disadvantages of this design become apparent if there are time-dependent changes in the physiological or pharmacological status of the animal. Examples of the former could include progressive changes in baseline haemodynamic parameters and of the latter include accumulation of parent compound or metabolites, metabolic enzyme induction, or the development of tolerance. There were no systematic daily changes in the baseline measurements of most physiological parameters (Tables 4 and 5). Although the levels of parent compound and metabolite were not measured in this study, the short duration of action of CNS 7056 and the high rates of metabolism of ester-based drug in vivo would suggest minimal accumulation on a day-to-day basis. Enzyme induction is a possibility that could not be excluded, but on theoretical grounds induction would be expected to have a minor impact on a high clearance (and therefore flow-limited) drug. The development of major tolerance would manifest as the same or less dynamic response as dose was escalated. This was not observed, but further studies testing for tolerance would be important for this compound.
Accepting these limitations of the experimental design, it was clear that CNS 7056 had powerful sedative properties in sheep and doses of 0.18 mg kg−1 and above caused rapid onset of LOC with minimal extraneous movement. The duration of the period of LOC was dose-dependent, but recovery, once begun, was rapid with a clear transition between the unconscious and conscious states. CNS 7056 rapidly caused respiratory depression and hypotension in a dose-dependent manner. However, the magnitude of these respiratory and cardiovascular effects was minor to moderate, and would not require clinical intervention (up to doses of 4.41 mg kg−1) by reasonable standards of clinical management. The upper limit to the dose (8.82 mg kg−1) was associated with the activation of compensatory responses to drug-induced respiratory and cardiovascular depression. The upper dose of CNS 7056 may be lower in individuals with compromised physiological control systems. However, there was no indication that the highest doses used in this study were a danger to the animals' welfare.
Midazolam is the closest comparator drug for CNS 7056 and has also been shown to produce hypotension in sheep (Upton et al., 2001) and in man (Choi et al., 2004) when administered as a intravenous bolus or short infusion. Respiratory and cardiovascular depression are common features of anaesthetics that depress the brainstem. Further studies at equi-anaesthetic doses are required to determine whether CNS 7056 differs quantitatively from midazolam in this regard. Like midazolam, any clinical use of CNS 7056 would benefit from the inherent safety margins afforded by the availability of benzodiazepine antagonists such as flumazenil, which has been shown to be an effective antagonist for CNS 7056 (Kilpatrick et al., 2007).
This study showed that CNS 7056 is a sedative in sheep, and that doses of 0.37–2.21 mg kg−1 produced short periods of sedation without excessive respiratory or cardiovascular depression. The alpha band of the EEG was a good quantitative indicator of the sedative effects of CNS 7056. Measurements of MAP (for cardiovascular effects) and arterial oxygen saturation (for respiratory effects) could be used to monitor for excessive dosing in future studies. Further investigations of the kinetics and dynamics of CNS 7056, and comparisons with other agents such as midazolam and propofol, will allow a better understanding of its potential role as a clinical sedative.
Acknowledgments
We acknowledge the contribution of the staff and infrastructure of the Surgical Research Facility of the Institute of Medical and Veterinary Science, Adelaide, Australia.
Abbreviations
- LOC
loss of consciousness
- MAP
mean arterial blood pressure
- RE
response entropy of the EEG
- ROC
recovery of consciousness
- SE
state entropy of the EEG
- total
average power in all bands (3–30 Hz) of the EEG
Conflict of interest
This research was funded by CeNeS Limited, Compass House, Vision Park, Chivers Way, Histon, Cambridge, CB24 9ZR, UK, the developers of CNS 7056. These data were presented at the SEAWP-RMP ASCEPT joint Annual Scientific Meeting, Adelaide, Australia, December, 2007.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 (Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YF, Wong TW, Lau CC. Midazolam is more likely to cause hypotension than etomidate in emergency department rapid sequence intubation. Emerg Med J. 2004;21:700–702. doi: 10.1136/emj.2002.004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolette DJ, Upton RN, Grant C. Agreement between ultrasonic Doppler venous outflow and Kety and Schmidt estimates of cerebral blood flow. Clin Exp Pharmacol Physiol. 1999;26:736–740. doi: 10.1046/j.1440-1681.1999.03109.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107:60–66. doi: 10.1097/01.anes.0000267503.85085.c0. [DOI] [PubMed] [Google Scholar]
- Ludbrook GL, Upton RN. A physiological model of induction of anaesthesia with propofol in sheep. 2. Model analysis and implications for dose requirements. Br J Anaesth. 1997;79:505–513. doi: 10.1093/bja/79.4.505. [DOI] [PubMed] [Google Scholar]
- Muncaster AR, Sleigh JW, Williams M. Changes in consciousness, conceptual memory, and quantitative electroencephalographical measures during recovery from sevoflurane- and remifentanil-based anesthesia. Anesth Analg. 2003;96:720–725. doi: 10.1213/01.ANE.0000040143.95962.36. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria; 2005. [Google Scholar]
- Schwilden H. Concepts of EEG processing: from power spectrum to bispectrum, fractals, entropies and all that. Best Pract Res Clin Anaesthesiol. 2006;20:31–48. doi: 10.1016/j.bpa.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Upton R, Grant C, Ludbrook G. An ultrasonic Doppler venous outflow method for the continuous measurement of cerebral blood flow in conscious sheep. J Cereb Blood Flow Metab. 1994;14:680–688. doi: 10.1038/jcbfm.1994.85. [DOI] [PubMed] [Google Scholar]
- Upton RN, Ludbrook GL. A physiological model of induction of anaesthesia with propofol in sheep. 1. Structure and estimation of variables. Br J Anaesth. 1997;79:497–504. doi: 10.1093/bja/79.4.497. [DOI] [PubMed] [Google Scholar]
- Upton RN, Ludbrook GL. A model of the kinetics and dynamics of induction of anaesthesia in sheep: variable estimation for thiopental and comparison with propofol. Br J Anaesth. 1999;82:890–899. doi: 10.1093/bja/82.6.890. [DOI] [PubMed] [Google Scholar]
- Upton RN, Ludbrook GL, Grant C, Martinez A. In vivo cerebral pharmacokinetics and pharmacodynamics of diazepam and midazolam after short intravenous infusion administration in sheep. J Pharmacokinet Pharmacodyn. 2001;28:129–153. doi: 10.1023/a:1011550915515. [DOI] [PubMed] [Google Scholar]
- Vakkuri A, Yli-Hankala A, Talja P, Mustola S, Tolvanen-Laakso H, Sampson T, et al. Time-frequency balanced spectral entropy as a measure of anesthetic drug effect in central nervous system during sevoflurane, propofol, and thiopental anesthesia. Acta Anaesthesiol Scand. 2004;48:145–153. doi: 10.1111/j.0001-5172.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S-plus. Springer: Germany; 2002. [Google Scholar]
- Viertio-Oja H, Maja V, Sarkela M, Talja P, Tenkanen N, Tolvanen-Laakso H, et al. Description of the entropy algorithm as applied in the Datex-Ohmeda S/5 Entropy Module. Acta Anaesthesiol Scand. 2004;48:154–161. doi: 10.1111/j.0001-5172.2004.00322.x. [DOI] [PubMed] [Google Scholar]