Abstract
Transcriptional regulation studies of CNS neurons are complicated by both cellular diversity and plasticity. Microdissection of specific functionally related populations of neurons can greatly reduce these issues, but typically excludes the use of many technologies due to tissue requirements, such as Chromatin Immunoprecipitation (ChIP), a powerful tool for studying in vivo protein-DNA interactions. We have developed a fast carrier ChIP (Fast CChIP) method for analyzing specific in vivo transcription factor-DNA interactions in as little as 0.2 mm3 brain tissue. Using an antibody against phosphorylated cyclic-AMP response element binding (CREB) protein, we confirmed phospho-CREB (pCREB) binding at the c-fos gene promoter. Then we further demonstrated the applicability of Fast CChIP in determining hypertension-induced pCREB binding at the c-fos gene promoter in the rat nucleus tractus solitarius (NTS), confirming CREB’s role in mediating hypertension-induced c-fos expression. This method will be broadly applicable to individual brain nucleus and biopsy/surgical samples.
Introduction
In the CNS, physiologically defined functional units - brain nuclei - are not only small, limited to hundreds to thousands of neurons, but are also composed of heterogeneous neuronal populations that receive input from different sources and show very different responses to any given stimuli. As such, any particular perturbation may activate tens to hundreds of cells in a background of thousands of non-responsive cells. Tissue samples of this heterogeneous nature, in contrast to relatively more homogeneous and readily available tissues such as liver or tissue cultured cells, are not amenable for chromatin immunoprecipitation analysis using any existing protocols. Current effort extending systems biology studies into CNS biology and disease depends on the development of such capability to understand the systems level transcription factor and target gene promoter interactions. ChIP has proven to be a powerful tool to study in vivo transcription factor binding at native promoter sites (Impey et al., 2004). However the conventional ChIP assay has several limitations: it takes several days to complete and it also requires a large number of cells (typically 107). It is especially challenging to adapt ChIP methods to small samples such as brain nuclei, micro-dissected tissues, biopsies, and/or surgical samples, where the amount of tissue is limited.
Conventional ChIP requires a large number of cells simply because: 1) the recovery rate of cross linked chromatin in ChIP varies from one to ten percent of the total cellular DNA content in the starting material; and 2) extensive wash steps during immunoprecipitation result in loss of specific interactions and therefore reduced signal to noise ratio. Recently, three new methods have been developed to address some of these limitations (Nelson et al., 2006; O’Neill et al., 2006; Dahl and Collas, 2007).
The Fast ChIP method reduces the time requirement by using a sonicating water bath to improve the rate of antibody-antigen binding and increases recovery efficiency by using a Chelex resin to combine cross-linking reversal and DNA purification (Nelson et al., 2006). These simple modifications reduced the amount of time required for ChIP assay from 2–3 days to 4 hours.
Carrier has been used in other nucleic acid isolation procedures to help recover small quantities of nucleic acid. Normally carrier consists of large polymers such as polysaccharide glycogen or non-specific nucleic acid such as tRNA. Its role is believed to be competition for non-specific interactions (enzymatic or binding), occupying significant aqueous space resulting in reduced reaction volume, and increasing efficiency of recovery from purification and concentration steps. Application of a carrier in ChIP has been seen in a sequential chromatin immunoprecipitation method (Geisberg and Struhl, 2004) to make the second immunoprecipitation similar to the first immunoprecipitation and minimize background signal. CChIP method uses a heterogeneous chromatin (Drosophila S2 cells) as a source of carrier to immunoprecipitate native chromatin from small number of mammalian cells (O’Neill et al., 2006). With CChIP, O’Neill et al were able to immunoprecipitate modified histone bound chromatin from ~200 cells (O’Neill et al., 2006).
More recently, Dahl and Collas (2007) reported a Q2ChIP method in which the authors demonstrated increased specificity by moving the IP reaction to a fresh tube prior to reversing the protein-chromatin cross linking, leaving behind non-specific plastic bound chromatin. They were able to immunoprecipitate modified histone associated chromatin from as few as 100 cells and transcription factor bound chromatin from ~1000 cells (Dahl and Collas, 2007).
Individually, these methods improved conventional ChIP in sensitivity and efficiency, but none were demonstrated to be directly applicable to analysis of transcription factor DNA binding in microdissected tissue samples. We have adapted Fast ChIP and CChIP and developed a fast carrier ChIP (Fast CChIP) method for detecting transcription factor DNA binding in a small number of heterogeneous cells from in vivo tissue samples. Using this method, we have successfully demonstrated its application in analyzing transcription factor DNA binding activity in an individual brain nucleus.
Material and Methods
Animals
Male adult Sprague Dawley rats obtained from Charles River Laboratory (Wilmington, MA) were housed in pairs under 12:12 light/dark cycles (lights on at 6 am). Food and water were available ad libitum. All animal protocols are approved by the Thomas Jefferson University IACUC.
Acute Hypertension Paradigm
Acute hypertension was induced as previously reported11. Briefly, animals were anesthetized with isoflurane dissolved in O2 (5% induction; 1.5% maintenance) and one femoral artery and vein were cannulated via a small medial incision with PE-50 tubing (Becton Dickinson, Franklin lakes, NJ) for measure of arterial pressure and infusion of drugs, respectively. The cannulae were run subcutaneously to an exit incision between the scapulae. The leg wound was sutured and topical anesthetic (Lidocaine) was applied to both skin incisions. Animals were then placed in cages and allowed to recover from the anesthetic for one hour. To induce hypertension in treated animals, intravenous infusion of approximately 1 ml of phenylephrine in saline (200µg/ml) was applied at a rate of 200µg/ml/hr.
Fast Carrier Chromatin Immunoprecipitation (Fast CChIP)
The chromatin immunoprecipitation method was adapted from a fast chromatin immunoprecipitation protocol (Fast ChIP) (Nelson et al., 2006) and a carrier chromatin immunoprecipitation protocol (CChIP) (O’Neill, 2006). Briefly, animals were killed by quick decapitation. Brains were quickly removed and placed in ice-cold ACSF (10 mM HEPES, pH 7.4, 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 24 mM D-Glucose). Coronal brain stem slices with a thickness of 250 µm were prepared using a McIlwain tissue chopper (Gamshall, England) and NTS were microdissected using a 1 mm micropunch (Stoelting Co., Wood Dale, IL). The tissue micropunches were fixed in 1.4% formaldehyde in ACSF for 15 minutes at room temperature on a rotating platform, followed by adding 1/10 volume of 1.25 M glycine and incubating on the rotating platform for 5 minutes, and two washes with cold PBS. The tissue was then mixed with about 107 1% formaldehyde treated yeast cells in 300 µl of IP buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 0.5% v/v NP-40, 1% v/v Triton X-100) with protease inhibitor (500 µM PMSF, 10 µg/ml leupeptin, 10 µg/ml aprotenin, 10 mM NaF, 100 µM Na3VO4). The tissue were then homogenized, incubated on ice for 10 minutes, and sonicated using a Fisher Scientific 60 Sonic Dismembrator (Waltham, MA) at 50% power for 10 rounds of 15 pulses in an ice-cold water bath. Sonication pulses were 1 second long with a 1 second interval in between. Between each round of sonication, samples were cooled on ice for two minutes. After sonication, the samples were centrifuged at 12,000x g for 10 minutes at 4°C. Next, the chromatin containing supernatant (120 µl) was incubated with 2 µg of antibody or normal IgG in an ice-cold sonicating water bath for 1 hour. DNA from the remaining 60 µl of chromatin was purified for use as input DNA. The antibody bound chromatin was precipitated with 50 µl of 50% protein-G-agarose bead slurry (Activ Motif, Carlsbad, CA) and washed with cold IP buffer 5 times. The precipitated chromatin was mixed with 100 µl of 10% Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA) in water and boiled for 10 minutes to reverse cross linking. After centrifugation at 14,000 rpm for 2 minutes, the supernatant was collected as chromatin DNA. The TF-specific antibody used was a rabbit monoclonal to pCREB (06-519, Millipore, Billerica, MA). Rabbit normal IgG (PP64, Millipore, Billerica, MA) was used for negative IP control.
PCR and real-time PCR detection of enrichment of promoter sequences
Enrichment of the c-fos gene promoter by Fast CChIP with an antibody against pCREB was detected using PCR and Hot Star Taq Polymerase Master Mix (Qiagen, Valencia, CA). Briefly, 5 µl of ChIP DNA or 1:10 diluted input DNA was mixed with the c-fos gene promoter primer pair in 25 µl total reaction volume and amplified in a first round PCR of 15 cycles on a DNA engine tetrad thermal cycler (PTC-225, Bio-Rad). Then a second round of PCR using 2 µl of the first round product and amplified for 40 cycles. For both rounds of PCR, the thermal cycling conditions were 15 minutes at 95°C and then cycles of 95°C for 30 seconds, 57°C for 1 minute, and 72°C for 1 minute. The resulting PCR products were run out on a 3 % agarose with 25 bp DNA ladder (Invitrogen). Fast CChIP enrichment of the c-fos gene promoter was quantified by real-time PCR. In brief, 5 µl of ChIP DNA was incubated with primer pairs using Qiagen HotStar Taq Polymerase Master mix in 25 µl for 15 cycles as above. Then 2.5 µl of the PCR product was mixed with primers and iTaq SYBR master mix (Bio-Rad) in a 10 µl of reaction volume overlaid with 10 µl of mineral oil. Real-time PCR amplification was carried out on an ABI Prism 7000 Sequence Detection system (ABI). The thermal cycling conditions for real-time PCR were first 2 minutes at 50°C and 10 minutes at 95°C and consisted of 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The sequences of primer pairs were 5’-TTCTCTGTTCCGCTCATGACGT-3’ (sense) and 5’-CTTCTCAGTTGCTAGCTGCAATCG-3’ (antisense) for the c-fos gene promoter. All PCR primers used were obtained from Sigma (St. Louis, MO).
Quantification of enrichment of promoter sequences
Enrichment of the c-fos gene promoter was quantified by real-time PCR using the comparative delta Ct method as described by Livak et al (2001). The relative fold enrichment was given by the formula 2−(ΔΔCt+/−SD), where ΔΔCt = (Ct hypertension IP − Ct hypertension input) − (Ct saline IP − Ct saline input). All experiments were performed three times and samples from each experiment were analyzed in triplicates.
Results and Discussion
The Fast CChIP procedure combines the Fast ChIP method with the CChIP method to benefit from their respective advantages, i.e., shortened time requirement of Fast ChIP and high sensitivity to detect protein and DNA interaction in a small number of cells of CChIP. It follows the basic procedure of Fast ChIP with the modification of using yeast Saccharomyces cerevisiae strain BJ5464 cells as a source of carrier chromatin. Microdissected rat brain tissues are fixed and mixed with pre-fixed BJ5464 cells before homogenization and preparation of chromatin. Chromatin preparation, immunoprecipitation, and ChIP DNA isolation follows exactly as described in Fast ChIP. The quantity and fragment size of chromatin is checked by agarose gel electrophoresis (data not shown). Immunoprecipitated DNA fragments are detected with either quantitative PCR or PCR and agarose gel electrophoresis.
A known site-specific interaction between a transcription factor and its target gene promoter is required to validate and determine the sensitivity of the procedure. We chose the interaction of the transcription factor CREB with the c-fos gene promoter. In the proximal promoter sequence of the rat c-fos gene there is a consensus CREB binding site (Fig. 1A) and CREB is known to mediate c-fos gene expression (Runkel et al., 1991). ChIP studies using anti-CREB antibody have demonstrated site specific binding at the c-fos gene promoter in mammalian cells (Impey et al., 2004). Because CREB transcription regulation activity is activated by phosphorylation (Papavasilliou, 1994) and the c-fos gene is expressed in adult rat brain tissue (Gubits et al., 1988) (also see Allen Brain Atlas http://www.brain-map.org), adult rat brain cortex tissue and anti-pCREB antibody (#9189, Cell Signaling) were used to validate the Fast CChIP method. Enrichment of the c-fos gene promoter by anti-pCREB from adult rat brain cortex tissue will confirm binding of pCREB at the c-fos gene promoter and therefore validate the Fast CChIP approach.
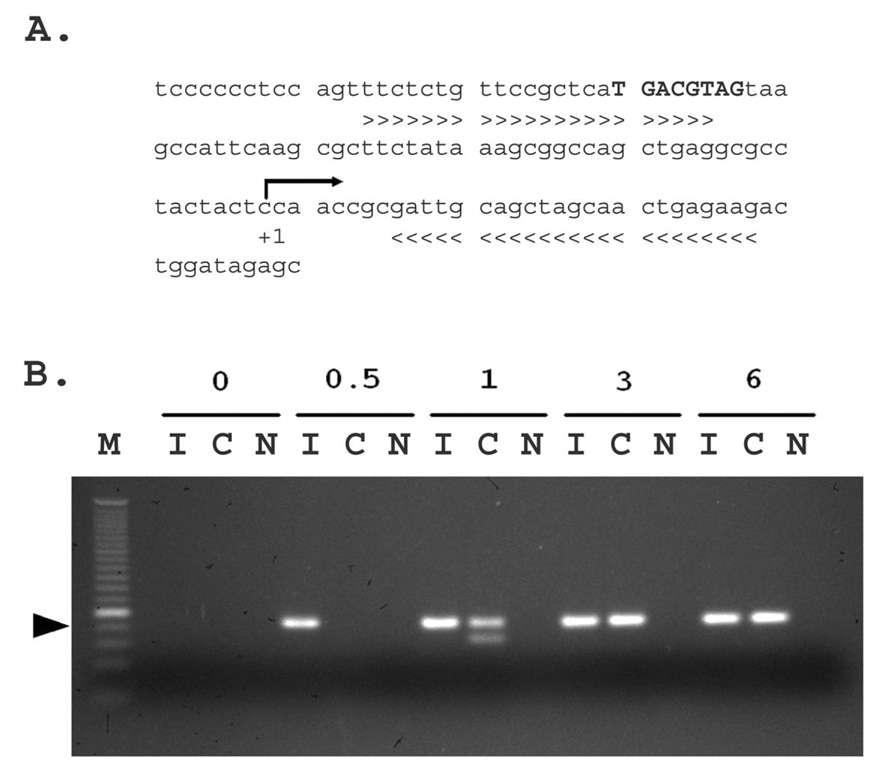
Figure 1. The c-fos gene promoter was enriched by pCREB immunoprecipitaion in microdissected rat cortical samples.
A. The partial rat c-fos gene promoter sequences (from accession #: AY786174) showing the locations of cAMP response element (CRE) site and PCR primers used for detecting enrichment of the promoter. +1 and the arrow indicate the transcription start site and transcription direction. Capital letters in bold denote the CREB binding site (from −58 to −51 bp). The > and < symbols underneath the sequences indicate the sequences of forward (>) and reverse (<) PCR primers used. The PCR amplicon size is 104 bp. B. PCR amplification of c-fos gene promoter sequences from Fast CChIP DNA obtained from different numbers of tissue micropunches. PCR product was visualized on a 3% agarose gel. One 30th Input DNA and one 20th of ChIP DNA were used as template for first round PCR reaction in a 25 µl volume for 15 cycles. Then 2 µl of each PCR product was used for a second round of amplification in a 25 µl volume and for 40 cycles. M: 25 bp DNA ladder (Invitrogen). Black arrow head indicates the 100 bp fragment. Numbers 0, 0.5, 1, 3, and 6 on top of the gel indicates the number of 0.2 mm3 micropunch tissues used as starting material. I: input chromatin; C: anti-pCREB IP; N: normal IgG IP.
Rat cortex tissue samples ranging from 0 to 6 punches (1 mm diameter from 250 µm thick tissue slices) were fixed, mixed with 107 pre-fixed yeast BJ5464 cells, and subjected to the Fast CChIP analysis (for detail see method section). Each sample was subdivided into three fractions: one was used as input chromatin DNA (one fifth of the total sample), one incubated with anti-pCREB antibody (two fifths of the total sample), and one incubated with normal IgG as negative control (two fifths of the total sample). Antibody-chromatin complexes were incubated with protein-G-agarose beads and non-specific complexes were removed by washing. Protein-DNA crosslinking was reversed and IP enriched chromatin DNA was isolated. DNA recovered from each fraction was subjected to PCR amplification for analysis of enrichment of the c-fos gene promoter (Fig. 1B). Differences in the amount of PCR product between pCREB specific and IgG non-specific immunoprecipitation is evidence of pCREB binding at the c-fos promoter in vivo. With tissue sample equivalent to one or more micropunches, the c-fos gene promoter was detected in both input DNA and anti-pCREB immunoprecipitated ChIP DNA, but not in normal IgG immunoprecipitated control ChIP DNA (Fig. 1B, 1/I-C-N, 3/I-C-N, and 6/I-C-N lanes). These results indicate specific precipitation from anti-pCREB antibody and no or very low non-specific precipitation from non-immune IgG. When tissue sample equivalent to one half of a micropunch was used as starting material, the c-fos gene promoter was only amplified in the input chromatin DNA, not from anti-pCREB or from normal IgG ChIP DNA (Fig. 1B, 0.5/I-A-C lanes). This may suggest that the amount of tissue used has fallen below the lower limit of the approach and the sensitivity of this method to detect pCREB binding at the c-fos gene promoter is one or more micropunches. A second smaller fragment was noticed in the one micropunch ChIP DNA lane (Fig. 1B, 1/C). It is probably a mispriming product due to the low template concentration and using a more stringent PCR amplification conditions might eliminate the band. When there is no rat cortex tissue punch used as starting material, no PCR product of the c-fos gene promoter was detected, indicating the specificity of the primers for the rat c-fos gene promoter sequences (Fig. 1B, 0/I-A-C lanes).
Based on rat brain density (1.04) (Weaver et al., 2001) and rat cortex cell density (Herculano-Houzel and Lent, 2005), one cortex tissue punch (250 um thick by 1mm diameter, 0.2 mm3) equals to approximately 20,000 total cells and 8000 neurons. The c-fos gene expression density in the cortex is about 30% at the coronal plane and about 60% at sagittal plane (Allen Brain Atlas http://www.brain-map.org). Therefore one cortex punch has approximately 2400–4800 cells expressing the c-fos gene and requiring pCREB bound at the c-fos promoter. While it is clear that this is a gross estimate, this implies that at basal expression level (without any stimulation) and using an antibody against pCREB in combination with c-fos promoter specific PCR, Fast CChIP is capable of detecting basal pCREB DNA binding activity to the c-fos promoter from approximately 2–5×103 cells.
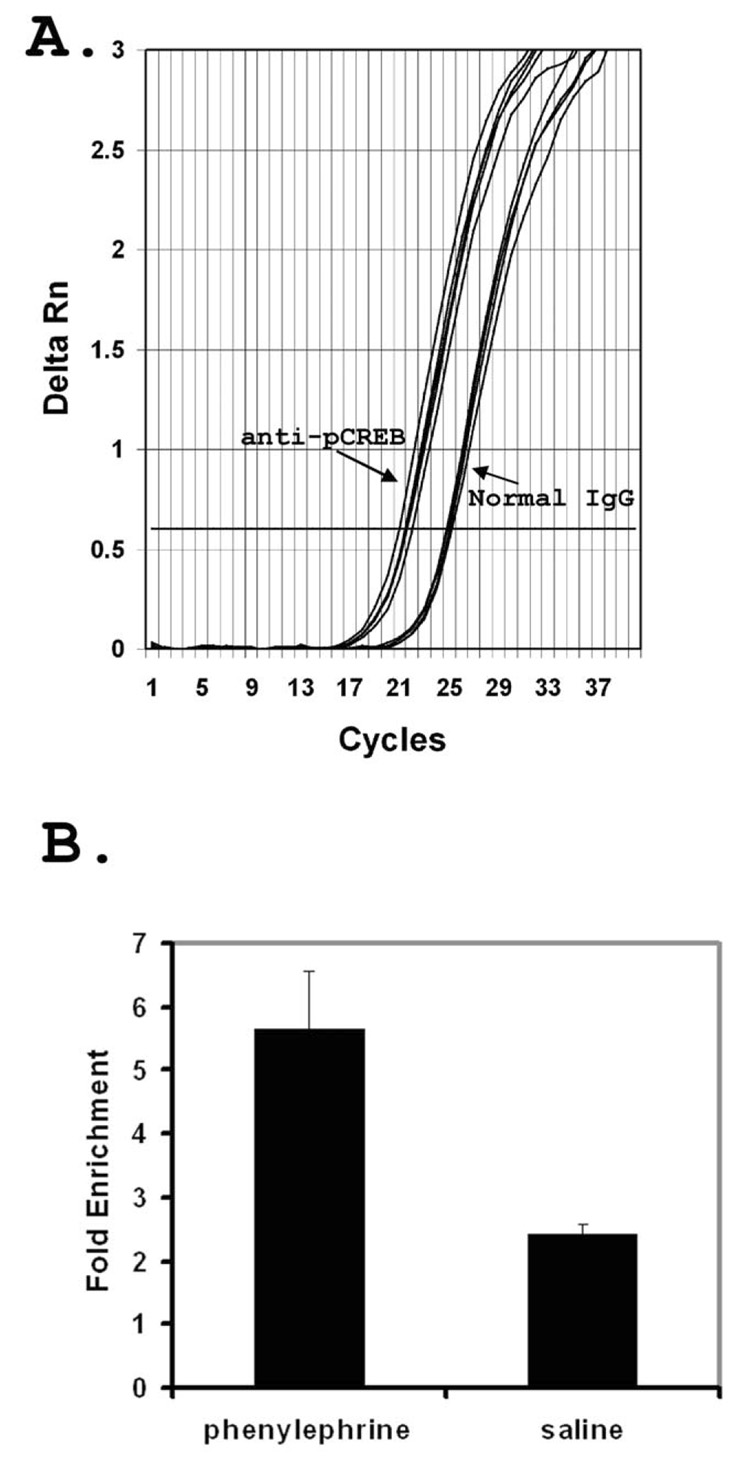
To further demonstrate the utility of Fast CChIP method in studying specific TF binding in an individual brain nucleus, we used phenylephrine (PE) induced hypertension in rat as a physiological stimulus and studied hypertension induced pCREB binding at the c-fos gene promoter in the nucleus tractus solitarius (NTS). The arterial baroreceptor reflex provides a rapid negative feedback mechanism in maintaining the homeostasis of cardiovascular function (Li et al., 1994; Chan and Sawchenko, 1998). The NTS plays an integrative role within the baroreflex function and either activates or inhibits the sympathetic and parasympathetic nervous systems to control heart rate, cardiac output, and peripheral vascular resistance, based upon the visceral and emotional inputs (Li et al., 1994; Chan and Sawchenko, 1998). It has been shown that PE induced acute hypertension leads to the immediate early gene c-fos induction and Fos protein accumulation in the NTS (Chan and Sawchenko, 1994; Li et al., 1994; Chan et al., 2004). Phosphorylation of CREB has been suggested to mediate the induction of the c-fos gene expression (Chan et al., 1999; Chan et al., 2004). This has been difficult to demonstrate directly because only a small percentage of the cells in the region respond to blood pressure change. Phenylephrine induced hypertension was combined with Fast CChIP to evaluate blood pressure dependent changes of pCREB binding at the c-fos promoter. NTS tissue was isolated by microdissection 30 minutes after PE induced hypertension and half of the NTS tissue (about three micropunches at 0.2 mm3 each) was subjected to Fast CChIP assay using anti-pCREB antibody and the enrichment of the c-fos gene promoter by Fast CChIP was tested using qPCR. Enrichment of c-fos gene promoter by anti-pCREB using Fast CChIP in the NTS tissue was demonstrated by earlier detection of amplification from anti-pCREB immunoprecipitated DNA as compared to normal IgG mock precipitated DNA (Fig. 2A). In saline infused animals the enrichment of the c-fos gene promoter by anti-pCREB immunoprecipitation was 2.43±0.147 fold (mean±SE) compared to normal IgG control, while as in PE induced hypertensive animals, the enrichment was 5.65±0.93 fold (Fig. 2B). Comparing PE induced hypertensive animals to saline infused animals, PE induced hypertensive increased the c-fos gene promoter enrichment by approximately 2 fold, indicating an increased pCREB binding on the c-fos gene promoter sequences. Such an increase is in agreement with increased gene expression (Chan et al., 2004) and Fos immunoreactivity (Chan and Sawchenko, 1994; Li et al., 1994) seen in the NTS after PE induced hypertension.
Figure 2. Hypertension induced increase in c-fos gene promoter enrichment by anti-pCREB immunoprecipitation in rat NTS microdissected tissue samples.
A. Amplification plot from quantitative real-time PCR demonstrating pCREB antibody enrichment of c-fos promoter as compared to normal IgG. anti-pCREB: indicates amplification curves from anti-pCREB immunoprecipitated DNA samples. Normal IgG: indicates amplification curves from normal IgG mock immunoprecipitated DNA. B. Phenylephrine: NTS samples from PE infused animals. Saline: NTS samples from saline infused control animals. Fold enrichment are relative to normal IgG control for each sample. Data are averages from three animals with standard error.
Following moderate hypertension, the number of Fos-IR neurons in the NTS has been estimated to be around 1000 in each animal (Chan and Sawchenko, 1998). As only half of the NTS tissue was used for Fast CChIP, the sensitivity of this method is estimated to be approximately 500 responsive cells (defined by c-fos protein accumulation in response to hypertension). Of course, the binding of pCREB to the c-fos promoter is not limited to the hypertension-responsive cells as pCREB was seen binding to the c-fos promoters at a basal level in the absence of stimulation. However, the increase in pCREB binding in hypertensive NTS compared to saline-treated NTS tissue is certainly due to hypertensive treatment and the increased enrichment observed presumably is from the hypertension responsive cells with increased c-fos expression (Chan et al., 2004; Chan and Sawchenko, 1994; Li et al., 1994). It is noted that this is in the context of a larger quantity of starting material (0.6 mm3) compared to the initial sensitivity testing with cortex tissue (0.2 mm3). Nonetheless, Fast CChIP was demonstrated to be suitable for analyzing specific transcription factor binding to target gene promoter sequences in microdissected animal tissues, more specifically a single brain nucleus – the NTS.
In summary, combining the speed of Fast ChIP and the sensitivity of CChIP, we have developed a Fast CChIP procedure. Using this method we have immunoprecipitated chromatin bound by pCREB in rat brain cortical microdissected tissue samples and in NTS samples from PE-induced hypertensive animals. We have demonstrated the utility of Fast CChIP in analyzing in vivo transcription factor:DNA binding activity in an individual brain nucleus. The same protocol has also been used to determine hypertension-induced dynamic DNA binding activities in the NTS by additional transcription factors (AP-1 (c-JUN), data to be presented elsewhere, Hao and Schwaber). These observations demonstrate that Fast CChIP is capable of determining changes in site-specific transcription factor binding from a small number of cells (~500) in a background of non-responding heterogeneous cell types (about 104–105 cells in the NTS punches used). While not directly tested herein, it is very likely that ChIP investigating much more abundant protein:DNA interactions, for example targeting polII, global accessory TFs (such as TFIIB or TBPs) and histone interactions, will require even less tissue using this protocol. Previously, people have pooled samples from several animals to perform ChIP experiments using brain tissues. This is the first report we are aware of where an individual brain nucleus from a single animal has been used for successful ChIP detection of transcription factor:promoter binding. This will have broad implications for analyzing transcription factor activities in a wide range of samples that are limited in quantity, such as biopsies, surgical samples, and even laser capture microdissected cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chan JYH, Chen W-C, Lee H-Y, Chang T-J, Chan SHH. Phosphorylation of transcription factor cyclic-AMP response element binding protein mediates c-fos induction elicited by sustained hypertension in rat nucleus tractus solitarii. Neuroscience. 1999;88:1199–1212. doi: 10.1016/s0306-4522(98)00273-5. [DOI] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cells groups induced by cardiovascular challenges in the rat. J Comp Neurol. 1994;348:433–460. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci. 1998;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SHH, Chang K-F, Ou C-C, Chan JYH. Nitric Oxide regulates c-fos expression in nucleus tractus solitarii induced by baroreceptor activation via cGMP-dependent protein kinase and cAMP Response element-binding protein phosphorylation. Mol Pharmcol. 2004;65:319–325. doi: 10.1124/mol.65.2.319. [DOI] [PubMed] [Google Scholar]
- Dahl JA, Collas P. Q2ChIP, a quick and quantitative chromatin immunoprecipitation assay, unravels epigenetic dynamics of developmentally regulated genes in human carcinoma cells. Stem Cells. 2007;25:1037–1046. doi: 10.1634/stemcells.2006-0430. [DOI] [PubMed] [Google Scholar]
- Geisberg JV, Struhl K. Quantitative sequential chromatin immunoprecipitation, a method for analyzing co-occupancy of proteins at genomic regions in vivo. Nucleic Acids Res. 2004;32:e151. doi: 10.1093/nar/gnh148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubits RM, Hazelton JL, Simontov R. Variations in c-fos gene expression during rat brain development. Brain Res. 1988;427:197–201. doi: 10.1016/0169-328x(88)90067-8. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotopic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, et al. Defining the CREB regulon: A genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Li Y-W, Dampney RAL. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61:613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc. 2006;1:179–185. doi: 10.1038/nprot.2006.27. [DOI] [PubMed] [Google Scholar]
- O’Neill LP, VerMilyea MD, Turner BM. Epigenitic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat Genet. 2006;38:835. doi: 10.1038/ng1820. [DOI] [PubMed] [Google Scholar]
- Papavassiliou AG. The CREB/ATF family of transcription factors: modulation by reversible phosphorylation. Anticancer Res. 1994;14:1801–1805. [PubMed] [Google Scholar]
- Runkel L, Shaw PE, Herrera RE, Hipskind RA, Nordheim A. Multiple basal promoter elements determine the level of human c-fos transcription. Mol Cell Biol. 1991;11(3):1270–1280. doi: 10.1128/mcb.11.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver BM, Staddon GE, Mapleson WW. Tissue/blood and tissue/water partition coefficients for propofol in sheep. Br J Anaesth. 2001;86:693–703. doi: 10.1093/bja/86.5.693. [DOI] [PubMed] [Google Scholar]