Abstract
This report describes the design of the eplet version of HLAMatchmaker to determine class II compatibility at the structural level. This matching algorithm is based on the hypothesis, developed from molecular modeling of crystallized antigen-antibody complexes, that functional epitopes are represented by patches of surface-exposed nonself-amino acid residues surrounded by residues within a 3-Å radius. Patch determinations with a molecular viewer of crystalline structural models downloaded from the Entrez Molecular Modeling Database Web site led to the identification of 44 DRB, 33DQB, 29 DQA, 20 DPB, and 9 DPA unique combinations of polymorphic positions. The residue compositions of these patches were then determined from amino acid sequences. This analysis resulted in a repertoire of 146 DRB, 74 DQB, 58 DQA, 45 DPB, and 19 DPA eplets. In many eplets, the residues are in short linear sequences, but many other eplets have discontinuous sequences of residues that cluster on or near the molecular surface. This analysis has also shown that all serologically defined DR and DQ antigens detectable by monospecific antibodies have unique eplets. Other eplets are present in groups of class II antigens, many of which appear as cross-reacting. The eplet version of HLAMatchmaker should be considered as a hypothetical model for the structural assessment of donor-recipient compatibility and the determination of mismatch acceptability for sensitized patients.
Keywords: HLAMatchmaker, HLA, epitope structure, histocompatibility, eplet
INTRODUCTION
Class II human leukocyte antigens (HLA) play an important role in determining donor-recipient compatibility in solid organ and stem cell transplantation. Class II mismatching elicits strong alloimmune responses that impair transplant success. Preformed donor-specific anti-class II antibodies increase the risk of transplant failure [1–5], and the posttransplant development of anti-class II antibodies is associated with a higher incidence of acute and chronic rejection [6, 7].
Current class II matching strategies in kidney transplantation consider only the serologically defined HLA-DR antigens controlled by the DRB1 locus, although mismatching for HLA-DQ and HLA-DP appears associated with lower graft survivals [8–13] and the development of clinically relevant alloantibodies in transplant recipients [14]. Moreover, molecular typing has revealed a high degree of heterogeneity of HLA-DR antigens. Newer serum screening methods, such as enzyme-linked immunosorbent assay, flow cytometry, and Luminex, have greatly enhanced the detection and specificity analysis of anti-class II antibodies in sensitized patients.
The evaluation of HLA compatibility and the characterization of anti-HLA antibodies require a better understanding of the HLA epitope repertoire. HLAMatchmaker is a structurally based matching program that considers each HLA antigen as a string of epitopes represented by short sequences (originally referred to as triplets) involving polymorphic amino acid residues in antibody-accessible positions [15]. HLAMatchmaker determines which triplets are different between donor and recipient, and this algorithm is clinically useful for matching purposes [16–20] and in determining HLA mismatch acceptability for sensitized patients [21–31]. Although many triplets correspond to serologically defined private and public epitopes, they provide an incomplete description of the HLA epitope repertoire.
A recent study has led to a new version of HLAMatchmaker that considers the hypothesis, developed from molecular modeling of crystallized antigen-antibody complexes, that functional epitopes are represented by patches of surface-exposed nonself-amino acid residues surrounded by residues within a 3-Å radius [32]. These patches are referred to as “eplets,” and many of them are short linear sequences common to triplets, but others have residues in discontinuous sequence positions that cluster together on the molecular surface. The eplet version of HLAMatchmaker therefore considers a more complete repertoire of structurally defined epitopes.
This report describes how eplets are assigned in determining HLA-DR, -DQ, and -DP compatibility at the humoral immune level. This analysis considers 4-digit alleles encoded by not only DRB1 and DQB1, but also DRB3, DRB4, DRB5, DQA1, DPA1, and DPB1, because all of them have antigenic determinants that can induce specific antibodies. This paper will show how eplets correspond to serologically defined class II antigens and how the eplet version of HLAMatchmaker can be used to determine structurally based class II compatibility at the humoral immune level.
METHODS AND RESULTS
Topography of Polymorphic Amino Acid Residues
The construction of the eplet repertoire is based on polymorphic amino acid residues on the HLA molecular surface. Their locations are easily determined with three-dimensional models of class II molecules. The Entrez Molecular Modeling Database of the National Center for Biotechnology Information stores on its Web site (http://www.ncbi.nlm.nih.gov/Structure) an extensive collection of crystallographic structures of HLA molecules that can be viewed with the Cn3D structure and sequence alignment software program [33, 34].
Five DRB structures have been downloaded from the Molecular Modeling Database Web site, all of them have the same monomorphic DRA1*0101 sequence. They are DRB1*0101 (PDB Number 1KG0) [35], DRB1*0301 (1A6A) [36], DRB1*0401 (1D6E) [37], DRB1*1501 (1BX2) [38], and DRB5*0101 (1H15) [39]. There are three models of HLA-DQ heterodimers: DQA1*0501, DQB1*0202 (1S9) [40], DQA1*0301, DQB1*0302(1JK8) [41], and DQA1*0102, DQB1* 0602 (1UVQ) [42].
Cn3D viewing of crystalline HLA-DR and HLA-DQ molecular models shows different patterns of surface expression of polymorphic residues (Figure 1). The structural polymorphisms of HLA-DR are restricted to the β chains. They are readily visible on the top of the molecule adjacent to the bound peptide, and many of them involve contiguous sequences. Polymorphic residues on the side of the molecule generally comprise distinct clusters in both β1 and β2 domains. A few polymorphisms are visible at the bottom part of the molecule that is nearby the cell membrane. DRB and DQB seem to show similar numbers of polymorphic positions. DQA displays somewhat contiguous polymorphic positions on the top of the molecule near the bound peptide and on the side of the α1 domain. The polymorphic positions in the α2 domain seem to be more in distinct clusters.
FIGURE 1.
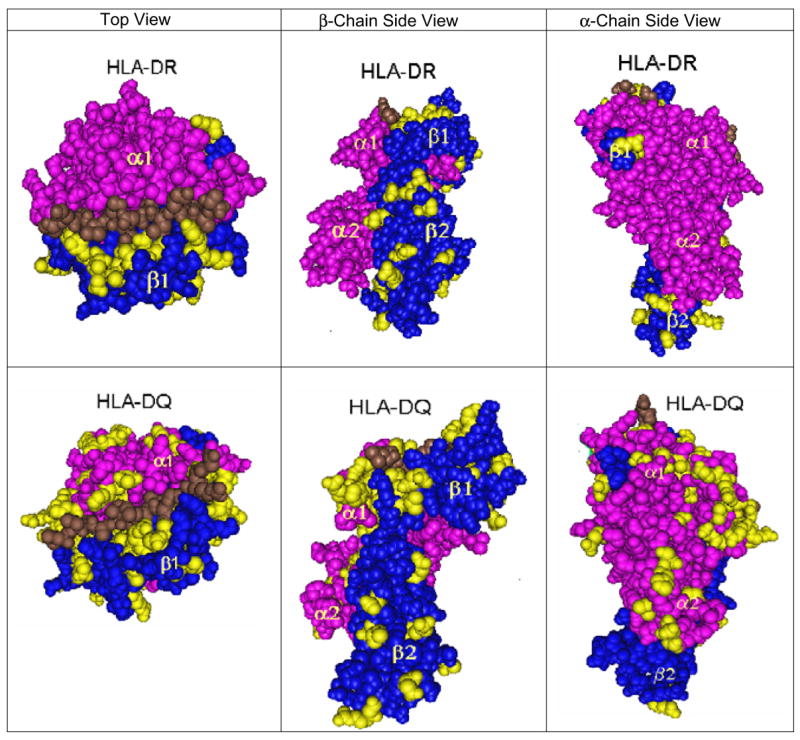
Visualization of polymorphic amino residue positions on HLA-DRB and HLA-DQ molecules. The following crystalline models are shown: DRA1*0101, DRB1*0101 (PDB # 1KG0), and DQA1*0301, DQB1*0302(PDB No. 1JK8). Left = top view; middle = β-chain side view right = α-chain side view.
No crystalline structures of HLA-DP molecules are available in the Molecular Modeling Database. Predicted three-dimensional models for HLA-DP and other DR and DQ class II alleles can be generated by submitting amino acid sequences to the Geno3D online molecular modeling server [43]. This web-based service is located at http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml. The Vector NTI-3D Molecular Viewer software (Invitrogen Life Science Software, Frederick, MD) can visualize the locations of polymorphic amino acid residues and also measure intermolecular distances. These prediction models yield incomplete information about molecular surface expression of polymorphic residues because they deal only with isolated α or β chains without any peptide in the peptide-binding groove.
Determination of Patches on HLA Class II Molecules
Each surface-exposed polymorphic residue represents an essential element of a potential epitope recognized by antibody. The “select by distance” command of the Cn3D molecular viewer has been used to identify residues within a 3-Å radius. A patch defined this way seems to provide the best estimate of the size of a functional epitope [32].
Sequence comparisons of 381 most common DRB1, 3, 4, 5 alleles have identified 49 polymorphic positions on the molecular surface. Cn3D viewing has shown that 13 of them are on the top (i.e., the α-helices) and 26 are on the side of the molecule (Table 1). Six positions have “underside” locations (i.e., underneath the groove) and four are at the “bottom” near the cell membrane; they become more readily visible if the molecule has been turned upside down. These positions seem less antibody-accessible if the HLA antigen is anchored in the cell membrane like in the lymphocytotoxicity test, but they might react with antibody if the HLA molecule is fixed to a different surface like in a solubilized antigen-binding assay. Molecular surface expression of polymorphic residues has been graded as prominent (++), readily visible (+), and somewhat visible (±).
TABLE 1.
Polymorphic and monomorphic residue positions in three-Angstrom patches on HLA-DRB1, -DRB3, -DRB4 and -DRB5 alleles*
| Class II Locus | Sequence position | Molecular location | Surface exposure | 3.0 Angstrom patches | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DRB | 4 | Side | ++ | 3 | 4 | 5 | |||||
| DRB | 6 | Side | + | 5 | 6 | 7 | A15 | A17 | |||
| DRB | 12 | Underside | ± | 11 | 12 | 13 | 29 | ||||
| DRB | 14 | Underside | + | 13 | 14 | 15 | 16 | 27 | 29 | ||
| DRB | 16 | Underside | + | 15 | 16 | 17 | 18 | 25 | |||
| DRB | 18 | Side | + | 17 | 18 | 19 | 23 | ||||
| DRB | 25 | Side | ++ | 16 | 24 | 25 | 26 | 43 | |||
| DRB | 26 | Side | ± | 25 | 26 | 27 | 42 | ||||
| DRB | 31 | Underside | + | 10 | 29 | 30 | 31 | 32 | |||
| DRB | 32 | Underside | + | 31 | 32 | 33 | 35 | ||||
| DRB | 33 | Underside | + | 8 | 32 | 33 | 34 | ||||
| DRB | 34 | Side | ++ | 33 | 34 | 35 | A83 | ||||
| DRB | 40 | Side | + | 28 | 39 | 40 | 41 | ||||
| DRB | 41 | Side | + | 40 | 41 | 42 | 43 | 44 | 45 | ||
| DRB | 44 | Side | + | 41 | 43 | 44 | 45 | ||||
| DRB | 47 | Side | + | 28 | 46 | 47 | 48 | 62 | |||
| DRB | 48 | Side | ++ | 47 | 48 | 49 | |||||
| DRB | 51 | Side | + | 37 | 50 | 51 | 52 | ||||
| DRB | 57 | Top | + | 56 | 57 | 58 | 61 | A76 | P13 | ||
| DRB | 58 | Top | + | 54 | 57 | 58 | 59 | 62 | |||
| DRB | 59 | Top | ++ | 58 | 59 | 60 | |||||
| DRB | 60 | Top | ++ | 59 | 60 | 61 | 63 | ||||
| DRB | 67 | Top | + | 66 | 67 | 68 | 71 | ||||
| DRB | 70 | Top | + | 67 | 69 | 70 | 71 | 73 | P11 | ||
| DRB | 71 | Top | ± | 70 | 71 | 72 | 73 | ||||
| DRB | 73 | Top | + | 69 | 72 | 73 | 74 | 76 | 77 | ||
| DRB | 74 | Top | + | 70 | 71 | 72 | 73 | 74 | 79 | P8 | P9 |
| DRB | 76 | Top | ++ | 73 | 75 | 76 | 77 | ||||
| DRB | 77 | Top | ++ | 73 | 76 | 77 | 78 | P6 | |||
| DRB | 81 | Top | + | 80 | 81 | 82 | 85 | P4 | |||
| DRB | 85 | Top | + | 84 | 85 | 86 | |||||
| DRB | 86 | Side | ± | 82 | 85 | 86 | 87 | 90 | |||
| DRB | 96 | Side | ++ | 95 | 96 | 97 | 180 | ||||
| DRB | 98 | Side | ++ | 97 | 98 | 99 | 120 | ||||
| DRB | 104 | Side | + | 103 | 104 | 105 | 107 | 114 | |||
| DRB | 105 | Side | ++ | 104 | 105 | 106 | 107 | ||||
| DRB | 108 | Side | ++ | 107 | 108 | 109 | |||||
| DRB | 112 | Bottom | ++ | 108 | 111 | 112 | 113 | ||||
| DRB | 120 | Side | + | 98 | 119 | 120 | 121 | ||||
| DRB | 133 | Bottom | ++ | 132 | 133 | 134 | |||||
| DRB | 135 | Bottom | ++ | 134 | 135 | 136 | |||||
| DRB | 140 | Side | ++ | 139 | 140 | 141 | |||||
| DRB | 142 | Side | ++ | 138 | 141 | 142 | 143 | ||||
| DRB | 149 | Side | ++ | 148 | 149 | 150 | |||||
| DRB | 180 | Side | + | 96 | 177 | 179 | 180 | 181 | |||
| DRB | 181 | Side | ++ | 180 | 181 | 182 | |||||
| DRB | 183 | Side | + | 182 | 183 | 184 | |||||
| DRB | 187 | Side | ++ | 186 | 187 | 188 | |||||
| DRB | 189 | Bottom | ++ | 188 | 189 | 190 | |||||
Polymorphic positions are marked in bold, underlined font.
Table 1 lists the sequence positions clustered within a 3-Å radius of each exposed polymorphic position. These patches are combinations of monomorphic and polymorphic positions (marked in bold, underlined font), and they have an average of 4.2 residues. About one third are continuous sequences, and the remaining are discontinuous sequences including several patches (e.g., positions 12, 14, and 16) with residues far removed in sequence. Three patches in positions 6, 34, and 57 have monomorphic DRA residues (prefixed with A), and five α helix patches (positions 57, 70, 74, 77, and 81) include residues of peptides bound to the groove; their positions have the prefix “P.” Exposed peptide residues might contribute to the functional epitope recognized by alloantibody. Several studies have shown the influence of HLA-bound peptides on the reactivity of class I and class II specific antibodies [44 – 47].
Sequence comparisons of 43 DQB1 alleles and Cn3D viewing of DQ molecules have identified patches for 36 polymorphic positions on the DQB chain surface (Table 2), 13 of them are on the top and 18 are on the side of the DQB molecule. There are three underside and two bottom locations. DQB patches have fewer residues than DRB patches (3.8 vs 4.2, p = 0.04 by two-tailed Student’s t-test), and one half of them are continuous sequences. Only one patch (in position 30) has a peptide residue and another patch (position 53) has a monomorphic α-chain residue.
TABLE 2.
Polymorphic and monomorphic residue positions in three-Angstrom patches on HLA-DQB1 alleles*
| Class II Locus | Sequence position | Molecular location | Surface exposure | 3.0 Angstrom patches | |||||
|---|---|---|---|---|---|---|---|---|---|
| DQB | 3 | Side | ++ | 3 | 4 | ||||
| DQB | 14 | Underside | ± | 13 | 14 | 15 | 27 | ||
| DQB | 23 | Side | ++ | 18 | 22 | 23 | 24 | ||
| DQB | 26 | Underside | + | 25 | 26 | 27 | 42 | ||
| DQB | 30 | Side | ± | 29 | 30 | 31 | 37 | 38 | P9 |
| DQB | 45 | Side | ++ | 41 | 44 | 45 | 46 | 72 | |
| DQB | 46 | Side | ++ | 45 | 46 | 47 | |||
| DQB | 47 | Side | + | 46 | 47 | 48 | 62 | ||
| DQB | 49 | Side | ++ | 48 | 49 | 50 | |||
| DQB | 52 | Side | ++ | 51 | 52 | 53 | 54 | ||
| DQB | 53 | Top | ++ | 52 | 53 | 54 | A78 | ||
| DQB | 55 | Top | ++ | 52 | 54 | 55 | 56 | ||
| DQB | 56 | Top | ++ | 55 | 56 | 57 | |||
| DQB | 57 | Top | ± | 56 | 57 | 58 | |||
| DQB | 66 | Top | ++ | 65 | 66 | 67 | |||
| DQB | 67 | Top | + | 64 | 66 | 67 | 68 | 71 | |
| DQB | 70 | Top | ++ | 69 | 70 | 71 | |||
| DQB | 71 | Top | + | 67 | 70 | 71 | 72 | ||
| DQB | 74 | Top | ± | 73 | 74 | 75 | |||
| DQB | 75 | Top | + | 74 | 75 | 76 | 80 | ||
| DQB | 77 | Top | ++ | 73 | 76 | 77 | 78 | 81 | |
| DQB | 84 | Top | + | 83 | 84 | 85 | |||
| DQB | 85 | Top | ++ | 84 | 85 | 86 | 89 | ||
| DQB | 87 | Side | + | 83 | 86 | 87 | 88 | 92 | |
| DQB | 89 | Side | + | 85 | 86 | 88 | 89 | 90 | 92 |
| DQB | 90 | Underside | ± | 86 | 89 | 90 | 91 | ||
| DQB | 116 | Side | + | 102 | 115 | 116 | 117 | ||
| DQB | 125 | Side | + | 124 | 125 | 126 | |||
| DQB | 126 | Side | ++ | 124 | 125 | 126 | 127 | ||
| DQB | 130 | Side | ++ | 129 | 130 | 131 | 174 | ||
| DQB | 135 | Side | ++ | 134 | 135 | 136 | |||
| DQB | 140 | Side | ++ | 139 | 140 | 141 | |||
| DQB | 167 | Bottom | ++ | 166 | 167 | 168 | 190 | ||
| DQB | 168 | Bottom | + | 167 | 168 | 169 | |||
| DQB | 182 | Side | ++ | 181 | 182 | 183 | |||
| DQB | 185 | Side | ++ | 184 | 185 | 186 | |||
Polymorphic positions are marked in bold, underlined font.
The 21 DQA1 alleles have almost the same number of polymorphic surface positions as the 43 DQB1 alleles, namely 37 including 12 on the top and 20 on the side of the molecule (Table 3). DQA patches have an average of 4.1 residues. One contains a peptide residue and two have monomorphic β-chain residues.
TABLE 3.
Polymorphic and monomorphic residue positions in three-Angstrom patches on HLA-DQA1 alleles*
| Class II Locus | Sequence position | Molecular location | Surface exposure | 3.0 Angstrom patches | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DQA | 2 | Side | ++ | 1 | 2 | 3 | ||||
| DQA | 18 | Side | ++ | 17 | 18 | 19 | B7 | |||
| DQA | 21 | Side | + | 20 | 21 | 22 | ||||
| DQA | 25 | Side | ± | 14 | 24 | 25 | 26 | 27 | 37 | P5 |
| DQA | 40 | Side | + | 39 | 40 | 41 | ||||
| DQA | 41 | Side | ++ | 38 | 40 | 41 | 42 | |||
| DQA | 44 | Side | ++ | 43 | 44 | 45 | ||||
| DQA | 45 | Side | + | 36 | 44 | 45 | 46 | |||
| DQA | 47 | Side | + | 34 | 46 | 47 | 48 | |||
| DQA | 48 | Side | + | 47 | 48 | 49 | 51 | |||
| DQA | 50 | Side | ++ | 49 | 50 | 51 | ||||
| DQA | 51 | Underside | + | 48 | 50 | 51 | 52 | 53 | ||
| DQA | 52 | Side | + | 49 | 51 | 52 | 53 | |||
| DQA | 53 | Top | ++ | 51 | 52 | 53 | 54 | |||
| DQA | 54 | Top | ++ | 51 | 53 | 54 | 55 | |||
| DQA | 55 | Top | + | 54 | 55 | 56 | ||||
| DQA | 56 | Top | ++ | 53 | 55 | 56 | 57 | |||
| DQA | 59 | Top | + | 58 | 59 | 60 | 61 | 62 | ||
| DQA | 61 | Top | ++ | 58 | 59 | 60 | 61 | 62 | 65 | |
| DQA | 64 | Top | ++ | 60 | 63 | 64 | 65 | |||
| DQA | 66 | Top | + | 62 | 65 | 66 | 67 | 70 | ||
| DQA | 69 | Top | ± | 65 | 68 | 69 | 70 | |||
| DQA | 75 | Top | ++ | 74 | 75 | 76 | 79 | |||
| DQA | 76 | Top | ± | 72 | 75 | 76 | 77 | 80 | ||
| DQA | 79 | Top | ++ | 75 | 78 | 79 | 80 | B32 | ||
| DQA | 80 | Side | ± | 75 | 79 | 80 | 81 | |||
| DQA | 107 | Side | + | 97 | 106 | 107 | 108 | |||
| DQA | 129 | Side | ++ | 126 | 128 | 129 | 130 | |||
| DQA | 130 | Side | + | 129 | 130 | 131 | ||||
| DQA | 138 | Side | + | 137 | 138 | 139 | 141 | |||
| DQA | 139 | Side | + | 33 | 138 | 139 | 140 | 141 | ||
| DQA | 153 | Side | + | 136 | 152 | 153 | 154 | B150 | ||
| DQA | 156 | Bottom | + | 106 | 155 | 156 | 157 | |||
| DQA | 160 | Bottom | + | 159 | 160 | 161 | ||||
| DQA | 161 | Bottom | ++ | 160 | 161 | 162 | ||||
| DQA | 163 | Bottom | + | 127 | 162 | 163 | 164 | |||
| DQA | 175 | Side | ++ | 174 | 175 | 176 | ||||
Polymorphic positions are marked in bold, underlined font.
The determination of patches on DP molecules was more difficult because no crystalline structures are available for DP, and the Vector NTI-3D Molecular Viewer can visualize predicted structures of only single DP chains rather the whole molecule with a peptide inserted. Therefore, we included Cn3D viewing of DR and DQ molecules and considered positions equivalent to the polymorphic positions on DP sequences. These positions were identified with the Basic Local Alignment Search Tool (BLAST) program [48] available on the National Center for Biotechnology Information Web site http://www.ncbi.nlm.nih.gov/BLAST.
This patch analysis was limited to the first domain (positions 1–91) of 95 DPB alleles. Insufficient sequence information of the second DPB domain precluded any patch determination. The number of patches is lower for DPB than for DRB and DQB: 19 versus 32 and 26 patches in the first domains of DRB and DQB, respectively (Table 4). The DPB patches have an average of 3.9 residues. DPA1 alleles (n = 13) have fewer patches than DQA1, 11 versus 37. DPA patches have an average of 4.4 residues (Table 4).
TABLE 4.
Polymorphic and monomorphic residue positions in three-Angstrom patches on HLA-DPB1 and HLA-DPA1 alleles*
| Class II Locus | Sequence position | Molecular location | Surface exposure | 3.0 Angstrom patches | |||||
|---|---|---|---|---|---|---|---|---|---|
| DPB | 8 | Underside | ± | 7 | 8 | 9 | 31 | ||
| DPB | 11 | Side | ± | 10 | 11 | 12 | A11 | ||
| DPB | 28 | Side | ± | 26 | 27 | 28 | 29 | 36 | P8 |
| DPB | 33 | Underside | ++ | 30 | 32 | 33 | 34 | ||
| DPB | 35 | Underside | + | 7 | 34 | 35 | 36 | 48 | |
| DPB | 36 | Underside | ± | 28 | 35 | 36 | 37 | ||
| DPB | 43 | Side | + | 39 | 42 | 43 | 44 | 70 | |
| DPB | 56 | Side | + | 52 | 55 | 56 | 57 | 60 | |
| DPB | 57 | Side | ++ | 56 | 57 | 58 | |||
| DPB | 64 | Top | + | 63 | 64 | 65 | |||
| DPB | 65 | Top | + | 64 | 65 | 66 | 69 | ||
| DPB | 69 | Top | + | 65 | 68 | 69 | 70 | ||
| DPB | 70 | Top | ++ | 43 | 66 | 69 | 70 | 71 | |
| DPB | 76 | Top | + | 72 | 75 | 76 | 77 | ||
| DPB | 84 | Side | + | 83 | 84 | 85 | 88 | ||
| DPB | 85 | Side | ++ | 81 | 84 | 85 | 86 | ||
| DPB | 86 | Side | + | 85 | 86 | 87 | |||
| DPB | 87 | Side | ++ | 86 | 87 | 88 | |||
| DPB | 91 | Side | ++ | 90 | 91 | 92 | |||
| DPA | 18 | Side | + | 14 | 16 | 17 | 18 | 67 | |
| DPA | 28 | Underside | + | 25 | 26 | 27 | 28 | 29 | |
| DPA | 31 | Underside | + | 20 | 30 | 31 | 32 | ||
| DPA | 50 | Top | ++ | 49 | 50 | 51 | |||
| DPA | 51 | Top | + | 47 | 50 | 51 | 52 | ||
| DPA | 72 | Top | + | 68 | 71 | 71 | 73 | 75 | 76 |
| DPA | 73 | Top | + | 72 | 73 | 74 | 75 | 76 | 77 |
| DPA | 83 | Side | + | 82 | 83 | 84 | B37 | ||
| DPA | 111 | Side | ++ | 86 | 110 | 111 | 112 | ||
| DPA | 127 | Side | + | 121 | 126 | 127 | 128 | ||
| DPA | 160 | Bottom | + | 124 | 159 | 160 | 161 | ||
Polymorphic positions are marked in bold, underlined font.
These findings on HLA-DR, DQ, DP patches are comparable in size and location to those reported for HLA-A, B, C patches [32]. The polymorphic positions determine residue variability within each patch. Class II patches have between 1 and 4 polymorphic positions. A few patches have the same polymorphic positions although there are differences between the monomorphic positions. For instance, the DRB patches in positions 85 and 86 (see Table 1) have the same two polymorphic positions but different monomorphic positions. Such patches are considered equivalent.
The patch information in Tables 1–4 yielded the following numbers of unique combinations of polymorphic positions: DRB: 46, DQB: 33, DQA: 29, DPB: 20, and DPA: 9; they are considered the positional basis for the class II HLA epitope repertoire. The residue compositions of these polymorphic patches were determined from amino acid sequences retrieved from the IMGT/HLA online database [49] with a Microsoft Excel macro (called HLA Patch Generator) developed by Grzegorz Dudek (Czestchowa University of Technology, Poland).
Assignments of Class II Eplets
DRB
An analysis of the most common four-digit DRB alleles (122 DRB1 and 18 DRB3, 4, 5 alleles) has yielded a total of 222 patches with different combinations of polymorphic residues, 91 of them are on the top of the molecule, and most of them have overlapping residues. The remaining 132 polymorphic patches are largely on the side of the molecule and include 58 at underside or bottom locations.
Further comparisons have shown that certain overlapping polymorphic patches can be grouped together because they belong to a single allele or a distinct group of alleles including cross-reacting antigens. The term eplet is used to describe a given patch or an overlapping group of patches.
For example, DRBI*1501, DRB1*1502, DRB1*1503 and DRB5*0202 share the same three overlapping patches: 70IQAA, 71QAA, and 74QAAA. They are collectively referred to as one eplet assigned as 71QAA. In other cases, an eplet represents a single polymorphic patch. For instance, the 98QS patch shared between DRB3*0101, DRB3*0201, DRB3*0202, and DRB3*0301 has been assigned as the 98QS eplet. This eplet corresponds to the serologically defined DR52 determinant.
Several polymorphic patches are shared between either all DRB1 alleles or all DBB3, 4, 5 alleles, and they cannot be considered immunogenic. For example, there are two polymorphic patches in position 108. One is 100T found on DRB5 molecules, and specific antibodies would react with the serologically defined DR51, which corresponds to DRB5. The other one is 108P present on all DRB1, DRB3, and DRB4 molecules. This patch cannot induce alloantibodies because it is always a self-sequence. The patches in position 34 represent another example: 34HQ is shared between all DR4 alleles and 34NQ is shared between all DRB3, DRB4, and DRB5 alleles plus all DRB1 alleles that are not DR4. Thus, 34NQ must be considered as a self-sequence and cannot be immunogenic. This analysis has yielded 17 self-DRB patches, and they have been deleted from the eplet repertoire.
This analysis yielded a total of 146 DRB eplets, 52 of them are on the α helices on the top of the molecule. There are 59 eplets on the side surface, including 8 at the bottom. A total of 38 eplets are beneath the peptide-binding groove and they cluster in two underside locations, namely positions 12, 14, and 16 (n = 19) and positions 31, 32, and 33 (n = 19). Eplets in bottom and underside locations seem less antibody accessible if the HLA molecule is bound to the cell membrane.
Table 5 shows serologically defined DR antigens that have one or more corresponding eplets. These antigens can be readily identified with monospecific allosera and/or monoclonal antibodies as demonstrated during the 1984–1997 International Histocompatibility Workshops [50–54]. Seven serologically defined DR antigens, namely DR5, DR6, DR13, DR14, DR15, DR16, DR17, and DR18, do not have corresponding eplets. None of them except DR15 and DR17 can be identified with monospecific antibodies; their serologic determination is based on reactivity patterns of antibodies specific for epitopes shared between different groups of antigens. For instance, serologic assignments of the serologic DR6 splits DR13 and DR14 can be deduced from antibodies reactive with different groups, such as DR2+6, DR3+6, DR3+5+6, DR5+13, DR3+8+13, and antigens, such as DR8 and DR11 [55]. The reaction patterns of these antibodies are often too complex for reliable serologic assignments of individual DR13 and DR14 alleles.
TABLE 5.
Serologically defined HLA-DR antigens with uniquely corresponding eplets
| DR Antgen | Unique eplets | ||||||
|---|---|---|---|---|---|---|---|
| DRB1 | |||||||
| DR1 | 12LKF | 31QCIY | |||||
| DR2 | 12PKR | 133L | 142M | ||||
| DR3 | 73GRDN | ||||||
| DR4 | 12VKH | 34HQ | 96YL | 180LT | |||
| DR7 | 14YKH | 25HQF | 31QLFY | 71DRE | |||
| DR8 | 25YRF | 73ALDT | |||||
| DR9 | 12DKF | 70FRRA | |||||
| DR10 | 12VKF | 31ERVH | 40EYD | 74RRAA | |||
| DR11 | 57DE | ||||||
| DR12 | 25YRL | 31YHFH | 47EFR | ||||
| DRB3,4,5 | |||||||
| DRB3 (DR52) | 98QS | ||||||
| DRB3*01 (DR52a) | 12RKS | 183A | |||||
| DRB3*02 (DR52b) | 31LHFH | 51R | |||||
| DRB4 (DR53) | 25HWN | 44NL | 48YQ | 81YV | 96QM | 180MM | 187Q |
| DRB5 (DR51) | 12DKY | 104AR | 108T | ||||
| DRB5*01 (DR51a) | 31QDIY | ||||||
| DRB5*02 (DR51b) | 6C | ||||||
The DR2 split DR15 and the DR3 split DR17 can be defined serologically by monospecific antibodies. It is possible that the DR15 and DR17 antigens correspond to a pair of eplets whereby one is the specific recognition site and the other functions as a critical contact site for antibody. As described elsewhere [32, 56], certain HLA antibodies react with epitopes represented by combinations of two eplets separated 6–15 Å from each other. For DR17, the most likely pair of eplets appears to be 73GRDN, present on all DR3 molecules, and 48FR, present on DR17, DR11, DR13, and DR15. These eplets are about 9.5 Å apart. Similarly, the DR15 specificity might be represented by the combination of 71QAA (on DR15 and DRB5*0202) with 40DFD (on DR4, DR8, DR11, DR13, DR14, DR15, DR16, DR17, and DRB3*01) or 48FR (on DR17, DR11, DR13, and DR15).
Table 5 also lists unique eplets that correspond to the serologically defined antigens encoded by the DRB3, DRB4, and DRB5 loci. DR53 has a relatively large number of unique eplets in nonoverlapping positions. There are also unique eplets that distinguish DRB3*01 from DRB3*02 alleles. These eplets appear to correspond to the DR52 subtypes serologically defined as DR52a and DR52b [57, 58]. Similarly, the 31QDIY and 6C eplets can differentiate between DRB5*01 and DRB5*02.
DQB1
An HLA Patch Generator analysis of 43 DQB1 alleles has yielded 106 DQB1 patches with different combinations of polymorphic residues, 48 of them are on the top and 46 are on the side of the molecule; 12 are in underside or bottom locations.
As shown for DRB, we have assigned eplets from groups of overlapping DQB1 patches shared between combinations of DQB1 alleles. For instance, the 55RPL eplet represents the combination of overlapping 55RPL, 56 RLD, and 57LD patches on DQB1*0401 and DQB1*0402.
This analysis has resulted in a repertoire of 74 distinct DQB1 eplets. Table 6 shows which eplets correspond to DQ1-DQ7 specificities; all of them can be defined serologically with monospecific antibodies. The DQ3 subtype DQ8 has a unique 56PPA eplet that is structurally similar to the 55PPP eplets that correspond to DQ3. There is no unique eplet for the DQ3 subtype DQ9, and this is consistent with the experience that there are no monospecific antibodies against DQ9 [54].
TABLE 6.
Serologically defined DQ antigens and 2-digit DQA1 alleles with their corresponding eplets
| DQ locus | Unique eplets | |||||
|---|---|---|---|---|---|---|
| DQ Antigen | ||||||
| DQ1 | 52PQ | |||||
| DQ5 | 30HYV | 71VGA | 87AY | 116I | ||
| DQ6 | 87AF | 125GQ | ||||
| DQ2 | 30SIV | 45GE | 52LL | 70RK | ||
| DQ3 | 55PPP | |||||
| DQ7 | 45EV | |||||
| DQ8 | 56PPA | |||||
| DQ4 | 55PRL | |||||
| DQA1 Allele | ||||||
| DQA1*01 | 18F | 48WF | 56KGG | 69A | 76IM | 175Q |
| DQA1*02 | 47EKL | 52FHR | ||||
| DQA1*03 | 25YS | 47EQL | 52FRR | 75IVR | ||
| DQA1*05 | 76SL | 107I | 156L | 163S | 175K | |
DQA1
Complete sequence information (positions 6–180) is available for 18 DQA1 alleles. HLA Patch Generator identified 91 DQA patches with different polymorphic residue combinations, 38 are on the top and 41 are on the side of the molecule. There are 12 polymorphic patches on underside and bottom locations. Similar to DRB and DQB, eplets have been assigned to describe single polymorphic patches and overlapping patches that belong to the same group of DQA1 alleles. Altogether, there are 58 distinct DQA1 eplets.
Although DQA1 polymorphisms have never been defined serologically, several two-digit DQA1 alleles have multiple unique eplets (Table 6). Especially, DQA1*01 can be distinguished with seven nonoverlapping eplets. No unique eplets have been identified for DQA1*04 and DQA1*06.
DPA and DPB
An HLA Patch Generator analysis of DPB1*01 through DPB1*99 has generated 62 polymorphic patches, 31 of them are on the top and 23 are on the side of the DPB1 chain. Further comparisons of overlapping patches led to the assignment of a total of 45 DPB eplets, 15 of which are on one or few DPB1 alleles all of which appear to have low frequencies. The identification of DPB eplets is confined to the first domain (positions 8–91), because insufficient sequence information is available for the second domain of DPB chains.
The 13 DPA1 alleles have only nine polymorphic positions, and an HLA Patch Generator analysis has led to the assignment of 19 eplets, 5 of which are on the top and 10 are on the side of the DPA chain. DQA1*02 has a unique 111R, and DQA1*04 has two unique eplets, 18T and 73IA. There are no unique eplets for DQA1*01 and DQA1*03.
Although no DPA1 or DPB1 allele-specific antibodies have been identified [59], there are several murine and human monoclonal antibodies that recognize DP epitopes that appear to eplets, such as 31M and 51RA on DPA1, and 56ED and 85DEA on DPB chains [59].
Example of Eplet-Based Determination of Class II Compatibility
Table 7 represents an illustration of eplet mismatches for HLA-DR and HLA-DQ. This analysis was done for the phenotype DRB1*1101, 1501; DRB3*0202; DRB5*0101; DQA1*0102, 0501; DQB1*0301, 0602, which is serologically equivalent to DR11,15; DR51,52; DQ6,7. The incompatible DR antigens in the left columns of Table 7 show varying numbers of mismatched eplets. The highest range of 9–12 eplet mismatches occurs with DR7, DR9, DR10, DR12, and DR14. The lowest range of 1–3 eplet mismatches is seen for DR3, DR13, and DR16. These low numbers are not surprising because DR3 and DR13 cross-react with DR11 since they share several epitopes that correspond to eplets. Moreover, DR15 and DR16 are serologic splits of DR2, whereas DRB1*1601 and DRB1*1602 have one and three mismatched eplets, respectively. It should be noted however, that DR12—which together with DR11 is a split of DR5—has nine mismatched eplets.
TABLE 7.
Example of an HLAMatchmaker Analysis to Determine HLA-DR and HLA-DQ Compatibility at the Eplet Level
| Mismatched Antigen | Number of mm Eplets | Mismatched Eplets | Same 2-Digit Allele | Number of mm Eplets | Mismatched Eplets |
|---|---|---|---|---|---|
| DRB1*0101 (DR1) | 8 | 12LKF, 14FEH, 25HRL, 26RL, 31QCIY, 67LR, 71QRA, 74QRAA | DRB1*1102 | 2 | 67IE, 71DEA |
| DRB1*0301 (DR3) | 3 | 25HRY, 31YYFH, 73GRDN | DRB1*1103 | 2 | 67IE, 71DEA |
| DRB1*0401 (DR4) | 6 | 12VKH, 34HQ, 47DYR, 71QKA, 96YL, 180LT | DRB1*1104 | 0 | |
| DRB1*0701 (DR7) | 9 | 4Q, 14YKH, 25HQF, 31QLFY, 59AES, 67IR, 71DRG, 98ES, 180VM | DRB1*1105 | 2 | 12STG, 14GEH |
| DRB1*0802 (DR8) | 5 | 12STG, 14GEY, 25YRF, 47DYR, 73ALDT | DRB1*1106 | 1 | 81HA |
| DRB1*0901 (DR9) | 12 | 4Q, 12DKF, 14FEH, 25HRY, 31QGIY, 59AES, 70FRRA, 71RRA, 73AEDT, 74RRAE, 98ES, 180VM | |||
| DRB1*1001 (DR10) | 11 | 12VKF, 14FEH, 25HRL, 26RL, 31ERVH, 40EYD, 67LR, 70LRRA, 71RRA, 74RRAA, 180VM | DRB1*1502 | 0 | |
| DRB1*1201 (DR12) | 10 | 12STG, 14GEY, 25YRL, 26RL, 31YHFH, 47EFR, 59AES, 67IR, 70IDRA, 81HA | DRB1*1503 | 1 | 31QHFY |
| DRB1*1301 (DR13) | 3 | 31YYFH, 67IE, 71DEA | DRB1*1504 | 1 | 67FA |
| DRB1*1401 (DR14) | 9 | 31 YYFH, 47DYR, 57AA, 67LR, 70LRRA, 71RRA, 73AEDT, 74RRAE, 112Y | |||
| DRB1*1601 (DR16) | 1 | 47DYR | |||
| DRB1*1602 (DR16) | 3 | 47DYR, 67LR, 70LDRA | |||
| DRB3*0101 (DR52a) | 7 | 12RKS, 25HRY, 31LYFH, 47DYR, 59AES, 73GRDN, 183A | DRB3*0201 | 0 | |
| DRB3*0301 (DR52c) | 2 | 31LYFH, 59AES | DRB3*0203 | 0 | |
| DRB4*0101 (DR53) | 16 | 4Q, 16HLW, 25HWN, 31QYIY, 40IYN, 44NL, 48YQ, 67LR, 70LRRA, 71RRA, 73AEDT, 74RRAE, 81YV, 96QM, 180MM, 187Q | DRB3*0204 | 1 | 73GRDN |
| DRB5*0202 (DR51b) | 3 | 6C, 31QGIY, 81HA | DRB5*0102
DRB5*0103 DRB5*0104 DRB5*0105 |
1
2 1 0 |
31QGIY
31QGIY, 67FT 73ALDT |
| DQB1*0201 (DQ2) | 8 | 30SIV, 45GE, 52LL, 56LPA, 57PA, 66DI, 70RK, 77DR | DQB1*0302 (DQ8) | 3 | 56PPA, 57PA, 185I |
| DQB1*0401 (DQ4) | 7 | 23L, 26G, 55PRL, 66DI, 70ED, 74SV, 185I | DQB1*0303 (DQ9) | 1 | 185I |
| DQB1*0501 (DQ5) | 10 | 14GL, 26G, 30HYV, 57PV, 70GA, 74SV, 77DR, 87AY, 116I, 125SQ | DQB1*0304
DQB1*0305 DQB1*0601 DQB1*0603 DQB1*0604 DQB1*0609 |
2
4 4 1 4 3 |
56PPA, 57PA
26G, 56PPA, 57PA, 185I 3P, 30YDV, 66DI, 67DIT 30HYA 30HYA, 57PV, 87GY, 130Q 57PV, 87GY, 130Q |
| DQA1*0201 | 7 | 25FT, 47EKL, 48LF, 52FHR, 76IL, 79IRS, 175E | DQA1*0101 | 1 | 47ERW |
| DQA1*0301 | 7 | 25YS, 47EQL, 48LF, 52FRR, 75IVR, 79IRS, 175E | DQA1*0103 | 3 | 25FT, 40EK, 129HA |
| DQA1*0401 | 4 | 69T, 76IL, 79IRS, 175E | DQA1*0104 | 2 | 2G, 47ERW |
| DQA1*0601 | 5 | 25FT, 69T, 76IL, 79IRS, 175E | DQA1*0502
DQA1*0503 |
0
1 |
160SE |
Recipient Type: DRB1*1101, 1501; DRB3*0202; DRB5*0101; DQA1*0102, 0501; DQB1*0301, 0602
As expected, all eplets that are unique for the antigens listed in Table 7 are mismatches for this phenotype. It may also be seen that many mismatched eplets have overlapping sequences. For instance, DR1 has this pair of overlapping eplets: 12LKF, which is unique on DR1 and 14FEH, which is present on DR1, DR9, and DR10. They may represent distinct epitopes because specifically reactive antibodies have been identified [54]. On the other hand, it is also possible that the combination of 12LKF and 14FEH comprises a single structural determinant that could induce an antibody that reacts with only DR1.
The right columns of Table 7 list examples of eplet mismatches for class II alleles with the same two-digit types as in the above phenotype. DRB1*1104 and DRB1*1502 are zero-eplet mismatches. The other DRB1*11 and DRB1*15 alleles have one or two—but not necessarily the same—mismatched eplets.
Antigens controlled by the DRB3, 4, 5 loci have varying numbers of mismatched eplets. DRB4*0401 (DR53) has the most (n = 16), and this is partially due to the large number of eplets unique to DR53 (Table 5). It should be noted that the other two-digit DRB3 and DRB5 alleles have mismatched eplets. Especially DRB3*0101, which corresponds to the serologically defined DR52a specificity [57, 58], has seven mismatched eplets, including two that are unique for this allele. These eplet differences are clinically relevant because our experience has shown several cases whereby a DRB3*0202- or DR52b-positive patient makes antibodies reactive with DRB3*0101 or DR52a (unpublished data). Good matches can be present for DRB3 and DRB5 alleles with the same two-digit types. For instance, DRB3*0201, DRB3*0203, and DRB5*0105 are zero-eplet mismatches, and others have one or two mismatched eplets.
DQ antigens seem structurally more mismatched than DRB antigens because they have two polymorphic chains: DQB antigens have 7–10 mismatched eplets and DQA alleles have 4–7 mismatched eplets (Table 7). Although DQ5 (DQB1*0501) and DQ6 (DQB1*0602) are serologic splits of DQ1, there is a high degree of structural incompatibility of DQ5 as indicated by 10 mismatched eplets. The phenotype of this example contains the DQ3 split DQ7 (DQB1*0301). With one mismatched eplet, the DQ3 split DQ9 (DQB1*0303) mismatches seems structurally the most compatible among the DQB1*03 mismatches. DQB1*0603, DQA1*0101, DQA1*0502, and DQA1*0503 are zero or one eplet mismatches.
DISCUSSION
This report describes the design of the eplet-based class II version of HLAMatchmaker and how this algorithm can be used to determine structural compatibility for antigens encoded by HLA-DR, HLA-DQ, and HLA-DP. For each of these loci, an eplet repertoire has been developed from patches of residues within a 3-Å radius of each polymorphic residue exposed on the molecular surface. In many eplets, the residues are in short linear sequences, but many other eplets have discontinuous sequences of residues that cluster on or near the molecular surface. This analysis has identified eplets that correspond to serologically defined DR and DQ antigens recognized by monospecific antibodies. Other eplets are present on groups of class II antigens, many of which are considered cross-reacting.
The class II version of HLAMatchmaker considers all polymorphisms in the HLA-D regions that lead to alloantibody responses, and they should be defined by DNA-typing methods, preferably at the four-digit allelic level. Most clinical laboratories type for DRB and DQB antigens and other class II alleles are not considered. Interestingly, DQA1 alleles can be putatively assigned to a given phenotype on the basis of common DRB1-DQB1-DQA1 haplotypes reported in various ethnic groups [60–62]. HLA-DP typing is almost never done in the clinical setting, although there is evidence that HLA-DP matching affects kidney transplant survival [12, 13] and that transplant patients can produce anti-HLA-DP antibodies [14, 63–65]. Such antibodies are specific for epitopes defined by short sequences and shared between groups of DP alleles [59, 64].
For the time being, the clinical use of HLAMatchmaker will primarily focus on determining structural compatibility for the HLA-DR and HLA-DQ loci. As illustrated in Table 7, certain antigens have many more mismatched eplets than others, and this might correlate to their ability of inducing specific antibodies. Applying the original HLAMatchmaker program, Dankers et al. have demonstrated that the frequency of HLA class I antibody production during pregnancy and after kidney transplantation correlates with the number of mismatched triplets on exposed HLA-A and HLA-B antigens [23]. No data are available on such correlations for mismatched class II eplet numbers.
This study has also generated information about the location, the surface expression, and the amino acid composition of each eplet. These factors undoubtedly play an important role in the immunogenicity of an eplet, i.e., its ability to induce a specific antibody response. Recent studies have shown considerable differences in eplet (or triplet) immunogenicity [21, 28, 66]. Highly immunogenic eplets will increase sensitization and the risk for antibody-mediated rejection. An international collaborative study is underway to determine class I and class II eplet immunogenicity in kidney transplant patients [66].
Eplet versions of HLAMatchmaker for antibody analysis are slightly different from the matching programs because they incorporate the notion that antibody reactivity patterns cannot determine the differential recognition of individual eplets within a group of eplets unique for a given antigen (see Tables 5 and 6) or a combination of alleles. In such cases, the program uses a single eplet that represents a group of eplets. HLA typing differences between antibody producer and immunizer will define the mismatched eplet repertoire, and this information facilitates the interpretation of antibody reactivity patterns with HLA panels. The identification of reactive and nonreactive eplets permits a determination of HLA mismatch acceptability for sensitized patients.
At present, the eplet version of HLAMatchmaker should be considered as a hypothetical model for determining structural HLA compatibility and mismatch acceptability. Its validation depends on the outcome of clinical data.
The eplet versions of HLAMatchmaker and the HLA Patch Generator can be downloaded from the website http://tpis.upmc.edu. There is also an Eplets and Patches file that shows how eplets have been assigned from polymorphic patches.
Acknowledgments
This study is supported by grant AI-55933 from the National Institutes of Health.
ABBREVIATION
- HLA
human leukocyte antigens
References
- 1.Schoenemann C, Groth J, Leverenz S, May G. HLA class I and class II antibodies: monitoring before and after kidney transplantation. Transplantation. 1998;65:1519. doi: 10.1097/00007890-199806150-00024. [DOI] [PubMed] [Google Scholar]
- 2.Mahoney RJ, Taranto S, Edwards E. B-Cell Crossmatching and kidney allograft outcome in 9031 United States transplant recipients. Hum Immunol. 2002;63:324. doi: 10.1016/s0198-8859(02)00363-4. [DOI] [PubMed] [Google Scholar]
- 3.Gebel H, Bray R, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk Amer J Transplant. 2003;3:1488. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 4.Iniotaki-Theodoraki AG, Boletis JN, Trigas G, Kalogeropoulou HG, Kostakis AG, Stavropoulos-Giokas CG. Humoral immune reactivity against human leukocyte antigen (HLA)-DQ graft molecules in the early posttransplantation period. Transplantation. 2003;75:1601. doi: 10.1097/01.TP.0000061611.51612.09. [DOI] [PubMed] [Google Scholar]
- 5.Itescu S, Tung TC, Burke EM, Weinberg A, Moazami N, Artrip JH, Suciu-Foca N, Rose EA, Oz MC, Michler RE. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation. 1998;98:786. doi: 10.1161/01.cir.98.8.786. [DOI] [PubMed] [Google Scholar]
- 6.McKenna RM, Takemoto S, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Terasaki PI. Humoral theory of transplantation. Amer J Transplant. 2003;3:665. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 8.Duquesnoy RJ, Annen K, Marrari M, Kauffman HM., Jr Association of MB compatibility with successful intrafamilial kidney transplantation. N Engl J Med. 1980;302:821. doi: 10.1056/NEJM198004103021501. [DOI] [PubMed] [Google Scholar]
- 9.Matsuno N, Hidetoshi I, Ando ATN, Sato T, Ichikawa S, Sonoda T, Tsuji K. Importance of DQB as indicator in living related kidney transplant. Transplantation. 1990;49:208. doi: 10.1097/00007890-199001000-00046. [DOI] [PubMed] [Google Scholar]
- 10.Tong JY, Hsia S, Parris GL, Nghiem DD, Cottington EM, Rudert WA, Trucco M. Molecular compatibility and renal graft survival–the HLA DQB1 genotyping. Transplantation. 1993;55:390. doi: 10.1097/00007890-199302000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Middleton D, Mytilineos D, Savage D, Ferrara GB, Angelini G, Ameroso A, Trainor F, Gaweco A, Mazzola G, Delfino L, Berrino M, Oplez G. Matching for HLA-DPB1 alleles in zero mismatched HLA-A, -B and -DR Renal Transplants. Transpl Proc. 1992;24:2439. [PubMed] [Google Scholar]
- 12.Mytilineos J, Deufel A, Opelz G. Clinical relevance of HLA-DPB locus matching for cadaver kidney retransplants: a report of the Collaborative Transplant Study. Transplantation. 1997;63:1351. doi: 10.1097/00007890-199705150-00025. [DOI] [PubMed] [Google Scholar]
- 13.Laux G, Mansmann U, Deufel A, Opelz G. A new epitope-based HLA-DP matching approach for cadaveric kidney transplantation. Transplantation. 2003;75:1527. doi: 10.1097/01.TP.0000061759.57702.8A. [DOI] [PubMed] [Google Scholar]
- 14.Arnold M-L, Pei R, Spriewald B, Wassmuth R. Anti-HLA class II antibodies in kidney retransplant patients. Tissue Antigens. 2005;65:370. doi: 10.1111/j.1399-0039.2005.00382.x. [DOI] [PubMed] [Google Scholar]
- 15.Duquesnoy RJ. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination. I Description of the algorithm. Hum Immunol. 2002;63:339. doi: 10.1016/s0198-8859(02)00382-8. [DOI] [PubMed] [Google Scholar]
- 16.Duquesnoy RJ, Takemoto S, De Lange P, Doxiadis IIN, Schreuder GMT, Claas FHJ. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination III. Effect of matching at the HLA-A,B amino acid triplet level on kidney transplant survival. Transplantation. 2003;75:884. doi: 10.1097/01.TP.0000055101.20821.AC. [DOI] [PubMed] [Google Scholar]
- 17.Boehringer D, Reinhard T, Duquesnoy R, Boehringer S, Enczmann J, de Lange P, Claas F, Sundmacher R. Beneficial effect of matching at the HLA-A and B amino-acid triplet level on rejection free survival in penetrating keratoplasty. Transplantation. 2004;77:417. doi: 10.1097/01.TP.0000110415.10401.94. [DOI] [PubMed] [Google Scholar]
- 18.Laux G, Mytilineos J, Opelz G. Critical evaluation of the amino acid triplet-epitope matching concept in cadaver kidney transplantation. Transplantation. 2004;77:902. doi: 10.1097/01.tp.0000114595.59168.3b. [DOI] [PubMed] [Google Scholar]
- 19.Duquesnoy R, Claas F. Is the application of HLAMatchmaker relevant in kidney transplantation? Transplantation. 2005;79:250. doi: 10.1097/01.tp.0000144327.92898.a6. [DOI] [PubMed] [Google Scholar]
- 20.Haririan A, Fagoaga O, Daneshvar H, Morawski K, Sillix D, El-Amm J, West M, Garnick J, Migdal S, Gruber S, Nehlsen-Cannarella S. Predictive value of HLA epitope matching using HLAMatchmaker for graft outcomes in a predominantly African-American renal transplant cohort. Clin Transplant. 2006;20:226. doi: 10.1111/j.1399-0012.2005.00473.x. [DOI] [PubMed] [Google Scholar]
- 21.Duquesnoy RJ, Marrari M. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination. II Verification of the algorithm and determination of the relative immunogenicity of amino acid triplet-defined epitopes. Hum Immunol. 2002;63:353. doi: 10.1016/s0198-8859(02)00381-6. [DOI] [PubMed] [Google Scholar]
- 22.Lobashevsky AL, Senkbeil RW, Shoaf JL, Stephenson AK, Skelton SB, Burke RM, Deierhoi MH, Thomas JM. The number of amino acid residues mismatches correlates with flow cytometry crossmatching results in high PRA renal patients. Hum Immunol. 2002;63:364. doi: 10.1016/s0198-8859(02)00371-3. [DOI] [PubMed] [Google Scholar]
- 23.Dankers MKA, Witvliet MD, Roelen DL, De Lange P, Korfage N, Persijn GG, Duquesnoy RJ, Doxiadis IIN, Claas FHJ. The number of amino acid triplet differences between patient and donor is predictive for the antibody reactivity against mismatched HLA antigens. Transplantation. 2004;I28:1236. doi: 10.1097/01.tp.0000120385.03278.28. [DOI] [PubMed] [Google Scholar]
- 24.Duquesnoy RJ, Witvliet MJ, Doxiadis IIN, de Fijter H, Claas FHJ. HLAMatchmaker-based strategy to identify acceptable HLA class i mismatches for highly sensitized kidney transplant candidates. Transpl Int. 2004;7:31. doi: 10.1007/s00147-003-0641-z. [DOI] [PubMed] [Google Scholar]
- 25.Claas FHJ, Witvliet M, Duquesnoy RJ, Persijn G, Doxiadis IIN. The acceptable mismatch program as a fast tool to transplant highly sensitized patients awaiting a post-mortal kidney: short waiting time and excellent graft outcome. Transplantation. 2004;78:190. doi: 10.1097/01.tp.0000129260.86766.67. [DOI] [PubMed] [Google Scholar]
- 26.Iniotaki-Theodoraki A, Kalogeropoulou E, Apostolaki M, Doxiadis I, Stavropoulos-Giokas C. Humoral sensitization against rejected grafts: specific antibodies to graft immunogenic amino acid triplets. Transplant Proc. 2004;36:1728. doi: 10.1016/j.transproceed.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Varnavidou-Nicolaidou A, Doxiadis I, Iniotaki-Theodoraki A, Patargias T, Stavropoulos-Giokas C, Kyriakides G. HLA class I donor-specific triplet antibodies detected after renal transplantation. Transplant Proc. 2004;36:1732. doi: 10.1016/j.transproceed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Adeyi O, Girnita A, Awadalla Y, Askar M, Shapiro R, Howe J, Martell J, Zeevi A, Nalesnik M, Rhandawa P, Demetris AJ, Duquesnoy RJ. Serum analysis after kidney transplant nephrectomy reveals restricted antibody specificity patterns against donor HLA class i antigens. Transpl Immunol. 2005;14:53. doi: 10.1016/j.trim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Doxiadis IIN, Duquesnoy RJ, Claas FHJ. Extending options for highly sensitized patients to receive a suitable kidney graft. Curr Opin Immunol. 2005;17:536. doi: 10.1016/j.coi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Goodman R, Taylor C, O’Rourke C, Lynch A, Bradley A, Key K. Utility of HLAMatchmaker and single-antigen HLA-antibody detection beads for identification of acceptable mismatches in highly sensitised patients awaiting kidney transplantation. Transplantation. 2006;81:1331. doi: 10.1097/01.tp.0000205202.56915.f5. [DOI] [PubMed] [Google Scholar]
- 31.Nambiar A, Duquesnoy RJ, Adams S, Oblitas J, Leitman S, Stroncek D, Marincola F. HLAMatchmaker-driven analysis of response to HLA matched platelet transfusions in alloimmunized patients. Blood. 2006;107:1680. doi: 10.1182/blood-2004-10-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duquesnoy R. A structurally based approach to determine HLA compatibility at the humoral immune level. Human Immunol. 2006;67:847–862. doi: 10.1016/j.humimm.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Anderson JB, DeWeese-Scott C, Fedorova ND, Geer LY, He S, Hurwitz DI, Jackson JD, Jacobs AR, Lanczycki CJ, Liebert CA, Lin C, Madej T, Marchler-Bauer A, Marchler GH, Mazumder R, Nikolskaya AN, Rao BS, Panchenko AR, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Vasudevan S, Wang Y, Yamashita RA, Yin JJ, Bryant SH. MMDB: Entrez’s 3D-structure database. Nucleic Acids Res. 2003;31:474. doi: 10.1093/nar/gkg086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogue C. Cn3D: a new generation of three-dimensional molecular structure viewer. Trends Biochem Sci. 1997;22:314. doi: 10.1016/s0968-0004(97)01093-1. [DOI] [PubMed] [Google Scholar]
- 35.Mullen MM, Haan KM, Longnecker R, Jardetzky TS. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol Cell. 2002;9:375. doi: 10.1016/s1097-2765(02)00465-3. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 37.Bolin DR, Swain AL, Sarabu R, Berthel SJ, Gillespie P, Huby NJ, Makofske R, Orzechowski L, Perrotta A, Toth K, Cooper JP, Jiang N, Falcioni F, Campbell R, Cox D, Gaizband D, Belunis CJ, Vidovic D, Ito K, Crowther R, Kammlott U, Zhang X, Palermo R, Weber D, Guenot J, Nagy Z, Olson GL. Peptide and peptide mimetic inhibitors of antigen presentation by HLA-DR class II MHC molecules. Design, structure-activity relationships, and X-ray crystal structures. J Med Chem. 2001;43:2135. doi: 10.1021/jm000034h. [DOI] [PubMed] [Google Scholar]
- 38.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA1*0101,DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang H, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, Stuart D, Bell J, Jones EY, Fugger L. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 40.Kim CY, Quarsten H, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci U S A. 2004;101:4175. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2:501. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 42.Siebold C, Hansen BE, Wyer JR, Harlos K, Esnouf RE, Svejgaard A, Bell JI, Strominger JL, Jones EY, Fugger L. Crystal structure of HLA-DQ0602 that protects against type 1 diabetes and confers strong susceptibility to narcolepsy. Proc Natl Acad Sci U S A. 2004;101:1999. doi: 10.1073/pnas.0308458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Combet C, Jambon J, Deleage G, Geourjon C. Geno3D: automatic comparative molecular modeling of protein. Bioinformatics. 2002;18:213. doi: 10.1093/bioinformatics/18.1.213. [DOI] [PubMed] [Google Scholar]
- 44.Viken HD, Paulsen G, Drover S, Marshall WH, Sollid LM, Gaudernack G, Thorsby E. Influence on antibody recognition of amino acid substitutions in the cleft of HLA-DQ2 molecules. Suggestive evidence of peptide-dependent epitopes. Hum Immunol. 1995;44:63. doi: 10.1016/0198-8859(95)00047-8. [DOI] [PubMed] [Google Scholar]
- 45.Smith KD, Mace BE, Valenzuela A, Vigna JL, McCutcheon JA, Barbosa JA, Huczko E, Engelhard VH, Lutz CT. Probing HLA-B7 conformational shifts induced by peptide-binding groove mutations and bound peptide with anti-HLA monoclonal antibodies. J Immunol. 1996;157:2470. [PubMed] [Google Scholar]
- 46.Takamiya Y, Sakaguchi T, Miwa K, Takiguchi M. Role of HLA-B*5101 binding nonamer peptides in formation of the HLA-Bw4 public epitope. Int Immunol. 1996;8:1027. doi: 10.1093/intimm/8.7.1027. [DOI] [PubMed] [Google Scholar]
- 47.Mulder M, Eijsink C, Kester MGD, Franke MEI, Kardol MJ, Heemskerk MHM, van Kooten C, Verreck FA, Drijfhout JW, Koning F, Doxiadis IN, Claas FJH. Impact of peptides on the recognition of HLA class i molecules by human HLA antibodies. J Immunol. 2005;175:5950. doi: 10.4049/jimmunol.175.9.5950. [DOI] [PubMed] [Google Scholar]
- 48.Altschul S, Madden T, Schäffer A, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson J, Waller M, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoer P, Marsh SG. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31:311. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albert ED, Baur MP, Mayr WR. Class II Antigen Reports. Berlin: Springer-Verlag; 1984. Histocompatibility Testing 1984. [Google Scholar]
- 51.Dupont B, editor. Histocompatibility Testing 1987. I. New York: Springer-Verlag; 1987. Immunobiology of HLA. [Google Scholar]
- 52.Tjuji K, Aizawa M, Sasazuki T, editors. Proceedings of the Eleventh International Workshop and Conference. Vol. 1. Oxford: Oxford University Press; 1991. HLA1991. [Google Scholar]
- 53.Charron D. Allele and Haplotype Society Reports #10-18. Proceedings of the Twelfth International Histocompatibility Workshop and Conference. Sevres, EDK Medical and Scientific International Publisher; 1997. [Google Scholar]
- 54.Navarrete C, Brown C, De Lange P, Schreuder GMT. 12th International Histocompatibility Workshop HLA Class II Monoclonal Antibodies Study. In: Charron D, editor. HLA Genetic Diversity of HLA. Functional and Medical Implication. Vol. 1. Paris: EDK Publisher; 1997. [Google Scholar]
- 55.Schreuder GMT. Serological Definition of HLA-DR and -DQ Polymorphisms. The Hague: CIP-Data Koninklijke Bibliotheek; 1989. [Google Scholar]
- 56.Duquesnoy RJ, Mulder A, Askar M, Fernandez-Vina M, Claas FHJ. HLAMatchmaker-based analysis of human monoclonal antibody reactivity demonstrates the importance of an additional contact site for specific recognition of triplet-defined epitopes. Hum Immunol. 2005;66:749. doi: 10.1016/j.humimm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Fuggle SV, Carter C, Watts F, Kirkley J, Morris PJ. Monoclonal antibody definition of multiple polymorphic epitopes on HLA-DRw52. Human Immunol. 1987;19:255. doi: 10.1016/0198-8859(87)90107-8. [DOI] [PubMed] [Google Scholar]
- 58.Taylor CJ, Ugozolli L, Tanigaki N, Tosi G, Bunce M, Ting A, Ferrara GB. Antigen Society #29 Report (DRw52) In: Dupont B, editor. Immunobiology of HLA Vol I Histocompatibility Testing. Vol. 1987. New York: Springer Verlag; 1987. p. 273. [Google Scholar]
- 59.Tongio MM, van der Berg-Loonen E, Bignon JD, Chandanayingyong D, Dormoy A, Eiermann T, Marshall W, Park MS, Scheuder GMT. HLA-DP Detected by Serology. Sevres: EDK Medical and Scientific International Publisher; 1997. [Google Scholar]
- 60.Fernandez-Vina M, Moraes JR, Moraes ME, Miller S, Stastny P. HLA class II haplotypes in Amerindians and in black North and South Americans. Tissue Antigens. 1991;38:235. doi: 10.1111/j.1399-0039.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 61.Clayton J, Lonjou C, Whittle D. DRB1-DQA1-DQB1 Haplotype Frequencies. In: Charron D, editor. HLA Genetic Diversity of HLA. Functional and Medical Implication. Paris: EDK Publisher; 1997. [Google Scholar]
- 62.Klitz W, Maiers M, Spellman S, Baxter-Lowe LA, Schmeckpeper B, Williams TM, Fernandez-Vina M. New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens. 2003;62:296. doi: 10.1034/j.1399-0039.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 63.Pfeffer K, Vogeler U, Albrecht KH, Eigler FW, Buchholz B, Grosse-Wilde H. HLA-DP antibodies in patients awaiting renal transplantation. Transpl Internat. 1995;8:180. doi: 10.1007/BF00336534. [DOI] [PubMed] [Google Scholar]
- 64.Youngs D. DP alloantibodies. American Society for Histocompatibility and Immunogenetics Quarterly. 2004;2:60. [Google Scholar]
- 65.Qiu J, Cai J, Terasaki PI, El-Awar N, Lee JH. Detection of antibodies to HLA-DP in renal transplant recipients using single antigen beads. Transplantation. 2005;80:1511. doi: 10.1097/01.tp.0000181384.49832.3a. [DOI] [PubMed] [Google Scholar]
- 66.Duquesnoy RJ, Claas FHJ. Progress report of 14th International Histocompatibility Workshop Project on the structural basis of HLA compatibility. Tissue Antigens. 2006 doi: 10.1111/j.1399-0039.2006.00766.x. In Press. [DOI] [PubMed] [Google Scholar]


