Abstract
Loss of α-catenin is one of the characteristics of prostate cancer. The catenins (α, β) associated with E-cadherin play a critical role in the regulation of cell-cell adhesion. Tyrosine phosphorylation of β-catenin dissociates it from E-cadherin and facilitates its entry into the nucleus, where β-catenin acts as a transcriptional activator inducing genes involved in cell proliferation. Thus, β-catenin regulates cell-cell adhesion and cell proliferation. Mechanisms controlling the balance between these functions of β-catenin invariably are altered in cancer. Although a wealth of information is available about β-catenin deregulation during oncogenesis, much less is known about how or whether α-catenin regulates β-catenin functions. In this study, we show that α-catenin acts as a switch regulating β-catenin’s cell-cell adhesion and proliferation functions. In α-catenin null prostate cancer cells, re-expression of α-catenin increased cell-cell adhesion and decreased β-catenin transcriptional activity, cyclin D1 levels, and cell proliferation. Further, Src-mediated tyrosine phosphorylation of β-catenin is a major mechanism for decreased β-catenin interaction with E-cadherin in α-catenin null cells. α-catenin attenuated the effect of Src phosphorylation by increasing β-catenin association with E-cadherin. We also show that α-catenin increases the sensitivity of prostate cancer cells to a Src inhibitor in suppressing cell proliferation. This study reveals for the first time that α-catenin is a key regulator of β-catenin transcriptional activity and that the status of α-catenin expression in tumor tissues might have prognostic value for Src targeted therapy.
Keywords: catenin, prostate cancer, adherens junction, src, TCF/LEF
Introduction
Cell-cell adhesion is critical to establish and maintain tissue architecture and function. In epithelial cells, molecules localized to adhesion structures, such as tight junctions, adherens junctions, and desmosomes, regulate cell-cell adhesion. The adherens junction is composed of the calcium-dependent cell-adhesion molecule E-cadherin and catenins (α,β, γ, and p120), which are linked to the actin cytoskeleton (1, 2). E-cadherin’s homophilic adhesion, mediated by both its extracellular domain and its cytoplasmic tail association with catenins (3, 4), is critical for adherens junction formation and function. During cancer progression, the function of the adherens junction is generally compromised, leading to loss of cell-cell adhesion and increased cell proliferation (5). Although the link between cell-cell adhesion and cell proliferation is well documented, mechanisms that regulate these two seemingly diverse functions are poorly characterized.
β-Catenin binds directly to both E-cadherin and α-catenin and plays a critical role in the cell-cell adhesion function of E-cadherin (6, 7). In addition to its role in cell-cell adhesion, β-catenin also plays a major role in the Wnt signaling pathway, which is involved in the regulation of cell proliferation, embryonic development, and cancer progression (6, 7). The levels of cytoplasmic (free) β-catenin are regulated by its association with the adenomatous polyposis coli (APC) protein, which targets β-catenin for phosphorylation by glycogen synthase kinase-3β (GSK-3β), marking it for degradation via the ubiquitin/proteosome pathway. Activation of the Wnt receptors, Frizzled and LRP5/6, inhibits APC/GSK-3β function prevents β-catenin degradation (8, 9). This inhibition stabilizes β-catenin within the cytoplasm and allows translocation to the nucleus, where it associates with the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of activators (10). β-catenin and the TCF/LEF transcriptional activators induce transcription of several target genes. One of these well-studied target genes is cyclin D1, a protein involved in cell growth and proliferation (11, 12). Mutations in either the APC or the GSK-3β phosphorylation sites of β-catenin also increase the level of free β-catenin by preventing its degradation by the proteosome pathway and result in aberrant transcription of β-catenin/TCF/LEF target genes (13). These mutations occur commonly in colon cancer and are associated with oncogenic activation of β-catenin transcription (14). In addition, recent studies indicate that phosphorylation of β-catenin also might play a role in oncogenic activation of β-catenin transcription. Tyrosine phosphorylation of β-catenin by either Src family tyrosine kinases or Met receptor disrupts β-catenin association with E-cadherin and α-catenin (15). Tyrosine phosphorylated β-catenin shows increased nuclear localization and transcriptional activity (15, 16). Thus, β-catenin is a key molecule of the adhesion machinery that controls cell-cell adhesion and cell proliferation when localized to adherens junctions and nucleus, respectively.
α-catenin binds to β-catenin, links the E-cadherin complex to the actin cytoskeleton and stabilizes E-cadherin at the adherens junction (17, 18). Lack of α-catenin expression results in reduced cell-cell adhesion and loss of the epithelial phenotype (19-22); these effects are reversed after repletion of α-catenin (19, 20, 22, 23). In addition to α-catenin’s role in cell-cell adhesion, α-catenin also is implicated in the regulation of cell proliferation, as expression of α-catenin reduced cell growth (22, 24) and suppressed tumor formation in in-vivo xenografts (19, 20). However, how α-catenin is involved in the control of cell proliferation remains to be determined.
In this study using α-catenin null prostate cancer cells, we show that repletion of α-catenin leads to formation of the adherens junctions, and reduced β-catenin transcriptional activity, cyclin D1 levels, and cell proliferation. We also show that small molecule inhibition of Src has a profound effect on cell proliferation in α-catenin-positive cells compared with α-catenin null cells, indicating that the α-catenin status of tumor tissues might have prognostic value for targeted therapy against Src.
Materials and Methods
Cell Lines and Cell Culture
The prostate cancer cell line, PC3, was obtained from the American Type Culture Collection (Manassas, VA). The PC3 cell line expressing α-catenin was generated by micro-cell transfer of chromosome 5 (PC3-α), and the α-catenin null revertant cell line (PC3-Rev) has been described previously (20). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, MEM nonessential amino acid solution (Invitrogen, Grand Island, NY), and penicillin/streptomycin.
Antibodies and Reagents
Monoclonal antibodies against cyclin D1 (Cell Signaling Technology, Danvers, MA), E-cadherin, β-catenin, phosphorylated tyrosine: PY20 (BD Biosciences, San Jose, CA), α-catenin (Vector Laboratories, Burlingame, CA), and 4G10-(Millipore, Carlsbad, CA), and polyclonal antibodies against β-catenin (Millipore), phosphorylated Src (Tyr416), Src (Cell Signaling Technology), and zonula occludens-1 (ZO-1) (Invitrogen, Carlsbad, CA) were obtained from indicated vendors. Fluorescein isothiocyanate (FITC) and CY3-labeled anti-mouse and anti-rabbit antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA), and horseradish peroxidase conjugated anti-mouse and anti-rabbit were obtained from Cell Signaling Technology. The TOPFLASH and FOPFLASH reporter plasmids were kindly provided by Dr. Marian Waterman (University of California-Irvine, Irvine, CA). PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine-#529573) was purchased from EMD Biosciences (San Diego, CA) and dissolved as a 5 mM stock in dimethyl sulfoxide (DMSO). The α-catenin cytomegalovirus (CMV) expression vector was kindly provided by Dr. Masatoshi Takeichi (RIKEN Center for Developmental Biology, 2-2-3 Minatojima Minamimachi, Chuo-ku, Kobe, Japan) and has been described previously (22).
Immunoblotting
Total protein lysates were prepared using either a lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml of antipain, leupeptin, and pepstatin (protease inhibitor cocktail) or a lysis buffer containing 25 mM Tris-HCl (pH 7.4), 95 mM NaCl, 3 mM EDTA, 2% SDS, and protease inhibitor cocktail. One hundred micrograms of cell lysate was separated by SDS-PAGE and transferred to nitrocellulose membrane. For immunoblotting, blots were blocked in 5% nonfat milk in TBS/0.1% Tween 20 (TBST). Primary and secondary antibodies were diluted in 5% nonfat milk/TBST or 5% bovine serum albumin/TBST and incubated overnight at 4°C, and blots were developed with ECL or ECL plus (GE Biosciences, Piscataway, NJ). The blots were quantified using a Bio-Rad VersaDoc. The densities of the bands were normalized with respect to actin.
Immunofluorescence and Confocal Microscopy
Immunofluorescence and confocal microscopy were performed as described earlier (25). Co-localization of β-catenin with propidium iodide (PI) or α-catenin was performed using a Zeiss LSM 5 Pascal laser scanning microscope (Carl Zeiss, Oberkochen, Germany) and analyzed by Pascal software.
Transmission Electron Microscopy (TEM)
Cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, for 2 h at room temperature and processed for transmission electron microscopy as described previously (26).
Triton X-100 Solubility
The prostate cancer cells, PC3 and PC3-α, were washed with cold PBS, pH 7.4, supplemented with 1 mM CaCl2 and 1 mM MgCl2 (PBS-CM), and lysed for 10 min at 4°C with 500 μl of extraction buffer (10 mM PIPES, pH 6.8, 50 mM NaCl, 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose, and protease inhibitor cocktail). Lysates were transferred into microfuge tubes, spun at 13,000 rpm for 30 min, and the supernatant (soluble fraction) was transferred to a fresh tube. The pellet (insoluble fraction) was resuspended in 500 μl 2X SDS sample buffer and sonicated. For immunoblotting, the soluble fraction (30 μl of supernatant + 30 μl of 2X SDS sample buffer) and the insoluble fraction (30 μl of pellet fraction + 30 μl of extraction buffer) were separated by SDS-PAGE and blotted as described above. The β-catenin levels in the insoluble fractions were normalized to the β-catenin levels in the corresponding soluble fractions.
Immunoprecipitation
Cell lysates were prepared as described above in lysis buffer (10 mM Tris, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 0.2 mM Na-vanadate, 1% Triton X-100, 0.50 % IGEPAL, 0.1% SDS, 1% deoxycholic acid, and protease inhibitor cocktail) and pre-cleared with protein G-sepharose. Antibodies were pre-coupled to protein G-sepharose (GE Biosciences) and incubated overnight with 1000 μg of total protein lysate. Immunoprecipitates were washed and separated by SDS-PAGE and immunoblotted as described above.
TOPFLASH/FOPFLASH Reporter Assay
The TOPFLASH/FOPFLASH luciferase reporter assay was performed as described previously (27). Briefly, 100,000 cells per well were plated onto six-well plates. One microgram per well of either TOPFLASH or FOPFLASH was transfected using LIPOfectamine PLUS (Invitrogen) according to manufacturer’s instructions. Per well, 0.5ng of Renilla control luciferase plasmid was co-transfected to normalize for transfection efficiency. Forty-eight hours post transfection, cells were lysed with 1X passive lysis buffer (Promega, Madison, WI), and the luciferase assay was performed using the Dual-Luciferase Reporter Assay Kit (Promega). TOPFLASH and FOPFLASH values were normalized to Renilla, and fold induction for each cell line was calculated as normalized relative light units of TOPFLASH divided by normalized relative light units of FOPFLASH. For Src inhibition, transfected cells were incubated with 10 μM PP2 in RPMI for 24 h before lysis, or an equal volume of DMSO was used as a vehicle control.
Measurement of Growth Curve
One hundred thousand cells per well of PC3, PC3-α, or PC3-Rev cells were plated onto six-well plates. At the indicated time points, cells were trypsinized and the number of cells was determined by a hemocytometer. The cell doubling time was calculated according to the equation Td = 0.693(t)/ln (N/No); t = time (in hours), N = cell number at time t, and No = cell number at initial time. For inhibition of Src, PP2 was diluted to a final concentration of 10 μM in complete RPMI, and treatment began 24 h after cells were plated. Fresh media and PP2 were added every 24 h. An equal volume of DMSO was used as a vehicle control.
Spheroid Growth in Matrigel
The PC3, PC3-α, and PC3-Rev cells were trypsinized and suspended at a final concentration of 20,000 cells/ml in ice-cold Matrigel (BD Biosciences). Two hundred microliters of the cell/Matrigel mixture (4000 cells per well) were layered onto filter inserts (Nalgene Nunc International, Rochester, NY) and allowed to gel at 37°C. The hardened gel was covered in RPMI 1640 and monitored daily for spheroid formation. Media were replenished every two to three days. At day 14, gels were washed with PBS and fixed in 4% paraformaldehyde in PBS. Spheroids were photographed and counted. Data were analyzed using a Student’s t-test for unequal variance.
siRNA Knockdown of β-catenin
SMARTpool® β-catenin siRNA (M-003482) and control siRNA (D-001206-13-05) were purchased from Millipore and diluted according to manufacturer’s instructions. Two hundred thousand PC3 cells were plated onto 60-mm dishes and allowed to attach overnight. Transfections with siRNA oligomers were performed using Oligofectamine according to manufacturer’s instructions (Invitrogen).
Expression of α-catenin in PC3 Cells
Two hundred thousand PC3 cells were plated onto 60-mm dishes and allowed to attach overnight. Five micrograms of α-catenin expression vector or empty vector were transfected with Fugene 6 (Roche Diagnostics, Indianapolis, IN). After 24 h, transfected cells were selected by incubation with 500 μg/ml of geneticin (G418) for 5 days. Cells were then either lysed for immunoblotting/immunoprecipitation or plated onto glass coverslips for immunofluorescence as described above.
3H-thymidine Incorporation Assay
Ten thousand PC3-α and PC3 cells were plated onto 12-well plates and allowed to grow for 24 h. The PP2 was diluted in complete RPMI at indicated concentrations, added to cells, and incubated for a total of 48 h. Both the media and PP2 were refreshed at 24 h. After 48 h, 6 μCi/ml of 3H-thymidine was added to each well, and cells were pulsed for 4 h. Cells were lysed in 0.125N NaOH/0.05% SDS lysis buffer and counted in a Beckman LS 6500 scintillation counter. To calculate percent inhibition, the average counts per minute (CPM) from vehicle-treated samples were considered as 0% (no inhibition). The resulting values were used to calculate IC50 values by plotting data (inhibition against concentration) to generate a dose response curve, which was used to calculate the concentration of PP2 at which inhibition of thymidine incorporation was 50%. This experiment was performed in triplicate and repeated once.
Results
α-Catenin Restoration Induces Tight Junctions, Adherens Junctions, and Desmosomes in PC3 Cells
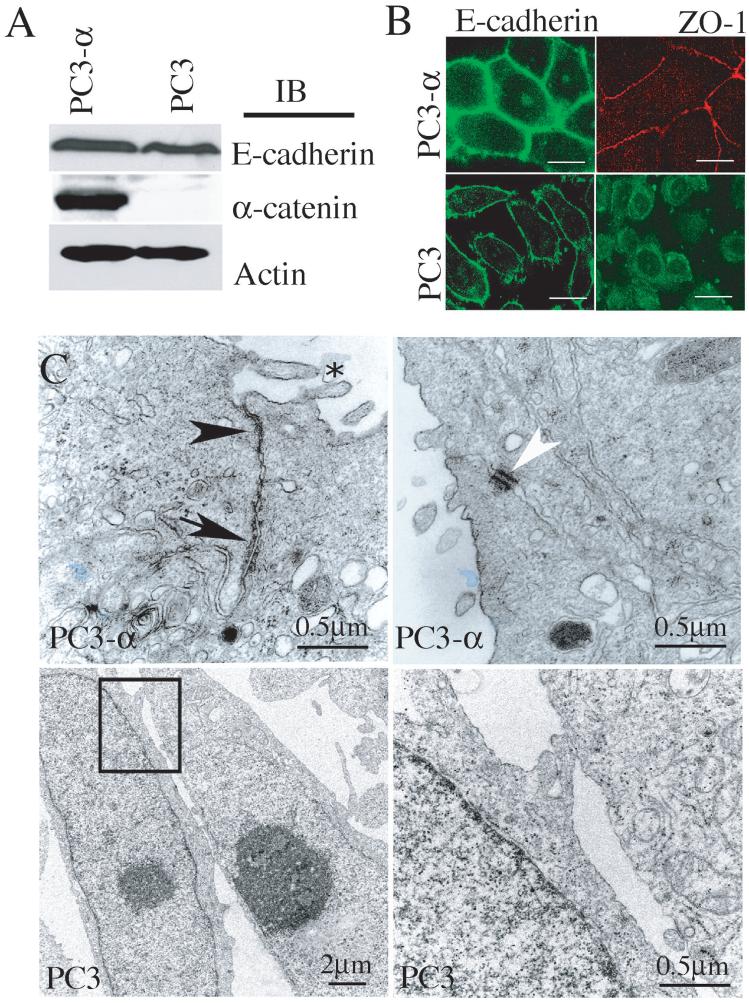
The prostate cancer cell line, PC3, was initially isolated from a bone metastasis of a patient diagnosed with androgen-independent prostatic adenocarcinoma (28). Cytogenetic analysis has revealed that PC3 cells exhibit dramatic chromosomal abnormalities; one of which is the absence of normal copies of chromosome 5 (20), which renders PC3 cells null for the expression of α-catenin (Fig. 1A). To induce α-catenin expression, normal chromosome 5 containing a neomycin resistance gene was re-introduced into PC3 cells using micro-cell transfer, followed by selection with neomycin (20). The resulting cell line, PC3-α, contains two normal copies of chromosome 5 and expresses α-catenin (Fig. 1A). While both PC3 and PC3-α cells differ in regards to α-catenin expression, both cell lines expressed similar levels of E-cadherin (Fig. 1A). Yet, despite E-cadherin expression, PC3 cells displayed a non-epithelial phenotype (data not shown), while PC3-α-cells showed epithelial morphology and grew as compact colonies. Immunofluorescence analyses of E-cadherin (adherens junction marker) and ZO-1 (tight junction marker) revealed localization at the cell-cell contact sites in PC3-α cells (Fig. 1B, top panel) while in PC3 cells E-cadherin was less intense at the plasma membrane, and ZO-1 was primarily intracellular (Fig. 1B, bottom panel). The dramatic differences in both E-cadherin and ZO-1 localization in PC3 and PC3-α cells suggested that the repletion of α-catenin in PC3 cells restored the adherens junctions and other epithelial junctional complexes. Transmission electron microscopy (TEM) confirmed tight junctions (arrowhead) and adherens junctions (arrow) in PC3-α cells (Fig. 1C, top panel), whereas these junctional complexes were absent in PC3 cells (Fig. 1C, bottom panel). In addition, PC3-α cells showed microvilli (Fig. 1C, top panel-asterisk) and desmosomes (Fig. 1C, top panel-white arrowhead), indicating that re-expression of α-catenin restored epithelial junctional complexes in PC3 cells. These results demonstrated that α-catenin has a critical role in supporting the cadherin cell-cell adhesion function and formation of epithelial junctional complexes.
Figure 1. Expression of α-catenin restores junctional complexes.
A, Immunoblot analysis of total protein lysate from PC3 and PC3-α cells. Actin was used as a loading control. B, Immunofluorescence analyses of E-cadherin and ZO-1 in PC3 and PC3-α cells. Bar represents 15 μm. C, TEM of PC3 and PC3-α cell lines. PC3-α cells show electron dense regions corresponding to the tight junctions (arrowhead), adherens junctions (arrow), desmosomes (white arrowhead), and microvilli (asterisk). Bottom panel, box in low magnification (left) highlights region shown at high power (right).
α-Catenin Restoration Reduces Nuclear β-Catenin Levels
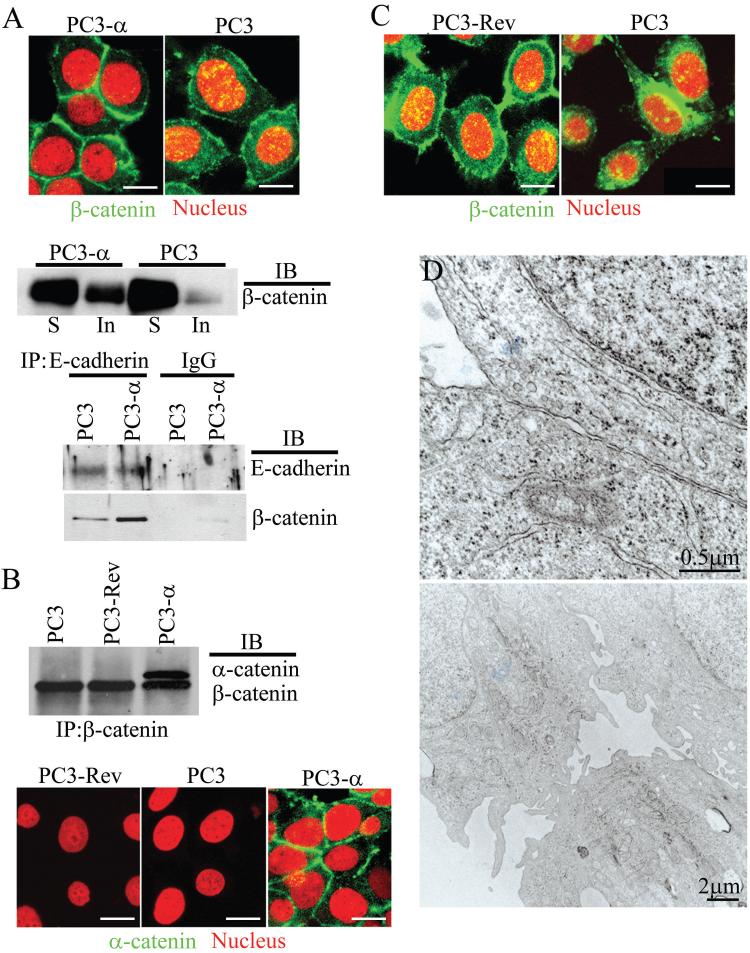
In epithelial cells, β-catenin is generally localized to the basolateral plasma membrane and the adherens junction (26). In the absence of adherens junctions, β-catenin localizes to the nucleus (29). Since re-expression of α-catenin in PC3 cells restored the adherens junction, we hypothesized that α-catenin re-expression might affect β-catenin localization. Confocal microscopy revealed intense β-catenin staining at the regions of cell-cell contact in PC3-α cells, with low levels in both the cytoplasm and nucleus (Fig. 2A). In contrast, PC3 cells revealed increased β-catenin staining in both the cytoplasm and the nucleus (yellow) compared with PC3-α cells (Fig. 2A). To further confirm the distribution of β-catenin in PC3 and PC3-α cells, we analyzed the β-catenin levels within the detergent-soluble (containing cytoplasmic and nuclear proteins) and insoluble (containing proteins associated with the actin cytoskeleton) fractions. A quantitative immunoblot analysis of the soluble (S) and insoluble (In) fractions revealed 6.9-fold more β-catenin in the detergent-insoluble fraction in PC3-α cells compared with PC3 cells (Fig. 2A, middle panel). In addition, immunoprecipitation analysis revealed that the amount of β-catenin co-immunoprecipitating with E-cadherin was 68% higher in PC3-α cells compared with PC3 cells (Fig. 2A, bottom panel), indicating that in the presence of α-catenin, the β-catenin interaction with E-cadherin is enhanced.
Figure 2. Nuclear/cytoplasmic localization of β-catenin in α-catenin positive and negative PC3 cells.
A, Top panel: Immunofluorescence staining of β-catenin (green) in PC3 and PC3-α cells. Propidium iodide (red) marks the nucleus. β-catenin localized to the nucleus appears in yellow. Bar represents 15 μm. Middle panel: Immunoblot analysis of β-catenin in detergent-soluble (S) and insoluble (In) fractions. Bottom panel: β-catenin co-immunoprecipitating with E-cadherin. IgG was used as a negative control. B, Top panel: Co-immunoprecipitation of α-catenin and β-catenin from PC3, PC3-Rev, and PC3-α cells. The blots were probed with both α- and β-catenin antibodies simultaneously. Bottom panel: Immunofluorescence staining of α-catenin (green) in PC3, PC3-Rev and PC3-α cells. Bar represents 15 μm. C, Immunofluorescence staining of β-catenin in PC3, and PC3-Rev cells. PC3-Rev cells show cytoplasmic (green) and nuclear (yellow) staining of β-catenin comparable to PC3 cells. Propidium iodide (red) marks the nucleus. Bar represents 15 μm. D, TEM of PC3-Rev cells.
Since PC3-α cells were generated by micro-cell transfer of an entire copy of chromosome 5, it is possible that other genes located on chromosome 5 also might contribute to the formation of junctional complexes and reduced β-catenin nuclear localization. We used a revertant clone of PC3-α cells (PC3-Rev) to investigate the specific role of α-catenin. The PC3-Rev cells were subcloned from PC3-α cells because of their reversion to the non-epithelial phenotype (20). The PC3-Rev cells are null for α-catenin expression (Fig. 2B, top and bottom panel) due to deletion of the α-catenin gene yet still retain chromosome 5 containing the neomycin resistance gene (20). Immunofluorescence analysis of β-catenin revealed both cytoplasmic and nuclear localization in PC3-Rev cells (Fig. 2C), and TEM revealed the absence of tight junctions, adherens junctions, and desmosomes in these cells (Fig. 2D). Together, these results demonstrated that re-expression of α-catenin induced junctional complexes and reduced nuclear β-catenin levels in PC3 cells.
Regulation of β-Catenin Transcriptional Activity and Cyclin D1 by α-Catenin
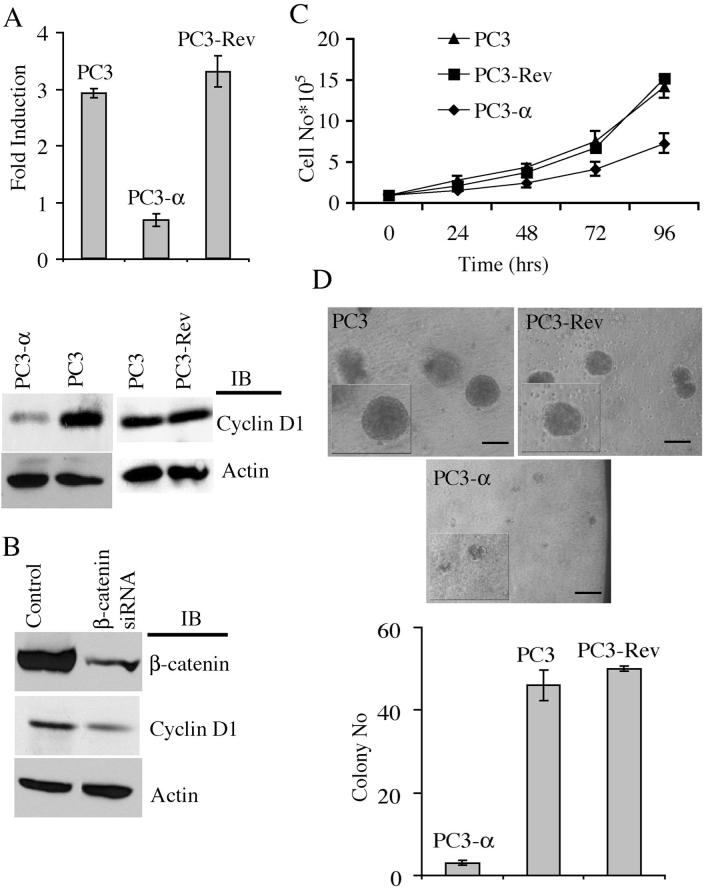
Nuclear localization of β-catenin is associated with β-catenin-mediated TCF/LEF transcriptional activity (10). The TOPFLASH and FOPFLASH luciferase assay is a well-established quantitative reporter assay for β-catenin/TCF/LEF transcriptional activity (30). We transiently transfected either TOPFLASH (positive control containing TCF/LEF binding sites for β-catenin) or FOPFLASH (negative control containing mutated TCF/LEF binding sites) plasmids into PC3, PC3-α, and PC3-Rev cells and measured the luciferase reporter activity. As expected, PC3 and PC3-Rev cells showed 2.91-fold (± 0.08) and 3.29-fold (± 0.68) more TOPFLASH over FOPFLASH activity, respectively, while PC3-α cells showed a minimal difference (0.67-fold, ± 0.11; P= 4.99 × 10-6) (Fig. 3A, top panel). This reduced TCF/LEF transcriptional activity in PC3-α cells was not due to reduced β-catenin levels as they are similar in these cell lines (Fig. 2B). These results indicated that α-catenin expression reduced the transcriptional activity of β-catenin in PC3 cells.
Figure 3. α-Catenin expression decreases β-catenin/TCF/LEF transcription activity and cell proliferation.
A, Top panel: β-catenin/TCF/LEF luciferase reporter assay. Fold induction corresponds to luciferase activity of positive TOPFLASH reporter over negative FOPFLASH reporter. Bars represent standard error of the average of three independent experiments. Bottom panel: Immunoblot of cyclin D1 in PC3, PC3-Rev, and PC3-α cells. B, Immunoblot of β-catenin and cyclin D1 upon siRNA knockdown of β-catenin in PC3 cells and in PC3 cells transfected with control siRNA. Actin was used as a loading control. Representative blot from two independent experiments is shown. C, Growth curve of PC3, PC3-Rev, and PC3-α cells. Bars represent standard error. Experiment was done in triplicate and repeated once. D, Top panel: Matrigel spheroid formation of PC3, PC3-Rev, and PC3-α cells. Inset shows cysts at a higher magnification. Bar represents 1 mm. Bottom panel: Quantification of PC3, PC3-Rev and PC3-α matrigel spheroids. Bar represents the standard error of the means of three independent experiments.
β-catenin/TCF/LEF transcriptional activity increases expression of cyclin D1, a protein that is involved in cell proliferation (11, 12, 31). The cyclin D1 levels in PC3 and PC3-Rev cells were higher compared to PC3-α cells (Fig. 3A, bottom panel). In addition, confocal microscopy detected nuclear localization of cyclin D1 in PC3 and PC3-Rev cells but not in PC3-α cells (data not shown), indicating activation of cyclin D1 in α-catenin null cells. To further validate that β-catenin is involved in the regulation of cyclin D1 expression, we used an siRNA approach to knockdown β-catenin levels in PC3 cells. As shown in Figure 3B, there was a 78% reduction of β-catenin in specific RNAi oligomer-transfected cells but not in PC3 cells transfected with the control RNAi oligomer. As expected, the targeted knockdown of β-catenin resulted in a 72% decrease in cyclin D1 levels compared with control cells (Fig. 3B), indicating that the increased cyclin D1 protein level in PC3 cells was due to enhanced β-catenin transcriptional activity.
Increased expression of cyclin D1 is associated with increased cell proliferation (32, 33). Consistent with cyclin D1 levels, PC3 and PC3-Rev cells showed a doubling time of 29 h and 28 h, respectively, compared with 41 h in PC3-α cells (Fig. 3C). As an independent test to verify cell proliferation, we compared the 3H-thymidine incorporation of PC3 and PC3-α cells. Again, PC3-α cells revealed a 54% decrease in the levels of 3H-thymidine incorporation compared with PC3 cells (data not shown). Re-expression of α-catenin into a α-catenin null variant of the DLD-1 colon carcinoma cell line decreases their growth in Matrigel into three-dimensional spheroids (24). Since re-expression of α-catenin reduced cell proliferation of PC3 cells in more conventional two-dimensional assays, we evaluated the ability of PC3-α, PC3, and PC3-Rev cells to form three-dimensional spheroids. The PC3, PC3-Rev, and PC3-α cells were suspended as single cells in Matrigel and allowed to grow as described in the Materials and Methods section. After day 14, both PC3 and PC3-Rev cells formed large spheroids within the Matrigel, while PC3-α cells either remained as primarily single cells or grew into small cell aggregates (Fig. 3D). Quantification of colonies revealed 46 colonies (±3), 50 colonies, and three colonies (P=2.72 × 10-7) for PC3, PC3-Rev, and PC3-α cells, respectively (Fig. 3D). Together, these results demonstrated that diminished β-catenin signaling is associated with reduced cyclin D1 levels and cell proliferation in PC3-α cells.
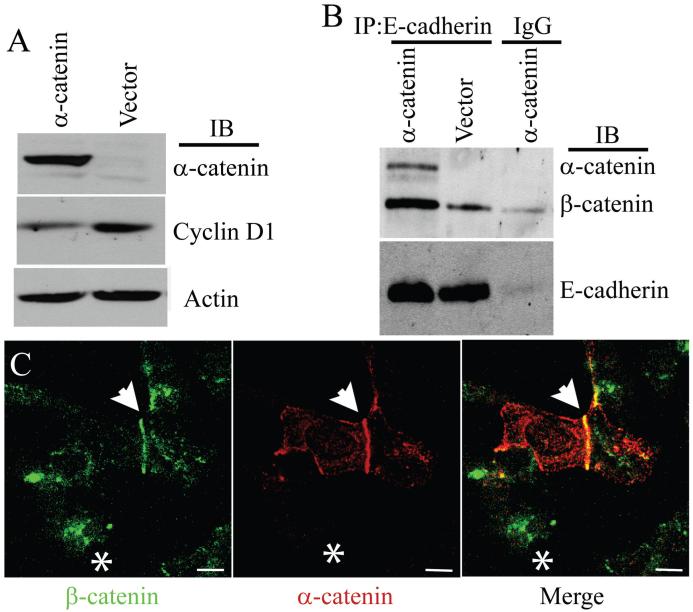
To address the specific role of α-catenin in the regulation of cyclin D1 levels, we attempted to generate stable clones of PC3 cells expressing α-catenin. However, these cells underwent cell-cycle arrest and failed to grow, a phenomenon which has also been observed by others (19, 20). Therefore, we transiently transfected α-catenin into PC3 cells and tested the levels of cyclin D1. Strikingly, an 80% reduction in the levels of cyclin D1 was observed in α-catenin transfected cells compared with control cells (Fig. 4A). In addition, the amount of β-catenin co-immunoprecipitated with E-cadherin was 3.6-fold higher in α-catenin transfected cells (Fig. 4B). Expression of α-catenin in PC3 cells did not affect the total levels of either E-cadherin or β-catenin (data not shown). Immunofluorescence analysis of β-catenin in α-catenin transfected PC3 cells showed enrichment of β-catenin at the cell-cell contact sites together with α-catenin (Fig. 4C). These results indicated that decreased cyclin D1 expressed in PC3-α cells is due primarily to enhanced association of β-catenin with E-cadherin at the adherens junction.
Figure 4. Exogenous expression of α-catenin reduces nuclear β-catenin levels.
A, Immunblot analysis of cyclin D1 levels in α-catenin expressing PC3 cells. Actin was used as a loading control. Blot represents two independent experiments. B, Co-immunoprecipitation of α-catenin and β-catenin with E-cadherin. The blots were probed with anti α- and β-catenin antibodies simultaneously. Blot represents three independent experiments. C, Immunofluorescence staining of β-catenin (green) and α-catenin (red) in PC3 cells transiently expressing α-catenin. Arrow highlights co-localization of β-catenin and α-catenin at the site of cell-cell contact in α-catenin expressing PC3 cells. Asterisk marks untransfected PC3 cell with nuclear and cytoplasmic staining of β-catenin. Bar represents 15 μm.
α-Catenin Overrides Src-induced Phosphorylation of β-Catenin and its Transcriptional Activity
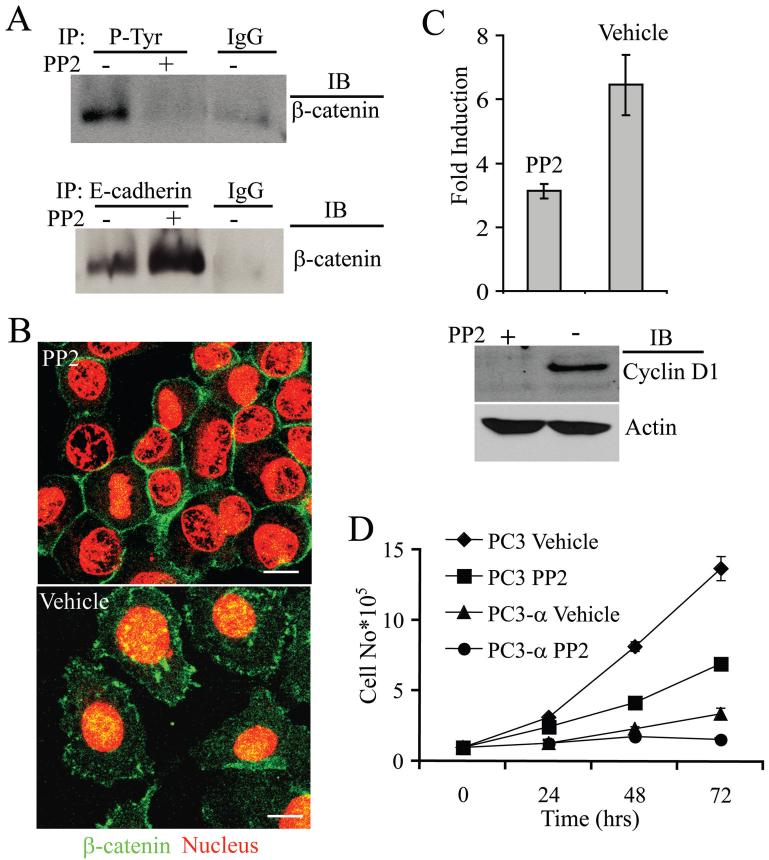
Recent studies indicate that tyrosine phosphorylation of β-catenin by Src family members reduces its association with E-cadherin and α-catenin (16, 34, 35). Inhibition of Src by PP2 dramatically reduced the tyrosine phosphorylation of β-catenin (Fig. 5A, top panel), with a 62% increase in the level of β-catenin associated with E-cadherin (Fig. 5A, bottom panel). The PP2 inhibition of Src did not affect the total levels of either E-cadherin or β-catenin (data not shown). Consistent with these biochemical data, PP2 treated PC3 cells grew in compact clusters compared with untreated cells, which grew as individual cells (Fig. 5B). Further, PP2-treated PC3 cells exhibited β-catenin distinctly localized at the sites of cell-cell contact (similar to PC3-α cells, see Fig. 2A) when compared with cytoplasmic and nuclear localization in vehicle (DMSO)-treated cells, correlating with the changes in the growth patterns. The distinct decrease in nuclear β-catenin observed in PP2-treated cells correlated with a 51% reduction in the transcriptional activity of β-catenin compared with untreated cells and reduced cyclin D1 levels (Fig. 5C). The decreases in both β-catenin transcription and cyclin D1 levels were accompanied by reduced cell proliferation (Fig. 5D). The PC3 cells treated with 10 μM of PP2 had a doubling time that was 35% higher than vehicle-treated cells. Taken together, these results indicated that Src plays an important role in β-catenin transcriptional activity and proliferation of PC3 cells lacking α-catenin.
Figure 5. Src regulates β-catenin localization and transcriptional activity in PC3 cells.
A, β-Catenin immunoblot of immunoprecipitates of tyrosine phosphorylated proteins from PC3 cells (upper blot) and of immunoprecipitates of E-cadherin (lower blot). Treatment with PP2 increased the amount of β-catenin associated with E-cadherin. IgG was used as control. Blots represent data from three independent experiments. B, Immunofluorescence staining of β-catenin (green) of PP2 and vehicle-treated PC3 cells. Propidium iodide stains the nucleus (red). Bar represents 15 μm. C, Src inhibition affects β-catenin mediated transcription in PC3 cells. Top Panel-β-catenin/TCF/LEF mediated transcriptional activity. Bars represent standard error of the means of two independent experiments performed in triplicate. Bottom panel: Immunoblot analysis of cyclin D1 levels in PC3 cells treated with either PP2 or vehicle (DMSO) for 48 h. Actin was used as a loading control. Blot represents two independent experiments. D, Effect of Src inhibition on growth of PC3 and PC3-α cells. Twenty-four hours after plating, PP2 or vehicle (DMSO) was added to the media and incubated for an additional 48 h before counting of the cells. Bars represent standard error of the means of two independent experiments done in triplicate.
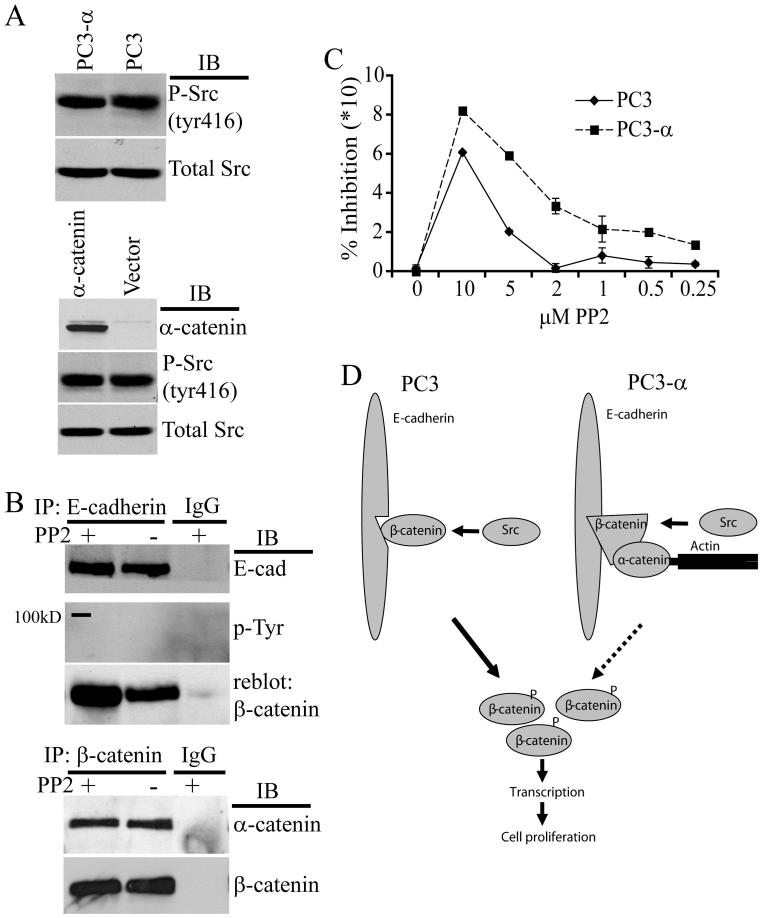
Interestingly, Src inhibition resembled the effects on β-catenin function induced by the expression of α-catenin in PC3 cells. Therefore, it is possible that α-catenin expression either reduces Src activity in PC3 cells or inhibits Src-induced cytoplasmic and nuclear translocation of β-catenin through stabilizing its association with E-cadherin. However, the levels of phosphorylated Src were similar in PC3 and PC3-α cell lines (Fig. 6A, top panel) as well as in control and α-catenin-transfected cells (Fig. 6A, bottom panel) indicating that α-catenin expression does not alter the activation status of Src.
Figure 6. α-Catenin reduces the effects of Src phosphorylation on β-catenin.
A, Top panel: Immunoblot for active, phosphorylated Src (Tyr 416) of PC3 and PC3-α cells. Immunoblot of total Src after stripping was used as a loading control. Bottom panel: Immunoblot for α-catenin, phosphorylated (Tyr 416) and total Src of cell lysates of PC3 cells transiently expressing α-catenin. Blot represents data from three independent experiments. B, Top panel: Src inhibition affects E-cadherin-β-catenin association in PC3-α cells. E-cadherin was immunoprecipitated from equal amounts of PC3-α cell protein lysate treated with either PP2 or vehicle (DMSO). Immunoprecipitates were blotted for E-cadherin (top blot) or for tyrosine phosphorylated β-catenin (middle blot). Blot from middle panel was reblotted for β-catenin. IgG was used as a negative control. Blot represents data from three independent experiments. Bottom panel: α-catenin-β-catenin association in PC3-α cells treated with PP2. β-catenin was immunoprecipitated from equal amounts of total PC3-α protein lysates treated with either PP2 or vehicle (DMSO) for 24 h. Immunoprecipitates were immunoblotted for α-catenin, stripped, and blotted for β-catenin. IgG was used as a negative control. Blot represents data from two independent experiments. C, Dose response effect of Src inhibition on DNA synthesis in PC3 and PC3-α cells. The 3H-thymidine incorporation in untreated PC3 and PC3-α cells was considered as 0% inhibition. Bars represent standard error of the means of two independent experiments done in triplicate. D, Model for α-catenin-mediated regulation of β-catenin. In α-catenin null PC3 cells, the unstable association between E-cadherin and β-catenin is further destabilized by phosphorylation of β-catenin by Src. Phosphorylated β-catenin disassociates from E-cadherin and enters the cytoplasm. Build up of β-catenin within the cytoplasm overwhelms APC/GSK-3β and enters the nucleus, leading to transcription and increased cell proliferation. In PC3-α cells, expression of α-catenin stabilizes the association between E-cadherin and β-catenin and reduces transfer of β-catenin into the cytoplasm/nucleus (indicated by dashed lines). The reduction of cytoplasmic levels of β-catenin by α-catenin reduces transcription and cell proliferation.
Next, to test whether α-catenin increases β-catenin association with E-cadherin, E-cadherin was immunoprecipitated from PC3-α cells treated with and without PP2 and immunoblotted with anti-phosphotyrosine antibody. No bands corresponding to the expected size of β-catenin (95kD) were observed (Fig. 6B, top panel), showing that β-catenin associated with E-cadherin was not tyrosine-phosphorylated. However, reblotting for β-catenin revealed a 30% increase in the levels of β-catenin associated with E-cadherin in PP2-treated cells when compared with untreated cells (Fig. 6B). This finding suggested that in PC3-α cells, inhibition of Src further increases the levels of β-catenin associated with E-cadherin. The levels of α-catenin associated with β-catenin were similar in PP2-treated and untreated control cells (Fig. 6B, bottom panel).
The increased amount of β-catenin associated with E-cadherin after PP2 treatment in PC3-α cells suggested that Src inhibiton in this cell line should have a more pronounced effect on cell proliferation. Consistent with this idea, treatment of PC3-α cells with PP2 resulted in an increase in the doubling time to 103 h compared with 43 h in vehicle-treated cells (Fig. 5D, P=0.05). In addition, a PP2 concentration of 10 μM resulted in an 82.1% (± 0.4) inhibition of 3H-thymidine incorporation in PC3-α cells compared with 60.9% (± 0.6) in PC3 cells (Fig. 6C). Strikingly, at low dose (2 μM) PC3-α cells showed a 40% inhibition of incorporation whereas there was hardly any inhibition in PC3 cells. The calculated IC50 for PP2 in PC3 and PC3-α cells were 8.4 μM and 3.9 μM, respectively (P=0.004, by student t-test), indicating that PC3-α cells are sensitive to small doses of PP2 compared with PC3 cells. These results demonstrated that Src inhibition has a more pronounced effect on cell proliferation in α-catenin-expressing cells.
Discussion
In this study, we reported that α-catenin expression in PC3 cells significantly reduced the levels of cytoplasmic/nuclear β-catenin, β-catenin/TCF/LEF transcriptional activity, cyclin D1 levels and cell proliferation. We confirmed that these effects are due to α-catenin expression by using a revertant clone lacking α-catenin as well as transient transfection of α-catenin into PC3 cells. The specific involvement of β-catenin was validated by RNAi-mediated knockdown of β-catenin. We also demonstrated that inhibition of Src in PC3 cells significantly reduced β-catenin transcriptional activity, cyclin D1 levels, and cell proliferation due to increased association of β-catenin with E-cadherin. Furthermore, we showed that α-catenin expression attenuated the effect of Src phosphorylation of β-catenin by increasing its association with E-cadherin and enhanced the sensitivity to a Src inhibitor in the suppression of cell proliferation. Thus, these studies demonstrated for the first time that α-catenin is a key regulator of β-catenin transcriptional activity and acts as a “switch” to modulate β-catenin’s cell-cell adhesion and cell proliferation functions.
Role of α-Catenin in Cell Proliferation
We showed that α-catenin regulates cell proliferation by reducing β-catenin transcriptional activity and cyclin D1 levels. Although previous studies have shown that α-catenin expression results in reduced proliferation of cells, the mechanism remains unknown (19, 20, 22, 24). Keratinocytes obtained from α-catenin knockout mice exhibited hyperproliferation, yet, in this case, it was due to sustained activation of the Ras-ERK pathway (21). Expression of α-catenin in colon carcinoma cell lines suppressed β-catenin transcriptional activity by interfering with the complex formation between β-catenin and TCF within the nucleus (36). We demonstrated a novel mechanism in which α-catenin restricts β-catenin to cell-cell contact sites and attenuates the effects of Src phosphorylation to reduce its transcriptional activity and cell proliferation. These results demonstrated that α-catenin is a key player in the regulation of β-catenin functions in prostate cancer cells.
The mechanism of cyclin D1 regulation in prostate cancer is complex (37, 38). Real time PCR analysis revealed similar transcript levels of cyclin D1 in PC3, PC3-Rev and PC3-α cells (data not shown), indicating that β-catenin modulates cyclin D1 levels post-transcriptionally in PC3-α cells. It is not uncommon that transcription factors regulate protein expression at the post-transcriptioal level (39). Experiments are in progress in our laboratory to identify mechanisms by which β-catenin regulates cyclin D1 in PC3-α cells.
In addition, the levels of c-myc transcript and protein were similar in PC3, PC3-Rev and PC3-α cells (data not shown), consistent with the amplification of myc in this cell line (40). A similar finding has been reported in human colorectal adenocarcinoma where aberrant activation of β-catenin resulted in increased protein levels of cyclin D1, but no change in c-myc levels, both at the protein and mRNA levels (41).
Src and β-Catenin Transcriptional Activity
Phosphorylation of β-catenin by Src reduces its ability to associate with E-cadherin (16, 34, 35). In this study we showed that inhibition of Src resulted in an enhanced association of β-catenin with E-cadherin with a concomitant reduction in cytoplasmic/nuclear β-catenin. This finding indicates that in PC3 cells inhibition of Src makes β-catenin competent to bind E-cadherin and reduces its transcriptional activity. Although APC is localized to the chromosome 5, the APC levels were similar in PC3, PC3-Rev and PC3-α cells (data not shown). Our results indicate that α-catenin expression in PC3 cells sequesters β-catenin at the adherens junction and reduces its accumulation in the cytoplasm. In the absence of α-catenin the APC/GSK-3β complex involved in β-catenin degradation might be saturated, allowing β-catenin to escape the degradation mechanism to enter the nucleus and activate transcription of target genes (Fig. 6D).
The most striking finding reported here is that PC3 and PC3-α cells had similar levels of active Src, yet the level of β-catenin associated with E-cadherin was 65% higher in PC3-α cells compared with PC3 cells. This indicated that α-catenin protects β-catenin from Src phosphorylation by increasing the stability of its interaction with E-cadherin (Fig. 6D), possibly by inducing changes in the conformation of β-catenin. It has been suggested that β-catenin exists in two distinct conformations for binding to E-cadherin and TCF and that binding of α-catenin to β-catenin enhances β-catenin’s affinity for E-cadherin while decreasing its affinity to TCF (42). Future experiments are necessary to validate whether Src phosphorylation and α-catenin binding of β-catenin alters its conformation to modulate binding to E-cadherin.
Clinical Relevance
Our study showed that loss of α-catenin might result in the activation of β-catenin signaling, providing a proliferative advantage to prostate cancer cells. Loss or altered expression of α-catenin exists in both prostate cancer cells and tissue samples (43, 44)(41,42) and correlates with patient survival (44-46). Since Src inhibition reduces invasion in cancers including prostate cancer, several Src inhibitors are being tested in clinical trials for their ability to reduce invasion and metastatic potential of cancer cells (47, 48). Treatment of various colon cancer cells with PP2 reduces in-vivo tumor formation and metastasis (49). Likewise, inhibition of Src activity in DU145 and LNCAP cells also reduces in-vivo tumor formation and metastasis (50, 51), while in-vitro experiments with PC3 cells demonstrate decreased growth and motility (52). Since PC3-α cells have lost their tumorigenicity (20), it is difficult to assess the effects of α-catenin and Src inhibition in vivo. However, our results for the first time revealed that Src inhibition, in combination with α-catenin expression, should substantially reduce β-catenin transcriptional activity and cell proliferation. Therefore, Src inhibitors should have tremendous clinical value in suppressing tumor growth in α-catenin expressing cancers. In addition, our data on the increased sensitivity of PP2 in α-catenin-positive cells indicated that patients without α-catenin expression might require higher doses of these compounds compared with patients positive for α-catenin expression in their tumors. One of the challenges of translational medicine is to identify patients who might respond positively to targeted therapy prior to treatment. We propose that α-catenin could serve as a biomarker for sensitivity to Src inhibitors in prostate and possibly other cancers. Further, due to the narrow range of options available to prostate cancer patients with advanced disease, the α-catenin status in prostate cancer patients would allow clinicians to better determine who might respond well to Src inhibitors and should be validated in future clinical trials.
Acknowledgments
This study was supported by NIH grants DK 56216, F31-GM068985 and DOD W81XWH-04-1-0113.
Abbreviations
- APC
adenomatous polyposis coli
- CMV
cytomegalovirus
- CPM
counts per minute
- DMSO
dimethyl sulfoxide
- FITC
fluorescein isothiocyanate
- GSK-3β
glycogen synthase kinase-3β
- LEF
lymphoid enhancer factor
- TBST
tris-buffered saline with Tween 20
- TEM
transmission electron microscopy
- ZO-1
zonula occludens-1
References
- 1.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 2.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–27. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 3.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255–68. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 6.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–9. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–47. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 9.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 10.Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 11.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 13.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 14.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–7. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 15.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–30. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coluccia AM, Benati D, Dekhil H, De Filippo A, Lan C, Gambacorti-Passerini C. SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of beta-catenin and its nuclear signaling. Cancer Res. 2006;66:2279–86. doi: 10.1158/0008-5472.CAN-05-2057. [DOI] [PubMed] [Google Scholar]
- 17.Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–25. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott JA, Yap AS. Cinderella no longer: alpha-catenin steps out of cadherin’s shadow. J Cell Sci. 2006;119:4599–605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- 19.Bullions LC, Notterman DA, Chung LS, Levine AJ. Expression of wild-type alpha-catenin protein in cells with a mutant alpha-catenin gene restores both growth regulation and tumor suppressor activities. Mol Cell Biol. 1997;17:4501–8. doi: 10.1128/mcb.17.8.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewing CM, Ru N, Morton RA, et al. Chromosome 5 suppresses tumorigenicity of PC3 prostate cancer cells: correlation with re-expression of alpha-catenin and restoration of E-cadherin function. Cancer Res. 1995;55:4813–7. [PubMed] [Google Scholar]
- 21.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–17. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 22.Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–56. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- 24.Matsubara S, Ozawa M. Expression of alpha-catenin in alpha-catenin-deficient cells results in a reduced proliferation in three-dimensional multicellular spheroids but not in two-dimensional monolayer cultures. Oncogene. 2004;23:2694–702. doi: 10.1038/sj.onc.1207423. [DOI] [PubMed] [Google Scholar]
- 25.Anilkumar G, Barwe SP, Christiansen JJ, Rajasekaran SA, Kohn DB, Rajasekaran AK. Association of prostate-specific membrane antigen with caveolin-1 and its caveolae-dependent internalization in microvascular endothelial cells: implications for targeting to tumor vasculature. Microvasc Res. 2006;72:54–61. doi: 10.1016/j.mvr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Rajasekaran SA, Gopal J, Espineda C, Ryazantsev S, Schneeberger EE, Rajasekaran AK. HPAF-II, a cell culture model to study pancreatic epithelial cell structure and function. Pancreas. 2004;29:e77–83. doi: 10.1097/00006676-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Espineda CE, Chang JH, Twiss J, Rajasekaran SA, Rajasekaran AK. Repression of Na,K-ATPase beta1-subunit by the transcription factor snail in carcinoma. Mol Biol Cell. 2004;15:1364–73. doi: 10.1091/mbc.E03-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 29.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 30.Korinek V, Barker N, Willert K, et al. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998;18:1248–56. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–21. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 32.Buckley MF, Sweeney KJ, Hamilton JA, et al. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–33. [PubMed] [Google Scholar]
- 33.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–71. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 34.Piedra J, Miravet S, Castano J, et al. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–97. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 36.Giannini AL, Vivanco MM, Kypta RM. Analysis of beta-catenin aggregation and localization using GFP fusion proteins: nuclear import of alpha-catenin by the beta-catenin/Tcf complex. Exp Cell Res. 2000;255:207–20. doi: 10.1006/excr.1999.4785. [DOI] [PubMed] [Google Scholar]
- 37.Casimiro M, Rodriguez O, Pootrakul L, et al. ErbB-2 induces the cyclin D1 gene in prostate epithelial cells in vitro and in vivo. Cancer Res. 2007;67:4364–72. doi: 10.1158/0008-5472.CAN-06-1898. [DOI] [PubMed] [Google Scholar]
- 38.Comstock CE, Revelo MP, Buncher CR, Knudsen KE. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br J Cancer. 2007;96:970–9. doi: 10.1038/sj.bjc.6603615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–85. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya N, Kondo Y, Takahashi A, et al. Mapping and gene expression profile of the minimally overrepresented 8q24 region in prostate cancer. Am J Pathol. 2002;160:1799–806. doi: 10.1016/S0002-9440(10)61126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang HL, Wang J, Xiao SY, et al. Elevated protein expression of cyclin D1 and Fra-1 but decreased expression of c-Myc in human colorectal adenocarcinomas overexpressing beta-catenin. Int J Cancer. 2002;101:301–10. doi: 10.1002/ijc.10630. [DOI] [PubMed] [Google Scholar]
- 42.Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–49. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Isaacs WB. Reduction of E-cadherin levels and deletion of the alpha-catenin gene in human prostate cancer cells. Cancer Res. 1993;53:3585–90. [PubMed] [Google Scholar]
- 44.Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E-cadherin and alpha-catenin expression in prostate cancer: correlation with patient survival. Cancer Res. 1997;57:3189–93. [PubMed] [Google Scholar]
- 45.Aaltomaa S, Lipponen P, Ala-Opas M, Eskelinen M, Kosma VM. Alpha-catenin expression has prognostic value in local and locally advanced prostate cancer. Br J Cancer. 1999;80:477–82. doi: 10.1038/sj.bjc.6690381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morita N, Uemura H, Tsumatani K, et al. E-cadherin and alpha-, beta- and gamma-catenin expression in prostate cancers: correlation with tumour invasion. Br J Cancer. 1999;79:1879–83. doi: 10.1038/sj.bjc.6690299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T, George JA, Taylor CC. Src tyrosine kinase as a chemotherapeutic target: is there a clinical case? Anticancer Drugs. 2006;17:123–31. doi: 10.1097/00001813-200602000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 49.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–6. [PubMed] [Google Scholar]
- 50.Evans CP, Lara PNJ, Kung H, Yang JC. Activity of the Src-kinase inhibitor AZD0530 in androgen-independent prostate cancer (AIPC): Pre-clinical rationale for a phase II trial. Clin Oncol. 2006;24:14542. [Google Scholar]
- 51.Goldenberg-Furmanov M, Stein I, Pikarsky E, et al. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64:1058–66. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 52.Recchia I, Rucci N, Festuccia C, et al. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur J Cancer. 2003;39:1927–35. doi: 10.1016/s0959-8049(03)00394-0. [DOI] [PubMed] [Google Scholar]