Abstract
To initiate infection, poliovirus must release its RNA genome to the cytoplasm of a target cell, a process called uncoating. How this occurs has remained uncertain, despite studies over several decades. Two new studies readdress the question of poliovirus entry. The results suggest that poliovirus enters different cells by different mechanisms, and point to a role for virus-induced intracellular signals in the process.
RNA delivery to the cytoplasm is the essential entry event
Poliovirus, the causative agent of paralytic poliomyelitis, is a small non-enveloped virus, with a single strand RNA genome encased in an icosahedral protein capsid. Before the development of an effective vaccine, poliovirus was a fearsome pathogen and the focus of intense scientific study [1]. Today polio is no longer a major threat to health in developed countries, but global efforts to eradicate the disease still face serious obstacles [2]. And despite landmark studies of poliovirus replication, structure, and receptor interactions, many aspects of poliovirus biology remain poorly understood [3].
Poliovirus replicates in the cytoplasm. Injection of naked viral RNA into a cell is sufficient to cause infection, so the essential event in virus entry is delivery of RNA to the cytoplasm. The capsid protects the viral genome from the environment, and— by binding to the specific poliovirus receptor (PVR)— facilitates delivery to appropriate target cells. As infection begins, viral RNA must be freed from the capsid (a process referred to as “uncoating”) and penetrate the cell. Either the viral particle (the virion) or the RNA itself must thus cross cellular membranes as entry occurs.
Controversies about poliovirus entry
Experiments in the 1980’s suggested that poliovirus entered cells in clathrin-coated vesicles, and that virus was delivered to endosomes, where uncoating was triggered by exposure to acidic pH. Poliovirus was observed within coated vesicles in electron micrographs [4], and drugs that interfered with endosomal acidification were reported to prevent uncoating and infection [5, 6]. However, subsequent experiments with bafilomycin, a powerful inhibitor of the endosomal proton pump, called into question the need for endosomal acidification [7]. And it was demonstrated that inhibition of dynamin, a GTPase required for internalization of clathrin-coated vesicles, does not block poliovirus replication in HeLa cells [8], suggesting that clathrin-mediated endocytosis is not essential for entry.
Although the conflicting experimental results are not fully explained, it is important to consider that drugs may have unexpected ("off-target") effects on cells, and that every virion within the cell is not necessarily infectious. As is true of many viruses, poliovirus is characterized by a very high ratio of physical particles to infectious units (particle/PFU ratio of 200 or more). Infection of a single cell may require its exposure to hundreds of viral particles, so it is difficult to tell whether the particle seen in an electron micrograph is destined for infection or headed toward a dead end.
Receptor-mediated endocytosis of many viruses (and other macromolecules) occurs in clathrin-coated vesicles, but a number of other entry routes have been identified [9]. Some viruses are internalized in caveolae, small vesicles marked by the transmembrane protein caveolin. Others appear to enter cells in less well-defined vesicles, containing neither clathrin nor caveolin, and characterized by their dependence on cellular factors such as membrane cholesterol, dynamin, flotillin, and small GTPases [10, 11].
It is conceivable that entry of the virion (as distinct from the RNA genome) is not required at all. After contact with soluble PVR in vitro, poliovirus undergoes conformational changes that culminate in release of RNA [12–14]. Hydrophobic domains within capsid proteins become exposed, and interact with membranes to form pores that may facilitate RNA transfer across the membrane [15]. Uncoating might occur at the cell surface: as poliovirus contacts PVR, conformational changes could lead to pore formation, release of RNA, and delivery across the plasma membrane.
Imaging poliovirus entry in live HeLa cells: a clathrin- and caveolin-independent pathway
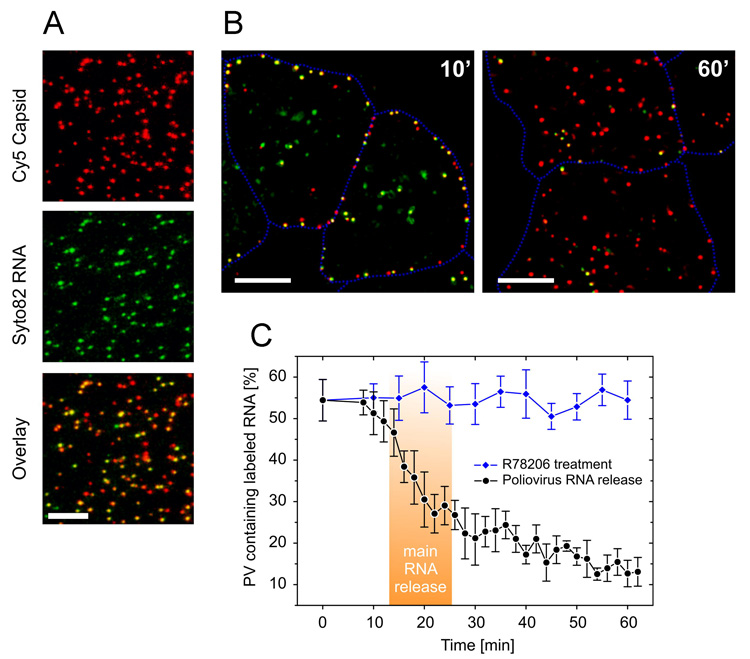
Two recent papers have revisited the mechanism of poliovirus entry, using fluorescence microscopy to trace the movement of virions, and a combination of approaches to correlate the movement of virions with the pathway of productive infection [16, 17]. In the first study, performed in the laboratories of James Hogle and Xiaowei Zhuang at Harvard, Brandenburg et al. [16] have used sophisticated imaging techniques to follow the entry of poliovirus virions, with capsid proteins and RNA marked by distinct fluorescent labels; the experiments were performed at low multiplicity of infection, to minimize the possibility that events on the pathway to infection were not obscured by non-productive events. Quite remarkably, these authors were able to watch as individual dual-labeled virions lost their RNA signal, indicating the release of RNA from the capsid and its delivery into the cell (Figure 1). Uncoating was seen to occur rapidly (within 20 min), and at the cell periphery. Most virions were seen to release RNA, suggesting that inefficiencies in the uncoating process are not likely to account for the high poliovirus particle/PFU ratio; over time, single-labeled (i.e., empty) capsids were seen to collect deeper within the cell, but dual-labeled virions were observed only at, or near, the cell surface. To determine whether uncoating occurred within the cell or on the surface, capsids were labeled with a pH-sensitive dye that loses fluorescence in alkaline medium, and cells were pulsed with alkaline medium during the entry process. RNA release was observed only from those virions that remained insensitive to pH change—virions that had already entered the cell. Thus uncoating occurred within the cell, but very close to the cell surface.
Figure 1. Poliovirus entry and RNA release in HeLa cells.
(A) Virions with capsid proteins labeled in red, and RNA in green. In the overlay (merged) image, dual-labeled virions appear yellow or orange. (B) Release of RNA between 10 and 60 minutes post-infection. At 10 minutes, yellow dual-labeled virions are concentrated at the cell periphery; by 60 minutes, most virions are red, indicating loss of RNA. (C) Kinetics of RNA release. By approximately 25 minutes, half of the dual labeled virions have lost RNA. RNA release is not seen when virions are treated with an uncoating inhibitor, R78206. (Figure adapted from reference 16).
A second approach was used to determine that RNA release observed by microscopy was, in fact, related to infection. Virus grown in the presence of neutral red incorporates the dye in close proximity to the RNA genome; when exposed to light, neutral red interacts with and damages the RNA, preventing infection [6]. However, once uncoating has occurred, RNA is no longer susceptible to inactivation by light. Given that RNA release occurs by 20 min, a light flash at 30 min should have no effect on infection. However, if uncoating is inhibited— by drugs, siRNAs, or other agents—infection should be blocked. (If the agent prevents infection in the absence of a light flash, one cannot determine whether it prevents uncoating or acts at another stage of replication.)
Brandenburg et al. found that both RNA release (determined by microscopy), and functional uncoating (determined by the neutral red infection assay, also performed at low multiplicity) were inhibited by agents that deplete cells of ATP, disrupt the actin cytoskeleton, or inactivate tyrosine kinases. In contrast, bafilomycin, and agents that interfere with clathrin-, caveolin-, and flotillin- dependent endocytosis, had no effect on poliovirus infection.
Taken together, the results indicate that uncoating does not occur on the cell surface, simply as the consequence of interaction with PVR. It takes place within the cell, and requires energy, an intact actin cytoskeleton, and the activity of cellular tyrosine kinases. The authors conclude that uncoating occurs within clathrin- and caveolin-independent vesicles in the cell periphery. The precise nature of the endocytic vesicles is likely to be the subject of further work, as will be identification of the intracellular cues that — in addition to contact with PVR— are required for the uncoating process.
Aside from the specific experimental results, the techniques employed by Brandenberg and colleagues are likely to be applicable to studies of other viruses; with improvements in RNA labeling it may be possible to view directly the trafficking of viral genomes to sites of replication.
Entry into microvascular endothelial cells: PVR-induced signals trigger caveolar endocytosis
In the second study, Carolyn Coyne (formerly in my lab, now at the University of Pittsburgh), examined poliovirus entry into human brain microvascular endothelial cells (HBMEC) [17]. HBMEC are polarized cells, with intercellular tight junctions that provide a barrier to the paracellular movement of ions and macromolecules; they serve as a model of the blood brain barrier, which prevents the movement of molecules and pathogens (such as poliovirus) from the bloodstream to the brain.
In HBMEC, as in HeLa cells, uncoating was found to occur inside the cell. A-particles were formed at the cell surface after contact with PVR, but they entered (a process requiring at least two hours) and persisted within the cell before uncoating was completed. However, unlike what was seen in HeLa cells, virus entry depended on both caveolin and dynamin, and virus was detected in caveolin-containing vesicles within the cytoplasm (Figure 2). It thus seems likely that poliovirus uses different mechanisms as it enters different cell types (Figure 3).
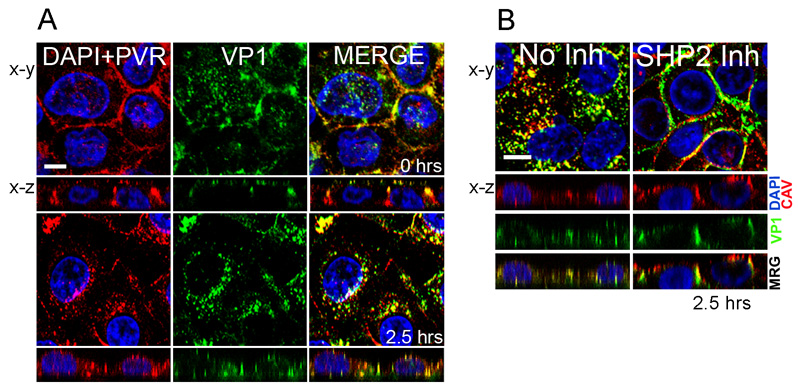
Figure 2. Poliovirus entry in brain microvascular endothelial cells.
(A) Virus enters the cell and moves to a perinuclear compartment. At 0 hrs, virus stained with anti-VP1 antibody (green) is seen at the cell surface most evident in x-z images. Poliovirus receptor (PVR, red) is also at the surface, largely concentrated at intracellular contacts. Cell nuclei are stained with DAPI (blue). By 2.5 hours post-infection, both virus and PVR have moved from the surface to a perinuclear compartment. (B) SHP-2 is required for virus entry. In control cells, virus (VP1, green) is seen within the cell, where it colocalizes with caveolin (CAV, red). In cells treated with an inhibitor of SHP-2 phosphatase, virus remains at the cell surface, largely at intercellular contacts. (Figure adapted from reference 17).
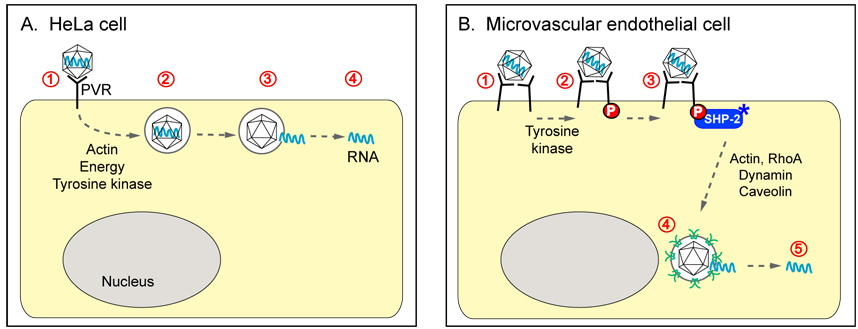
Figure 3. Poliovirus entry events in two cell types.
(A) In HeLa cells, virus interacts with poliovirus receptor (PVR) on the cell surface, then enters rapidly. RNA is released from virions contained in vesicles located just beneath the plasma membrane. Virus entry does not require either clathrin or caveolin, but RNA release depends on ATP, an intact actin cytoskeleton, and tyrosine kinase activity. (B) In brain microvascular endothelial cells, virus ligates PVR, leading to PVR phosphorylation by an unidentified tyrosine kinase, and the recruitment and activation of SHP-2. At approximately 2.5 hrs post-infection, virus enters the cell and concentrates with caveolin in vesicles near the nucleus. Entry and infection depend SHP-2, as well as Rho GTPase, actin rearrangements, dynamin GTPase, and caveolin.
The internalization of caveolar vesicles is not constitutive, but occurs in response to signals from specific ligands. And remarkably, a variety of virus-induced signaling events were required for entry into HBMEC. Interaction of virus with PVR led to phosphorylation of the PVR cytoplasmic domain (by an unidentified tyrosine kinase), which, in turn, induced the recruitment and activation of a protein tyrosine phosphatase, SHP-2. Although the precise function of SHP-2 is not yet clear, its activation was clearly required for virus entry and infection. In addition, PVR ligation was found to trigger — through activation of Rho GTPase— dramatic rearrangements of the actin cytoskeleton that were also required for the entry process. Thus PVR, already known to mediate virus attachment to target cells, and to initiate the uncoating process, serves an additional vital function— induction of intracellular signals for virus entry.
Given that tyrosine kinase activity is also required for entry into HeLa cells, it is likely that receptor-induced signals will prove to be important for infection in a variety of cell types. Defining the signaling pathways involved, and the roles of specific signals in the entry process, is likely to provide new insights into poliovirus biology. Because viruses have evolved to make use of cellular machinery, identifying their diverse entry mechanisms will extend our understanding of normal endocytic processes. After all these years, we still have something to learn from poliovirus.
References
- 1.Oshinsky DM. Polio: an American story. Oxford University Press; 2006. [Google Scholar]
- 2.Fine PE, Griffiths UK. Global poliomyelitis eradication: status and implications. Lancet. 2007;369:1321–1322. doi: 10.1016/S0140-6736(07)60533-9. [DOI] [PubMed] [Google Scholar]
- 3.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344:9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Zeichhardt H, et al. Entry of poliovirus type 1 and Mouse Elberfeld (ME) virus into HEp-2. Cells: Receptor-mediated endocytosis and endosomal or lysosomal uncoating. J. Virology. 1985;66:483–492. doi: 10.1099/0022-1317-66-3-483. [DOI] [PubMed] [Google Scholar]
- 5.Madshus IH, et al. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J. Cell Biol. 1984;98:1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madshus IH, et al. Requirements for poliovirus entry into cells at low pH. EMBO J. 1984;3:1945–1950. doi: 10.1002/j.1460-2075.1984.tb02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J. Virology. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. Embo J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1746:349–363. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Glebov OO, et al. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 12.Fricks CE, Hogle JM. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virology. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yafal A, et al. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 14.Xing L, et al. Distinct cellular receptor interactions in poliovirus and rhinoviruses. EMBO Journal. 2000;19:1207–1216. doi: 10.1093/emboj/19.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danthi P, et al. Genome delivery and ion channel properties are altered in VP4 mutants of poliovirus. J Virol. 2003;77:5266–5274. doi: 10.1128/JVI.77.9.5266-5274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandenburg B, et al. Imaging poliovirus entry in live cells. PLoS Biol. 2007;5:e183. doi: 10.1371/journal.pbio.0050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyne CB, et al. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. EMBO J. 2007;26:4016–4028. doi: 10.1038/sj.emboj.7601831. [DOI] [PMC free article] [PubMed] [Google Scholar]