Summary
When humans attempt to perform two tasks at once, execution of the first task usually leads to postponement of the second one. This task delay is thought to result from a bottleneck occurring at a central, amodal stage of information processing that precludes two response selection or decision-making operations from being concurrently executed. Using time-resolved functional magnetic resonance imaging (fMRI), here we present a neural basis for such dual-task limitations: the inability of the posterior lateral prefrontal cortex, and possibly the superior medial frontal cortex, to process two decision-making operations at once. These results suggest that a neural network of frontal lobe areas act as a central bottleneck of information processing that severely limits our ability to multi-task.
Introduction
Despite the impressive complexity and processing power of the human brain, it exhibits severe capacity limits in information processing. Nowhere is this better illustrated than when we attempt to perform two tasks at once, as such conditions will almost invariably lead to interference between the tasks. This is not only evident when executing such demanding tasks as talking on the cell phone while driving (Beede and Kass, 2006; Strayer and Drews, 2004), but also when attempting such simple tasks as selecting the appropriate motor responses for two distinct sensory events.
Dual-task costs have been extensively studied with the psychological refractory period (PRP) paradigm (Pashler, 1994a; Welford, 1952). In this paradigm, subjects are required to select different motor responses for two distinct sensory stimuli presented at variable stimulus onset asynchronies (SOAs). The dual-task interference is revealed by the increasingly longer response time (RT) to the second task as the SOA between the two tasks decreases. This response delay is thought to result from an inability to select two responses or make two decisions at once, thereby leading to the serial postponement of the second task at short SOAs (Pashler, 1994a; Welford, 1952). Importantly, this ‘bottleneck’ does not occur at perceptual or motor stages of information processing, which can proceed in parallel for the two tasks, but at a central amodal stage of information processing (Pashler, 1998; Sigman and Dehaene, 2005) (Fig. 1A, upper row).
Figure 1. Model, task design and behavioural results for the dual-task experiment.
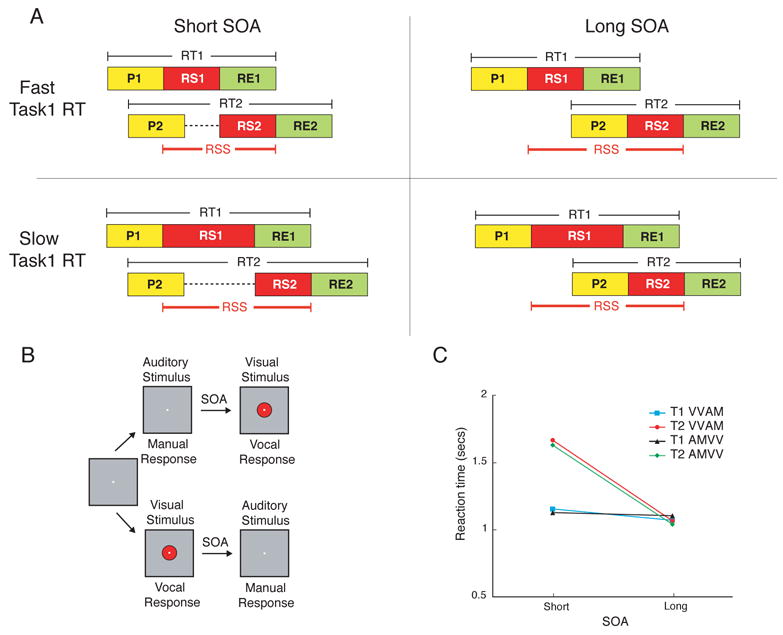
(A) Central Bottleneck Model. According to this model, sensory information proceeds through a series of processing stages, including stimulus perception (P), response selection (RS) and response execution (RE). Cognitive operations that require central processing (e.g. RS) can only proceed serially, whereas other operations (i.e. P and RE) can occur in parallel (Pashler, 1994a). At short SOAs, response selection for Task1 (RS1) overlaps with that for Task2, causing Task2 response selection (RS2) to be postponed and hence Task2 RT to be prolonged (upper left panel). At long SOAs, RS1 is completed before RS2 commences and, as a result, Task2 reaction time (RT2) is faster than at short SOAs (upper right panel). The Central Bottleneck model makes strong predictions regarding the influence of Task1 reaction time (RT1) on the response selection span (RSS: onset of RS1 to offset of RS2) in dual-task trials. At the short SOA, an increase in Task1 RT leads to a proportional increase in RSS (left column of panels). At the long SOA, increases in Task1 RT do not affect RSS as the variability in Task1 RT occurs before onset of Task2 processing (right column of panels). (B) Task Design. In the dual-task experiment each trial commenced with the presentation of one of eight visual (or auditory) stimuli for 200 ms followed by, after either a short or long SOA, Task2’s auditory (or visual) stimulus. Subjects responded vocally to the visual stimulus and manually to the auditory stimulus. (C) Behavioral Results. Task2 RT was increased at the short SOA relative to the long SOA (PRP effect). By contrast, Task1 RT was minimally affected by the SOA manipulation. There was no effect of task order (AMVV vs. VVAM).
Despite the pervasiveness of this capacity-limited process in human cognition (Pashler, 1998), its neural basis remains unknown (Jiang et al., 2004; Marois and Ivanoff, 2005). Investigations of dual-task slowing (Herath et al., 2001; Ivry et al., 1998; Jiang, 2004; Jiang et al., 2004; Luck, 1998; Marois et al., 2005; Osman and Moore, 1993; Pashler et al., 1994; Szameitat et al., 2002) have highlighted the lateral frontal, prefrontal, dorsal premotor, anterior cingulate and intra-parietal cortex as putative neural substrates of dual-task interference. A recent review of the literature particularly points to the lateral frontal/prefrontal and dorsal premotor cortex as key neural substrates of the central bottleneck of information processing (Marois and Ivanoff, 2005). However, the localization of this central bottleneck has been hampered by the limited spatial and/or temporal resolutions of these investigations. In particular, previous neuroimaging studies have relied on BOLD response amplitude as a measure of dual-task interference when the PRP actually reveals a fundamental temporal limitation in concurrently processing two tasks.
Time-resolved fMRI, the application of fMRI to discern the timing and duration of neural activity across brain regions (Formisano and Goebel, 2003), provides a potentially fruitful approach to unraveling the neural basis of dual-task limitations. By rapidly sampling brain activity while subjects performed a task that generated a prolonged PRP, we were able to bring this dual-task limitation within the temporal resolution of fMRI, thereby revealing the spatio-temporal hemodynamics of the central bottleneck. In particular, we present evidence that the posterior lateral prefrontal cortex (pLPFC) fulfilled three key criteria expected of the neural substrates of the central bottleneck of information processing: it was co-activated by tasks that shared neither sensory nor output modalities, it was involved in response selection and, crucially, it exhibited serial queuing of response selection activity under dual-task interference conditions, as predicted by the central bottleneck model of the PRP. In addition to the pLPFC, the superior medial frontal cortex (SMFC) also showed an activation pattern that was generally consistent with that expected of a neural substrate of the central bottleneck.
Results
Localizer Task
For each experiment, we first localized in individual subjects brain regions that were commonly activated by two single sensorimotor tasks that did not overlap either in their sensory or motor modalities, as would be expected of the neural substrates underlying the central bottleneck (Jiang and Kanwisher, 2003; Marois and Ivanoff, 2005). One task consisted of choosing the appropriate manual (finger) response to an auditory stimulus (AM Task), while the other consisted of choosing the appropriate vocal response to a visual stimulus (VV Task). Each task involved an eight alternative forced choice (AFC). The following brain regions, which are considered neither sensory nor motor and which have all been observed in previous fMRI studies of the PRP, were activated by each of the two tasks relative to a fixation baseline condition: pLPFC centered in the posterior extent of Brodmann area 9 (BA 9) of the left and right hemispheres (Figs. 2A, 3A, 4A) (Marois et al., 2005; Schubert and Szameitat, 2003), left/right inferior frontal gyrus (IFG) (BA44, Figs. 2D and 4C) (Herath et al., 2001; Jiang et al., 2004; Marois et al., 2005), dorsal pre-motor cortex (PMC) (Marois et al., 2005), anterior cerebellum (Ivry et al., 1998; Pashler et al., 1994), anterior cingulate cortex (ACC) (Marois et al., 2005; Schubert and Szameitat, 2003), and SMFC centered in pre-SMA/SMA of BA6 (Marois et al., 2005;(Schubert and Szameitat, 2003), as well as left Intra-Parietal Sulcus (IPS) (BA7, no right activation foci) (Szameitat et al., 2002). These regions of interest (ROIs) were defined in individual subjects and then probed in the dual-task, single-task and response selection load experiments described below.
Figure 2. Left LPFC and IFG activity in the VVAM Dual-task experiment (Experiment 1).
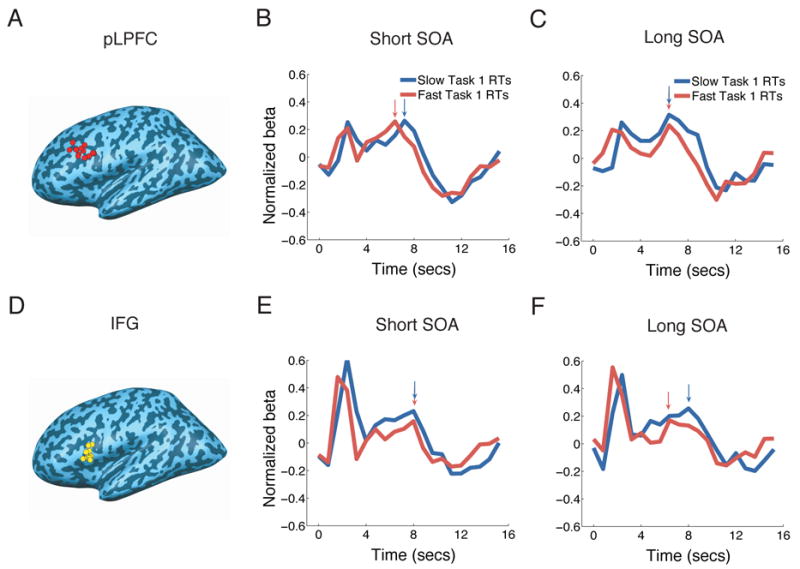
(A) and (D) Peak foci of individual left pLPFC (A, BA9) and IFG (D, BA44) ROIs isolated in the localizer task. Left pLPFC and IFG ROIs could be isolated in 12 and 13 of the 14 subjects, respectively. (B), (C), (E) and (F) BOLD time-courses for the fast and slow Task1 RTs at the short (B and E) and long (C and F) SOAs in the left pLPFC and IFG. pLPFC (upper row) activity peaked earlier in the fast RT than in the slow RT condition at the short SOA, but not at the long SOA. By contrast, the IFG (lower row) did not display serial postponement of activity at the short SOA. Arrows indicate peak latencies for each condition. Timecourses are time-locked to Task1 stimulus presentation. The early signal peaks near the onset of the time courses are due to vocal artifacts. These artifacts do not affect the main activation peaks (Birn et al., 2004).
Figure 3. Left LPFC activity in the Single-task experiment (Experiment 2).
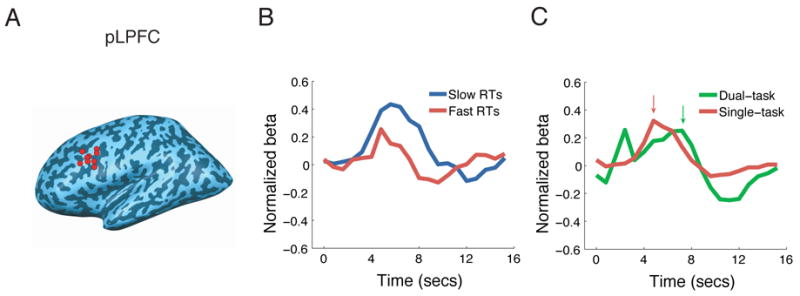
(A) Peak foci of individual left pLPFC ROIs isolated in the localizer task. All nine subjects had their ROI in BA9. (B) BOLD timecourses for the fast and slow RTs in pLPFC in the AM task. The RT condition affected peak latency, but not onset latency, of the BOLD response. Arrows indicate peak latency for each condition. Timecourses are time-locked to stimulus presentation. (C) Comparison of the BOLD time course in the single-task experiment to that in the short SOA VVAM condition of the dual-task experiment. The activation peaked later in the Dual-task than in the Single-task condition. The activation peak at the onset of the dual-task time course is due to vocal artifacts.
Figure 4. Response selection load experiment (Experiment 3).
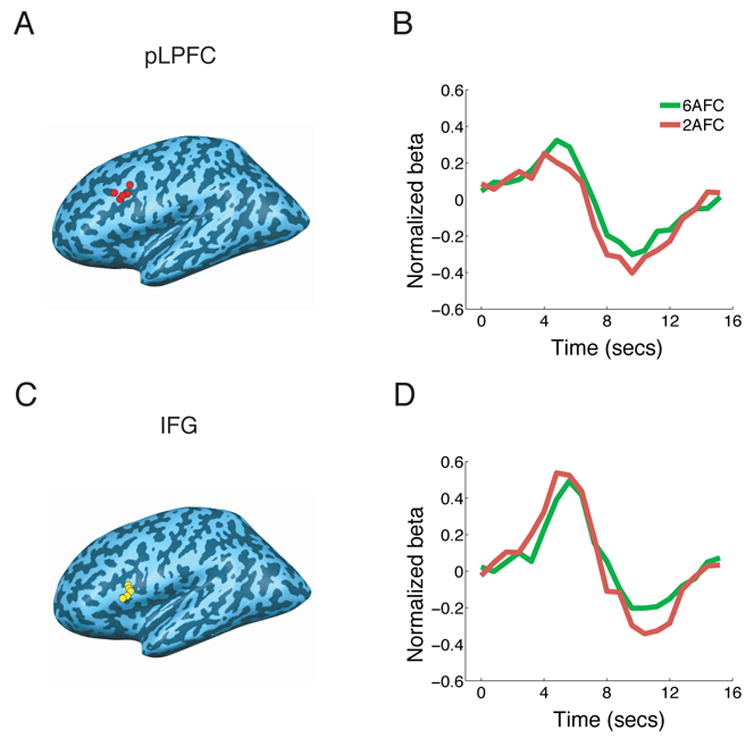
(A) and (C) Peak foci of individual left pLPFC (A, BA9) and IFG (C, BA44) ROIs isolated in the localizer task. Left pLPFC and IFG ROIs could be isolated in five of the six subjects. (B) and (D) BOLD timecourses for the 2AFC and 6AFC conditions of the AM task. Peak amplitude was greater in the 6AFC than in the 2AFC condition in pLPFC, but not in IFG. Although pLPFC peak latency tended to occur later in the 6AFC than the 2AFC condition, the RT difference between these conditions was too small (312ms) to be reliably detected with the present fMRI conditions.
Experiment 1: Dual-Task Experiment
The dual-task experiment employed 8AFC VV and AM tasks (Fig. 1B). For these dual-task trials, the SOA between the two tasks was either short (300ms) or long (1100ms for 6 subjects and 1900ms for 8 subjects, mean 1560ms). Because reaction time to Task1 was generally shorter than the duration of the long SOA (see below), significant dual-task interference was expected at the short but not the long SOA. In addition, the high number of response alternatives (eight) for each task was expected to generate long reaction times (Hick, 1952) and, consequently, prolonged PRPs (Karlin and Kestenbaum, 1968; Marois et al., 2005; Van Selst and Jolicoeur, 1997), thereby bringing the time course of dual-task interference within the temporal resolution of fMRI.
The behavioral data revealed a robust PRP (Fig. 1C) that was virtually identical for the AMVV and VVAM tasks: Task2 RT was much longer at the short than at the long SOA (n = 14, p = 0.0001, paired-samples t-test, two-tailed, this applies to all subsequent statistical tests except where noted), with no effect of accuracy (Task2 short SOA = 94.6%, Task2 long SOA = 95.2%; p = 0.32). By contrast, RT differences between Task1 and Task2 were marginal at the long SOA (Fig. 1C, p = 0.082), suggesting that dual-task interference was negligible at that SOA. Task1 RT was far less influenced by SOA, revealing an SOA effect that was only 9% that of Task 2 (Fig. 1C). Taken together, these results not only demonstrate that the present experimental design produced robust dual-task interference, but that this interference is largely revealed by a postponement of Task2, as predicted by the central bottleneck model (Pashler, 1994a; Welford, 1952) and other capacity-limited models of the PRP (Logan and Gordon, 2001; Navon and Miller, 2002; Tombu and Jolicoeur, 2003).
To assess whether any of the ROIs may be neural substrates of the central bottleneck, we tested whether they exhibited serial queuing of activity. The central bottleneck model predicts that, at the short SOA, response selection for Task2 is postponed until response selection for Task1 is completed (Fig. 1A), a prediction our data supports given the strong correlation between Task1 and Task2 RTs at the short SOA (r2 = 0.55). As a consequence, the span of response selection activity, as measured from onset of Task1 response selection to offset of Task2 response selection, should be proportional to reaction time to Task1 because response selection to Task2 is queued until completion of Task1 response selection. By contrast, because the mean RT to Task1 is shorter than the long SOA (1113ms vs. 1560ms), the model predicts that response selection for Task2 is largely independent of response selection for Task1, a hypothesis again supported by our data which showed a marginal correlation between Task1 and Task2 RT at the long SOA (r2 = 0.09). Thus, at the long SOA, the span of response selection activity should not be proportional to Task1 RT, as trial-to-trial variability in Task1 RT should be largely absorbed in the ‘slack period’ between the completion of response selection for Task1 and commencement of response selection for Task2 (Fig. 1A, lower row).
To test this prediction we compared, for both short and long SOAs, the BOLD response timecourses in the first (Fast Task1 RTs) and third (Slow Task1 RTs) tertiles of the Task1 RT distribution. Importantly, the mean RT difference between fast and slow RTs at the short (720ms) and long SOA (680ms) were statistically indistinguishable (p = 0.13). Yet, as there is strong evidence of serial postponement of Task2 at the short SOA but not at the long SOA (Fig. 1C), the central bottleneck model predicts that duration of BOLD activity in a bottleneck area should be prolonged for Slow RTs relative to Fast RTs at the short SOA, but not at the long SOA (see Supplemental Modeling). Duration of activity was estimated by measuring peak amplitude latency - a sensitive measure of the duration of the BOLD response (Henson et al., 2002; Miezin et al., 2000; Ruge et al., 2003) - in the VVAM task, as peak latency can be unambiguously distinguished from vocal artifacts (Birn et al., 1999; Birn et al., 2004) in this task order.
We observed an activation pattern consistent with the serial postponement prediction of the central bottleneck model in the left pLPFC (Fig. 2 and Table 1). Peak latency occurred later for Slow RTs than for Fast RTs at the short SOA (p = 0.01), but not at the long SOA (p = 0.8). Indeed, the Slow-Fast RT latency difference was larger at the short than at the long SOA (p = 0.02). We confirmed these results with a behavioral measure of central processing other than Task 1 RT, namely the time between the onset of Stimulus 1 and the response to Task2 (S1R2). Unlike the Task 1 RT measure, S1R2 takes into account response times to both tasks as a measure of the duration of central processing time. As would be expected, this measure is strongly correlated with Task1 RT at the short SOA (r2 = 0.9), but not at the long SOA (r2 = 0.2). Furthermore, when the S1R2 response time was subjected to the same tertile analysis as Task1 RT, it produced the same latency effects. Specifically, there was a peak latency difference between short and long S1T2 RTs at the short SOA (p = 0.04, one-tailed), but not at the long SOA (p = 0.14, one-tailed). Thus, two behavioral estimates of central processing time, Task1 RT and S1R2 RT, provide converging evidence for the role of pLPFC in a central bottleneck of information processing.
Table 1. Anatomical location and statistical assessment of activation for the regions of interest (ROIs) in Experiments 1–3.
For the Dual-task experiment (Experiment 1), the two t-statistics columns reflect the peak latency difference between the Slow RT and Fast RT conditions at short and long SOA, respectively. For the Single-task experiment (Experiment 2), the first and second t-statistics columns list the onset and peak latency differences, respectively, between the Slow RT and Fast RT conditions. For the response selection load experiment, the t-statistics column reflects the amplitude difference between the 2AFC and 6AFC conditions. In all cases * denotes statistically significant t-values. The “No.Subjects” column lists the number of subjects for whom an ROI could be identified in a given brain region in the localizer task. pLPFC = Posterior Lateral Prefrontal Cortex, IFG = Inferior Frontal Gyrus, SMFC = Superior Medial Frontal Cortex, ACC = Anterior Cingulate Cortex, PMC = pre-Motor Cortex, IPS = Intra-Parietal Sulcus, Cereb = Cerebellum.
| Experiment 1: Dual-Task
|
Experiment 2: Single-Task
|
Experiment 3: Response-Load
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Hemi | Mean Tal Co-Ords | Peak Latency (t) | Mean Tal Co-Ords | Latency Diff (t) | Mean Tal Co-Ords | Peak Amplitude (t) | |||||
| No. Subj | X, Y, Z | Short SOA | Long SOA | No. Subj | X, Y, Z | Onset | Peak | No. Subj | X, Y, Z | |||
| pLPFC | ||||||||||||
| Left | 12 | −37,14,25 | 3.0* | −0.3 | 9 | −44, 24, 30 | 0.5 | 3.2* | 5 | −44, 13, 29 | 2.7* | |
| Right | 12 | 42, 18, 28 | 0.2 | −0.3 | 8 | −44, 14, 35 | 1.1 | 1.8 | 5 | 35, 12, 25 | 2.2* | |
| IFG | ||||||||||||
| Left | 13 | −42, 10, 8 | 0 | 0.6 | 9 | −44, 11, 7 | 0.2 | 1 | 5 | −51, 11, 13 | −0.5 | |
| Right | 14 | 44, 12, 9 | 0.8 | 0.7 | 8 | 51, 51, 8 | 1.7 | 2.2* | 4 | 50, 13, 8 | 1.3 | |
| SMFC | ||||||||||||
| Bilateral | 13 | 0, 1, 57 | −0.2 | 0 | 9 | 0, 1, 54 | 1.4 | 3.0* | 6 | 1, 1, 52 | 0.9 | |
| ACC | ||||||||||||
| Bilateral | 14 | 1, 20, 30 | −0.3 | −0.2 | 9 | 1, 15, 37 | −0.3 | 0.7 | 5 | 2, 16, 37 | −2.2 | |
| PMC | ||||||||||||
| Left | 14 | −25, −5, 50 | −0.2 | 3.2* | 8 | −22, −6, 50 | −0.2 | 0.9 | 5 | −23, −5, 50 | 0.7 | |
| Right | 10 | 27, −5, 49 | 0.2 | 1.1 | 6 | 27, −5, 54 | −1.5 | −0.5 | 2 | 32, −9, 62 | 0.1 | |
| IPS | ||||||||||||
| Left | 13 | −24, −55, 41 | −0.2 | −0.3 | 7 | −26, −49, 47 | 3.2* | 1.5 | 6 | −27, 54, 51 | −1.3 | |
| Cereb | ||||||||||||
| Left | 11 | −7, −49, −13 | 0.8 | 0.8 | 9 | −8, −49, −14 | 0.2 | 0.2 | 6 | 14, −41, −16 | 0.3 | |
| Right | 11 | 7, −50, −14 | 2.0* | 2.7* | 8 | 7, −44, −12 | 0.9 | 2.3* | 5 | 13, −48, −16 | 0.1 | |
As exemplified by the inferior frontal gyrus (IFG) (Fig. 2), most other ROIs failed to show evidence of serial queuing of activity at the short SOA (Table 1), with the exception of the superior medial frontal cortex (SMFC) (see below). The right pLPFC did not exhibit significant serial queuing of activity with the Task1 RT analysis, although it did so with the S1R2 analysis (short SOA p = 0.03, one-tailed, long SOA p = 0.4, one-tailed). However, given that this ROI also failed to show significant effects in Experiment 2 (see Table 1), it exhibits few of the characteristics expected of the neural substrates of a central bottleneck.
Experiment 2: Single-Task Experiment
Since the vocal artifacts prohibited the accurate assessment of onset latencies in dual-task conditions, it is possible that the peak latency shifts were accompanied with comparable shifts in onset latency. A shift of the entire time course would suggest that pLPFC is more involved in response execution than in central processing (Menon et al., 1998). We therefore tested in an additional experiment whether a rightward shift in peak latency, but not in onset latency, can be obtained with increased Task RT in left pLPFC under artifact-free conditions. We scanned nine subjects while they performed single AM task trials. When the data was submitted to the same RT analysis used in the dual-task experiment (mean RT difference between Fast and Slow RTs: 815ms), we again observed a peak latency difference between Slow and Fast RTs (p = 0.007, one-tailed), but no difference in onset latency (p = 0.3, one-tailed, Fig. 3B).
These results corroborate model simulations of pLPFC activity under single-task conditions (see Supplemental Modeling) and are inconsistent with the region performing either a motor (as its entire time course would have been affected by Task1 RT) or a sensory function (as neither its onset nor peak latencies would have been affected by Task1 RT) (Menon et al., 1998). Instead, these findings are most consistent with pLPFC’s involvement in response selection.
Single-Task and Dual-Task Comparison
Comparison of the time courses in pLPFC for the dual- and single-task conditions provides further evidence that this region is a key neural substrate of the central bottleneck. The central bottleneck model predicts that duration of neural activity in pLPFC should be longer under dual-task than under single-task conditions. This should occur even at the short SOA since response selection for Task2 is postponed until completion of response selection for Task1. By contrast, a strictly parallel model of response selection, in which response selection can proceed simultaneously in both tasks, chiefly predicts a change in response amplitude in dual-task situations compared to single task conditions, with only a slight change in peak latency due to the 300ms SOA between the two tasks (see Supplemental Modeling). The results clearly support the central bottleneck model: The peak latency in left pLPFC was greater under dual-task than under single-task conditions (Fig. 3C, p = 0.01, one-tailed, independent-samples t-test). By contrast, the left IFG failed to show such peak latency difference (p = 0.2, one-tailed, independent-samples t-test, see Supplemental Table 1). We should caution however, that because the two tasks were presented with a 300ms delay instead of simultaneously in the dual-task condition, our experimental design was slightly biased for the hemodynamic response to peak later in the dual-task than in the single task condition. Nevertheless, these results reveal a pattern of activity in pLPFC that is consistent with what is expected of a central bottleneck of information processing, namely serial queuing of response selection activity under dual-task conditions.
Experiment 3: Response Selection Load Experiment
To provide converging evidence for pLPFC’s involvement in response selection, we performed an additional experiment that manipulated response selection load, a variable that affects the magnitude of the PRP (Karlin and Kestenbaum, 1968; Marois et al., 2005; Van Selst and Jolicoeur, 1997). Brain regions involved in response selection should be increasingly engaged as the number of response choices increases (Marois et al., 2005; Schumacher et al., 2003; van Eimeren et al., 2006). We scanned six subjects performing single AM tasks that required choosing between either two or six response alternatives, with the 2AFC and 6AFC trials separately blocked. As expected, subjects’ RTs were longer in the 6AFC than in the 2AFC condition (968 ms vs. 656ms, p = .001, one-tailed). Consistent with the left pLPFC’s involvement in response selection, its activity was stronger in the 6AFC than in 2AFC condition (p = 0.03, one-tailed; Fig. 4). Importantly, since the activity difference between the 6AFC and 2AFC conditions arose from a comparable baseline at trial onset (Fig. 4B), it is independent of any activity effects that could result from maintaining a different number of sensorimotor pairings in working memory in the two conditions (Marois et al., 2005). The differential activity we observed therefore likely reflects differential processing demands in the 2AFC and 6AFC conditions for selecting the appropriate response to a given stimulus (a process that may involve retrieval from working memory). Furthermore, since the same manipulation had no effect on some of the other ROIs (e.g. left IFG, Fig. 4 and Table 1), the pLPFC results cannot be accounted for by differences in general task difficulty or effort between the two response selection loads. Taken together, these findings are consistent with a key role for pLPFC in stimulus-response mapping (Passingham and Sakai, 2004; Rowe et al., 2000), the prototypical process associated with the central stage of information processing (Pashler, 1994a).
Other candidate ‘bottleneck’ regions
Although only the left pLPFC exhibited significant serial queuing of activity in the dual-task experiment (Experiment 1), another brain region - SMFC - displayed a similar, albeit non-significant, pattern (Figs. 5B and 5C, Table 1). Consistent with the activation trend in the dual-task experiment, SMFC exhibited a peak latency difference between Slow and Fast RTs (p = 0.005, one-tailed), but no onset latency difference (p = 0.1, one-tailed, Fig. 5D) in the single-task experiment (Experiment 2). Furthermore, peak latency was greater under dual-task than single-task conditions (p = 0.01, one-tailed, independent-samples t-test, Fig. 5E). Finally, SMFC showed a non-significant trend towards greater activity in the 6AFC than 2AFC conditions in the response selection load experiment (Fig. 5F).
Figure 5. Bilateral SMFC activity in the dual-task (Experiment 1), single-task (Experiment 2) and response selection load (Experiment 3) experiments.
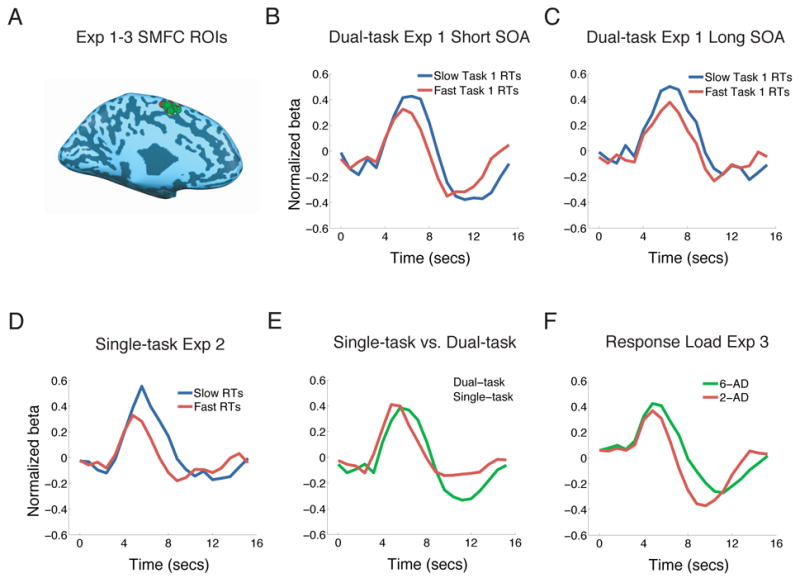
(A) Peak foci of individual bilateral SMFC ROIs (left-hemisphere view) isolated in the localizer tasks of the three experiments (ROIs for Experiments 1, 2 and 3 appear in green, red and yellow respectively). All ROIs were located in medial BA6. (B) and (C) Dual-task experiment. Activity in bilateral SMFC tended to be delayed in Slow Task 1 RT trials relative to Fast Task 1 RT trials at the short SOA (B) but not at the long SOA (C, n.s., Table 1). (D) Single-task experiment. Activity in Fast RT trials peaked earlier than in Slow RT trials, with no difference in the onset of activity between these two conditions. (E) Comparison between single-task and dual-task experiments indicates that activity peaked earlier in single-task trials than in dual-task, short SOA trials. (F) Response selection load experiment. SMFC activity amplitude tended to be greater in 6AFC trials than in 2AFC trials (n.s., Table 1).
The other ROIs did not show a pattern of activity consistent with a central bottleneck of information processing in the dual-task experiment, although a few displayed significant effects in one of the three experiments. Specifically, the left pre-motor cortex showed a peak latency difference at the long but not at the short SOA in Experiment 1 (Table 1), a pattern opposite to what is expected from a region exhibiting serial queuing of activity. On the other hand, the right cerebellum showed both onset and peak latency differences in Experiment 1 (Table 1), and only a peak latency difference in Experiment 2. Finally, while the right IFG exhibited a peak latency difference in Experiment 2, the IPS only showed an onset latency difference in that same experiment (Table 1). Some of these ROIs also showed a difference between single and dual-task conditions (Supplemental Table 1). The inconsistent pattern of activity observed across experiments in these brain regions makes it difficult to ascribe to them any specific role in dual-task limitations (see below).
Discussion
In this study we showed that the pLPFC fulfilled three criteria expected of a neural substrate of the central bottleneck of information processing. It was co-activated by tasks sharing neither sensory nor output modalities, it was highly sensitive to response selection demands and, most importantly, it exhibited serial queuing of response selection activity under dual-task conditions. The SMFC also showed evidence of serial queuing of activity, while the other ROIs failed to exhibit a bottleneck-like pattern of activity.
While our study implicates one, if not two, brain regions in the central bottleneck, it does not imply that the other ROIs are not involved in dual-task interference. Whereas previous dual-tasking studies used activity strength (peak amplitude) as a measure of dual-tasking interference (Marois and Ivanoff, 2005), the present study used activity duration (peak latency). Dual-task interference could lead to changes in the strength but not in the duration of neural activity in some ROIs, in which case these regions would not be highlighted by our time-resolved analysis. In addition, because the localizer task was designed to isolate foci commonly activated across sensorimotor tasks (compared to a fixation baseline), some of the isolated brain regions may not be involved in an amodal stage of response selection and may therefore not be expected to exhibit serial queuing of activity. Finally, brain regions exhibiting complex activity patterns in dual-tasking, either because they contribute to more than one stage of information processing or because they participate in both feed-forward and feed-back sweeps of activity, may have blurred hemodynamic responses that preclude detection of peak latency differences.
Lateral Prefrontal Cortex
While this study does not rule out the possibility that other brain regions may be involved, it strongly suggests that the pLPFC is a key neural substrate underlying the central bottleneck, as hypothesized in a recent review of dual-task limitations (Marois and Ivanoff, 2005). Interestingly, this area, which is located along the inferior frontal sulcus at the border between prefrontal and premotor cortex, overlaps extensively with the inferior frontal junction (IFJ) (Brass et al., 2005) and ‘periarcuate’ region of the frontal lobe (Diamond, 2006). The IFJ area is thought to be critical for cognitive control, decision-making, and modality-independent selection of task-relevant information (Badre et al., 2005; Brass et al., 2005; Bunge et al., 2003; Diamond, 2006), functions that are highly consistent with our suggestion that this brain region acts as a bottleneck of information processing in decision-making and response selection. By the same token, since we observed posterior LPFC activation even under single-task conditions (e.g., Adcock et al., 2000; Erickson et al., 2005), our results also indicate that the involvement of prefrontal cortex in dual-tasking is not exclusively related to strategic dual-task coordination or dual-task conflict resolution (D'Esposito et al., 1995; Dreher and Grafman, 2003; Szameitat et al., 2002).
Although our results suggest that a posterior region of the prefrontal cortex is involved in central processing, it is likely not the only prefrontal region associated with cognitive control, decision-making, and general selection of task-relevant information (e.g., Badre et al., 2005; Brass et al., 2005; Desimone and Duncan, 1995; Miller and Cohen, 2001). Interestingly, anterior regions of lateral prefrontal cortex are often co-activated with IFJ (Brass et al., 2005). This finding raises the possibility that more anterior foci of PFC may also prove to be neural constituents of a central bottleneck of information processing. Such foci may not have been observed in the present study because they may be preferentially activated in more complex tasks than simple sensorimotor associations (Koechlin et al., 2002), consistent with the view that more anterior regions of the prefrontal cortex process hierarchically higher behavioral functions (Fuster, 1989).
In addition to its posterior location, the LPFC ROI was also predominantly left lateralized. Indeed, the right hemisphere counterpart did not exhibit a robust pattern of activation expected of a central bottleneck of information processing. This predominantly left lateralization is unlikely to be related to linguistic processing, as the brain region most implicated in language, the left IFG (BA 44) (Gernsbacher and Kaschak, 2003), did not show a bottleneck-like activity pattern. However, language is not the only cognitive operation that has been localized to the left hemisphere. In particular, the selection of learned actions has been proposed to be preferentially left lateralized (Rushworth et al., 1998; Schluter et al., 2001). Furthermore, regions of lateral frontal/prefrontal cortex localized to the left hemisphere and near our pLPFC ROI (mean Talairach coordinates across three experiments: −42 14 28) have been implicated in cognitive control (Derrfuss et al., 2004), task-relevant selection of information (Bunge et al., 2003), and cue-mediated response preparation (Braver et al., 2003, −46 15 21). However, while these findings are consistent with this left posterior region of LPFC exerting an important function in dual-task limitations, they do not imply that all or even most executive processes are lateralized to the left prefrontal cortex. Indeed, several studies have found right-lateralized (Braver et al., 2003; Rowe et al., 2000; Yeung et al., 2006), or bilateral (Dosenbach et al., 2006; Koechlin and Jubault, 2006) control regions in prefrontal cortex. In the absence of a consensus on the functional organization of the prefrontal cortex (e.g. Thompson-Schill et al., 2005; Wood and Grafman, 2003) it is reasonable to conclude that the discrepancies across investigations in regards to the localization of prefrontal control functions likely depend on the specific cognitive processes under investigation and/or on the experimental methods employed to investigate them.
Superior Medial Frontal Cortex
A region of SMFC, centered at the pre-SMA/SMA, also exhibited an activation pattern that was generally consistent with a bottleneck of information processing, although we could only observe non-significant patterns of serial queuing (Experiment 1) and response selection (Experiment 3) activity in this brain region. These results suggest that while this region may be involved in the central bottleneck, its contribution may be weaker and/or more complex than that of pLPFC, thereby leading to a blurred hemodynamic trace of its involvement in serial queuing of activity under dual-task conditions. A role for SMFC in dual-tasking is consistent with work suggesting that the pre-SMA and subjacent dorsal anterior cingulate cortex are involved in cognitive control, decision making, sensori-motor association, and task-set implementation (Boxer et al., 2006; Dosenbach et al., 2006, Kurata et al., 2000; Picard and Strick, 2001; Rushworth et al., 2004).
Together with the pLPFC, the SMFC may form the core of a neural system underlying the central bottleneck. It is probably through the interaction of these two brain regions, with perhaps some additional areas, that the bottleneck of information processing arises, although the nature of this interaction remains to be established. For instance, this interaction may not only include feed-forward flow of information, but also performance feedback from the superior medial frontal regions onto lateral prefrontal cortex (e.g., Botvinick et al., 2004; Miller and Cohen, 2001). Indeed, the greater activity measured in long RT trials (Experiment 2, Fig. 5), which presumably involved greater processing demands than short RT trials, is consistent with such feedback mechanism.
Implications for the nature of the central bottleneck of information processing
The pLPFC and SMFC regions correspond very well to the mid-dorsolateral prefrontal and dorsal anterior cingulate areas that are recruited by diverse cognitive tasks (Duncan and Owen, 2000). Apart from a difference in the regional location of the prefrontal cortex activation, our neural network is also analogous to a core system of prefrontal and superior medial frontal areas important for the implementation of task sets across a large cohort of cognitive tasks (Dosenbach et al., 2006). In general, these findings point to the prefrontal and dorsal medial frontal cortex as a frontal lobe network recruited to meet a wide variety of cognitive demands, making this system well suited to act as a central, amodal bottleneck of information processing. Consistent with this hypothesis, similar pLPFC and SMFC regions as those identified in the present study are also recruited by such diverse cognitive processes as mental rotation (Cohen et al., 1996), memory retrieval (Dobbins et al., 2002) and task switching (Yeung et al., 2006), processes that have all been shown to generate dual-task slowing due to central processing limitations (Carrier and Pashler, 1995; Chun and Potter, 2001; Ruthruff et al., 1995). Interestingly, it has been suggested that these lateral prefrontal and superior medial frontal regions are recruited across a diverse array of tasks because these regions can adaptively code in a distributed and densely overlapping manner a wide range of task-relevant information and operations (Duncan, 2001). It is therefore tempting to speculate that dual-task limitations may derive from an inability to fully segregate the coding of behaviorally relevant information for two distinct tasks in the prefrontal cortex. Evidently, even the prefrontal cortex, the seat of much of our higher cognitive functions, has its humbling limitations.
Experimental Procedures
Experiment 1: Dual-Task Experiment
Subjects
Fourteen right-handed individuals (5 males, 19–31 years) with normal or corrected-to-normal vision participated for financial compensation. The Vanderbilt University Institutional Review Board approved the experimental protocol and informed consent was obtained from the subjects after the nature and possible consequences of the studies were explained to them.
Behavioral Paradigm
In each trial, subjects executed two distinct sensorimotor tasks. One task consisted of selecting the appropriate manual (finger) response to a complex auditory stimulus (AM Task), while the other consisted of selecting the appropriate vocal response to a visual stimulus (VV Task).
There were 8 possible stimuli and responses (8AFC) for both the AM and VV tasks. The visual stimulus was a disk presented centrally, with a diameter of approximately 1.5° visual angle, and was colored light green (109 205 119, RGB), brown (167 106 48), pink (255 57 255), light blue (79 188 220), dark green (10 130 65), red (237 32 36), navy (44 71 151) or yellow (255 235 30). Each visual stimulus required a distinct vocal response, consisting of the following pseudo-syllables: “Bah”, “Koe”, “Tay”, “Dee”, “Poe”, “Gah”, “Yee” or “Noo”. The auditory stimuli were eight discriminable sounds that consisted of complex tones and man-made or natural sounds edited by adding noise and/or reversing the waveform. Each sound required a distinct key press response, mapped on to every finger but the thumbs. The visual and auditory stimuli were each presented for 200 ms. The visual stimulus was presented on a grey background and at all times a white fixation square, subtending 0.3° of visual angle, was present in the centre of the screen.
The stimulus onset asynchrony (SOA) between the two tasks was either short (300ms) or long (1100ms ms [subjects 1–6] or 1900 ms [subjects 7–14]; the long SOA was increased after the first 6 subjects in order to reduce the number of trials where the Task1 response overlapped with the presentation of the second stimulus). Importantly, the behavioral and fMRI results obtained from the first 6 subjects were qualitatively identical to the results obtained with the whole group. Task order and SOA was randomized for each trial, leading to four different trial types (AMVV Short SOA, AMVV Long SOA, VVAM Short SOA, VVAM Long SOA). Trial onset asynchronies (TOAs) followed an exponential distribution (27 trials at 6.4 s TOA, 12 x 8.0 s TOA, 6 x 9.6 s TOA and 3 x 11.2 s TOAs) (Serences, 2004). Subjects completed 6 event-related dual-task runs (1 subject completed only 4 runs due to time restrictions). Each run contained 48 trials, 12 for each trial type.
The randomization of task order was used to prevent subjects from systematically prioritizing one task over the other (Levy and Pashler, 2001; Pashler, 1994b; Ruthruff et al., 2003; Ruthruff et al., 2001). Indeed, response reversals (responding to Task2 before Task1) were rare (9%) and occurred only at the short SOA, indicating that subjects responded according to the order of stimulus presentation. In addition, response grouping was minimized by instructing subjects to perform each task as soon as they heard/saw each of the two stimuli. Subjects were further encouraged to emphasize both speed and accuracy by being offered a financial reward (5 cents per trial, for a maximum of $14.40 per session) for each trial in which both tasks were responded to correctly and within the 75th percentile of each tasks’ reaction time as assessed from the single task blocks during the localizer runs (see below). These procedures ensured that the ensuing dual-task costs resulted from intrinsic limitations in concurrently processing two sensorimotor tasks instead of from strategic response deferment (Levy and Pashler, 2001; Meyer and Kieras, 1997; Pashler, 1994b; Ruthruff et al., 2003; Ruthruff et al., 2001).
Practice Session
Prior to the scanning session, subjects participated in an hour-long practice session outside the scanner. A Plantronics DSP digital headset (Plantronics, Santa Cruz, CA) was used for auditory stimulus presentation and vocal response recording, and manual responses were collected using a computer keyboard.
The first part of the practice session consisted of practice with the single tasks to learn the eight stimulus-response mappings. For the VV task, subjects initially studied a response diagram sheet that showed the colored disks and the corresponding syllable responses. After 10 minutes, subjects then performed two blocks of 80 trials, with trials being automatically initiated every 4 s. During these trials, subjects vocalized the appropriate response to each visual stimulus presentation. For the first block of trials, visual stimuli were presented for 500 ms, and the response diagram sheet was at hand. For the second block, stimulus duration was reduced to 200 ms and the response diagram was removed. For the AM task, subjects first familiarized themselves with the eight auditory stimuli-finger press pairings by pressing the computer keys associated with the sounds (keys ‘a’.’s’,’d’,’f’ for the four fingers of the left hand and ‘j’,’k’.’l’,’;’ for the four fingers of the right hand). After 10 mins, they then completed two blocks of 80 trials with a TOA of 4 s. For each trial, the sound lasted 200 ms and no response diagram was present. Order of the single task blocks was counterbalanced across subjects. Accuracy was stressed, and performance was comparably high for the two single tasks by the end of practice (mean 94% accuracy).
Following the single-task blocks, subjects then performed 5 blocks of dual-task trials, each containing 40 trials. The blocks contained 10 trials of each of the 4 conditions (2 Task Order x 2 SOA), with trial type randomly ordered. Trials lasted for 6 s and each was automatically initiated. Subjects were instructed as in the scanning session, except that there were no rewards in the practice session.
The experiment was programmed in Matlab (MathWorks, Natick MA), using the Psychophysics Toolbox extension (Brainard, 1997; Pelli, 1997) and was presented using a Pentium IV PC.
fMRI Paradigm
Data Acquisition
Anatomical 2D and 3D high-resolution T1-weighted images were acquired with conventional parameters on a 3T Philips Intera Achieva scanner at the Vanderbilt University Institute of Imaging Science. The visual display was presented on an LCD panel and back-projected onto a screen positioned at the front of the magnet. Subjects lay supine in the scanner and viewed the display on a mirror positioned above them. The auditory stimuli were presented, and the vocal responses were recorded, using a Commander XG MR compatible headset (Resonance Technology Inc, Northridge CA). Manual responses were recorded using two 5-key keypads (one for each hand; Rowland Institute of Science, Cambridge, MA). Functional (T2*) parameters were as follows: TR 800 ms, TE 30 ms, FA 55°, FOV 24 cm, 64x64 matrix with 16 slices (7 mm thick, 0.5 mm skip) acquired parallel to the AC-PC line. Stimulus presentation was synchronized with each fMRI volume acquisition.
Data Analysis
Image analysis was performed using Brain Voyager QX 1.4 (Brain Innovation, Maastricht, The Netherlands) and with custom Matlab software (MathWorks, Natick MA). Data preprocessing included 3D motion correction, slice scan time correction and linear trend removal. All functional data were aligned to the first localizer run and anatomical T1-weighted data were transformed into standardized Talairach space (Talairach and Tournoux, 1988).
Time courses were extracted from the ROIs isolated with the localizer task for each subject using a deconvolution analysis (Serences, 2004). Only VVAM trials in which both responses were correct and reported in the correct order were analyzed. This was done to avoid confounding peak activations with the magnetic susceptibility and motion artifacts associated with a vocal response. Since the vocal artifact is limited to within the first 3 seconds of responding, it does not affect the later peak hemodynamic response (Birn et al., 2004). In the deconvolution analysis, z-transformed beta estimates, corrected for serial auto-correlations, were extracted for 20 volumes following Task1 stimulus presentation. Individual timecourses were averaged across subjects, and the resulting averaged timecourses were plotted in Figs. 2–5. The peak volume of a time-course was defined as the volume with the greatest signal amplitude between the first volume after the vocal artifact (identified individually within the orbitofrontal cortex) and the 12th volume following T1 presentation. For statistical testing of peak latency (or amplitude) differences, the peak volume time points (or amplitudes) of each of two conditions (e.g., Fast vs. Slow Task 1 RTs) were extracted for each subject, and a t-test was applied to determine if the time points (or amplitude) were significantly different in the two conditions, using a random effects model.
Localizer Task
The dual-task experiment included two localizer runs in order to isolate regions that responded to both sensorimotor tasks and that have previously been hypothesized to be involved in response selection (see below). The behavioral paradigm and fMRI data acquisition and analysis for the localizer task are as described in the Dual-Task Experiment section above except where otherwise stated below.
Behavioral Paradigm
Subjects performed separate blocks of trials of single AM and VV tasks, dual-tasks, and fixation blocks. These blocks were ordered so that across both localizer runs each block type preceded and followed one another an equal number of times. Fixation blocks lasted for 21.6 secs, during which subjects were required to passively view the fixation square. The single VV, AM and the dual-task blocks lasted for 25.6 secs, with single task blocks containing 8 trials (3.2 secs per trial) and the dual-task blocks 4 trials (6.4 secs per trial). There were 4 blocks of fixation and 3 blocks each of single VV trials, single AM trials and dual-task trials per localizer run. Subjects were visually cued about the block identity for 3.2 s before first trial onset, and were instructed to perform each task as quickly and as accurately as possible. The dual-task condition was included to determine whether there were regions that may have been specifically activated in the dual-task condition relative to the single-task conditions (D'Esposito et al., 1995; Szameitat et al., 2002). No such regions were isolated in a random-effects statistical parametric map (SPM) analysis (q(FDR)<0.05). The dual-task condition in the localizer run was therefore not further analyzed.
fMRI Data Analysis
Data preprocessing was done as in the Dual-Task Experiment section except that in addition to 3D motion correction, slice scan time correction and linear detrending, spatial smoothing with an 8-mm Gaussian kernel (FWHM) and Gaussian temporal filtering (1 sec FWHM) was also performed.
To isolate ROIs that were engaged by both of the sensorimotor tasks, SPMs were created using a multiple regression analysis, with regressors defined for the VV, AM and fixation conditions and convolved with a double gamma hemodynamic response function (SPM2, http://www.fil.ion.ucl.ac.uk/spm), consisting of a positive gamma function and a small, negative gamma function reflecting the undershoot. Subject-specific ROIs were isolated by first identifying the peak voxel in an area of interest that was significantly activated by both the AM and VV tasks relative to fixation (i.e., AM-fixation and VV-fixation, see below) using a voxel-wise analysis thresholded at p < 0.05, Bonferonni corrected, or at a false discovery rate (FDR) of q < 0.05, when activation was not present at the first threshold. An ROI was then defined around that peak and included all significant voxels above threshold up to a maximum size of 1.33 cm3. ROIs were defined in left IPS (BA7), in left and right pLPFC (BA9), IFG (BA44), dorsal PMC (BA6), cerebellum (anterior lobe), and in bilateral SMFC (medial BA6 corresponding to preSMA/SMA and extending into dorsal anterior cingulate cortex) and ACC (BA32) (see Table 1).
Experiment 2: Single-Task Experiment
The behavioral paradigm and fMRI data acquisition and analysis for this experiment are as described in the Dual-Task Experiment section except where otherwise noted below.
Subjects
Eight right-handed individuals and 1 left-handed individual (6 males, 23–32 years) with normal or corrected-to-normal vision participated in the experiment for financial compensation (1 subject had previously participated in Experiment 1).
Behavioral Paradigm
The fast event-related runs contained randomly intermixed trials of single AM and VV tasks. TOAs followed an exponential distribution: 45 trials with a 3.2 s TOA, 20 trials with a 4.8 s TOA, 10 trials with a 6.4 s TOA and 5 trials with a 8.0 s TOA. There were 80 trials per run and subjects completed 6 runs each (one subject completed only 4 runs due to time restrictions). The localizer and practice sessions were as in Experiment 1 except that there were no dual-task conditions.
fMRI Data Analysis
Data analysis was only carried out on the AM trials as there were no vocal artifacts in this task. Peak amplitude volumes were isolated between the 3rd and 12th volumes post stimulus presentation. Activity onset was defined as the first volume that contributed to the positive slope (activation increase) reaching to the peak volume. Since the results of the RT manipulation for the single-task experiment were expected to replicate those of the dual-task experiment (slower RTs leading to longer peak latencies), a one-tailed paired-samples t-test was used to compare peak latency differences between Slow and Fast RTs. Similarly, a one-tailed t-test was also applied for comparing the peak latencies of dual-task and single-task conditions because of the a priori prediction that executing two response selections instead of one may only increase the duration of BOLD activity.
Experiment 3: Response Selection Load Experiment
The behavioral paradigm and fMRI data acquisition and analysis for this experiment were as described in the Dual-Task Experiment except where otherwise stated below.
Subjects
Five right-handed individuals and 1 left-handed individual (3 males, 19–32 years) with normal or corrected-to-normal vision participated in the experiment for financial compensation (Three of the subjects had previously participated in Experiments 1 or 2. Old and new subjects showed similar activity patterns).
Behavioral Paradigm
In each fMRI run, subjects were presented with three blocks of 2AFC trials interleaved with three blocks of 6AFC trials. Each block lasted 57.6 sec, including 3.2 sec of instructions. Each block contained 12 trials presented according to an exponential distribution of TOAs (six trials at a 3.2 s TOA, three at 4.8 s, two at 6.4 s, and one at 8.0 s). Half of the subjects completed three runs of the VV task followed by three runs of the AM task, and the other half completed the tasks in reverse order. The matching between stimuli and responses were arbitrarily selected, except that for the AM task, the 2AFC condition included the left and right index fingers for three subjects and the left and right pinky fingers for the other three subjects. The remaining 6 fingers made up the 6AFC condition. In any given AM block, subjects removed the fingers from the keys that were not in use for that block (i.e., 2AFC fingers removed during 6AFC blocks, and vice versa) (Marois et al., 2005).
The practice and localizer sessions were identical to those of Experiment 2. Thus, subjects received equal amounts of practice for all sensorimotor pairings, as they were not informed of the 2AFC vs. 6AFC manipulation until the event-related fMRI session.
fMRI Data Analysis
Since manipulations of response selection load have previously been shown to strongly affect signal amplitude (Marois et al., 2005; van Eimeren et al., 2006), peak amplitude was used as the primary measure of activity difference between the 2AFC and 6AFC conditions. The peak amplitude for each subject was derived by collapsing time courses for each condition and subject and identifying the time-point of greatest signal amplitude in the grand average (Todd and Marois, 2004). Peak amplitude differences between the 2AFC and 6AFC conditions were then compared using a one-tailed paired-samples t-test since greater activation with the larger AFC condition was predicted from prior results in our laboratory (Marois et al., 2005).
Supplementary Material
Acknowledgments
This research was supported by NIMH grant R01 MH70776 to R.M. and an NSERC Canada fellowship to J.I. We thank Dan Shima for programming assistance.
Footnotes
Supplemental Material: Supplemental material for this article can be found at http://www.neuron.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock RA, Constable RT, Gore JC, Goldman-Rakic PS. Functional neuroanatomy of executive processes involved in dual-task performance. Proc Natl Acad Sci USA. 2000;97:3567–3572. doi: 10.1073/pnas.060588897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Beede KE, Kass SJ. Engrossed in conversation: The impact of cell phones on simulated driving performance. Accid Anal Prev. 2006;38:415–421. doi: 10.1016/j.aap.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23:1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, Lisberger SG. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26:6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits cubserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Carrier LM, Pashler H. Attentional limits in memory retrieval. J Exp Psychol Learn Mem Cogn. 1995;21:1339–1348. doi: 10.1037//0278-7393.21.5.1339. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. The attentional blink and task switching within and across modalities. In: Shapiro K, editor. The limits of attention: temporal constraints in human information processing. NewYork: Oxford University Press; 2001. pp. 20–35. [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ. Changes in cortical activity during mental rotation: A mapping study using functional MRI. Brain. 1996;119:89–100. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diamond A. Bootstrapping conceptual deduction using physical connection: rethinking frontal cortex. Trends Cogn Sci. 2006;10:212–218. doi: 10.1016/j.tics.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive Control during Episodic Retrieval Multiple Prefrontal Processes Subserve Source Memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kramer AF. Neural correlates of dual-task performance after minimizing task-preparation. Neuroimage. 2005;28:967–979. doi: 10.1016/j.neuroimage.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Formisano E, Goebel R. Tracking cognitive processes with functional MRI mental chronometry. Curr Opin Neurobiol. 2003;13:174–181. doi: 10.1016/s0959-4388(03)00044-8. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. 2. New York: Raven Press; 1989. [Google Scholar]
- Gernsbacher MA, Kaschak MP. Neuroimaging Studies of Language Production and Comprehension. Annu Rev Psychol. 2003;54:91–114. doi: 10.1146/annurev.psych.54.101601.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- Hick WE. On the rate of gain of information. Q J Exp Psychol. 1952;4:11–26. [Google Scholar]
- Ivry RB, Franz EA, Kingstone A, Johnston JC. The psychological refractory period effect following callosotomy: Uncoupling of lateralized response codes. J Exp Psychol Hum Percept Perf. 1998;24:463–480. doi: 10.1037//0096-1523.24.2.463. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Resolving dual-task interference: an fMRI study. Neuroimage. 2004;22:748–754. doi: 10.1016/j.neuroimage.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. J Cogn Neurosci. 2003;15:1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Saxe R, Kanwisher N. Functional magnetic resonance imaging provides new constraints on theories of the psychological refractory period. Psychol Sci. 2004;15:390–396. doi: 10.1111/j.0956-7976.2004.00690.x. [DOI] [PubMed] [Google Scholar]
- Karlin L, Kestenbaum R. Effects of number of alternatives on the psychological refractory period. Q J Exp Psychol. 1968;20:167–178. doi: 10.1080/14640746808400145. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Danek A, Burnod Y, Grafman J. Medial prefrontal and subcortical mechanisms underlying the acquisition of motor and cognitive action sequences in humans. Neuron. 2002;35:371–381. doi: 10.1016/s0896-6273(02)00742-0. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kurata K, Tsuji T, Naraki S, Seino M, Abe Y. Activation of the dorsal premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J Neurophysiol. 2000;84:1667–1672. doi: 10.1152/jn.2000.84.3.1667. [DOI] [PubMed] [Google Scholar]
- Levy J, Pashler H. Is Dual-Task Slowing Instruction Dependent? J Exp Psychol Hum Percept Perf. 2001;27:862–869. [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Sources of dual-task interference: evidence from human electrophysiology. Psychol Sci. 1998;9:223–227. [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Marois R, Larson JM, Chun MM, Shima D. Response-specific sources of dual-task interference in human pre-motor cortex. Psychol Res. 2005 Nov;11:1–12. doi: 10.1007/s00426-005-0022-6. [DOI] [PubMed] [Google Scholar]
- Menon RS, Luknowsky DC, Gati JS. Mental chronometry using latency-resolved functional MRI. Proc Natl Acad Sci USA. 1998;95:10902–10907. doi: 10.1073/pnas.95.18.10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 2. Accounts of psychological refractory-period phenomena. Psychol Rev. 1997;104:749–791. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Navon D, Miller J. Queuing or Sharing? A Critical Evaluation of the Single-Bottleneck Notion. Cognit Psychol. 2002;44:193–251. doi: 10.1006/cogp.2001.0767. [DOI] [PubMed] [Google Scholar]
- Osman A, Moore CM. The locus of dual-task interference: Psychological refractory effects on movement-related brain potentials. J Exp Psychol Hum Percept Perf. 1993;19:1292–1312. doi: 10.1037//0096-1523.19.6.1292. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: Data and theory. Psychol Bull. 1994a;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pashler H. Graded capacity-sharing in dual-task interference? J Exp Psychol Hum Percept Perf. 1994b;20:330–342. doi: 10.1037//0096-1523.20.2.330. [DOI] [PubMed] [Google Scholar]
- Pashler H, Luck SJ, Hillyard SA, Mangun GR, O'Brien S, Gazzaniga MS. Sequential operation of disconnected cerebral hemispheres in split-brain patients. Neuroreport. 1994;5:2381–2384. doi: 10.1097/00001756-199411000-00042. [DOI] [PubMed] [Google Scholar]
- Pashler HE. The Psychology of Attention. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol. 2004;14:163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Lohmann G, von Cramon DY. Event-related analysis for event types of fixed order and restricted spacing by temporal quantification of trial-averaged fMRI time courses. J Magn Reson Imaging. 2003;18:599–607. doi: 10.1002/jmri.10397. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Wade DT, Renowden S, Passingham RE. The left hemisphere and the selection of learned actions. Neuropsychologia. 1998;36:11–24. doi: 10.1016/s0028-3932(97)00101-2. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ruthruff E, Miller J, Lachmann T. Does mental rotation require central mechanisms? J Exp Psychol Hum Percept Perf. 1995;21:552–570. doi: 10.1037//0096-1523.21.3.552. [DOI] [PubMed] [Google Scholar]
- Ruthruff E, Pashler HE, Hazeltine E. Dual-task interference with equal task emphasis: graded capacity sharing or central postponement? Percept Psychophys. 2003;65:801–816. doi: 10.3758/bf03194816. [DOI] [PubMed] [Google Scholar]
- Ruthruff E, Pashler HE, Klaassen A. Processing bottlenecks in dual-task performance: Structural limitation or strategic postponement? Psychon Bull & Rev. 2001;8:73–80. doi: 10.3758/bf03196141. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral Dominance for Action in the Human Brain: The Selection of Actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Schubert T, Szameitat AJ. Functional neuroanatomy of interference in overlapping dual tasks: an fMRI study. Cogn Brain Res. 2003;17:733–746. doi: 10.1016/s0926-6410(03)00198-8. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D'Esposito M. Neural evidence for representation-specific response selection. J Cogn Neurosci. 2003;15:1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Serences JT. A comparison of methods for characterizing the event-related BOLD timeseries in rapid fMRI. Neuroimage. 2004;21:1690–1700. doi: 10.1016/j.neuroimage.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S. Parsing a cognitive task: a characterization of the mind's bottleneck. PLoS Biol. 2005;3:e37. doi: 10.1371/journal.pbio.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer DL, Drews FA. Profiles in driver distraction: effects of cell phone conversations on younger and older drivers. Hum Factors. 2004;46:640–649. doi: 10.1518/hfes.46.4.640.56806. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Muller K, von Cramon DY. Localization of Executive Functions in Dual-Task Performance with fMRI. J Cogn Neurosci. 2002;14:1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perf. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Wolbers T, Munchau A, Buchel C, Weiller C, Siebner HR. Implementation of visuospatial cues in response selection. Neuroimage. 2006;29:286–294. doi: 10.1016/j.neuroimage.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Van Selst M, Jolicoeur P. Decision and response in dual-task interference. Cognit Psychol. 1997;33:266–307. doi: 10.1006/cogp.1997.0662. [DOI] [PubMed] [Google Scholar]
- Welford AT. The "psychological refractory period" and the timing of high-speed performance: A review and theory. Brit J Psychol. 1952;43:2–19. [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD. Between-Task Competition and Cognitive Control in Task Switching. J Neurosci. 2006;26:1429–1438. doi: 10.1523/JNEUROSCI.3109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


