Abstract
Geohistorical records reveal the long-term impacts of climate change on ecosystem structure. A 5-myr record of mammalian faunas from floodplain ecosystems of South Asia shows substantial change in species richness and ecological structure in relation to vegetation change as documented by stable isotopes of C and O from paleosols. Between 8.5 and 6.0 Ma, C4 savannah replaced C3 forest and woodland. Isotopic historical trends for 27 mammalian herbivore species, in combination with ecomorphological data from teeth, show three patterns of response. Most forest frugivores and browsers maintained their dietary habits and disappeared. Other herbivores altered their dietary habits to include increasing amounts of C4 plants and persisted for >1 myr during the vegetation transition. The few lineages that persisted through the vegetation transition show isotopic enrichment of δ13C values over time. These results are evidence for long-term climatic forcing of vegetation structure and mammalian ecological diversity at the subcontinental scale.
Keywords: faunal turnover, isotope ecology, mammals, paleocommunities, flood plain paleoecology
Long records of organisms and environments provide unique opportunities to evaluate the ecological and evolutionary responses of populations and ecological communities to environmental change over hundreds of thousands to millions of years. This historical perspective is essential for linking the dynamics of biotic change from ecological to evolutionary time scales and for understanding processes that transform ecosystems over geologic time. Here, we present evidence for the impact of local and regional climatic change on the species richness and trophic structure of Miocene mammals that inhabited sub-Himalayan alluvial plains. Stable isotopes of carbon and oxygen from paleosols document climate and vegetation, whereas carbon and oxygen isotopes from mammalian tooth enamel provide evidence for diets of herbivorous lineages. From 10.5 to 5.5 Ma, isotopic histories of species-level mammalian lineages reveal their responses to substantial changes in regional vegetation and climate.
The effects of climate on species' geographic ranges and the ecological structure of terrestrial biotas have been documented since the time of von Humboldt. For terrestrial mammals, climatic change has been linked to geographic-range shifts, fragmentation of populations, and selective filtering of populations over time and space—circumstances that can result in extinction or allopatric speciation as well as biotic turnover within ecosystems (1–5). Data from the fossil record do not, however, consistently match the predictions of this scenario of climate as a driver of mammalian evolution. This mechanism has been challenged when changes in taxonomic richness, origination rates, or extinction rates of regional or continental mammal faunas fail to track changes in the marine temperature record (as a proxy for global temperature) (6–8). On the other hand, notable changes in ecomorphological attributes, such as mean cheek-tooth height (hypsodonty) of ungulate species from Neogene fossil assemblages, and in species richness and ecological structure of extant mammalian faunas can track climatic gradients over time (9) and space (10). These contrasting perspectives reflect the different spatial, temporal, and taxonomic scales of analysis. Resolving these divergent views requires long fossil records with high temporal and taxonomic resolution, as well as faunal and climatic data from the same geohistorical system. The Siwalik record of northern Pakistan is the longest, best documented sequence of terrestrial mammalian faunas of the last 20 myr (11). Multiple lines of geologic, isotopic, and ecomorphological evidence from this continuous record of floodplain ecosystems reveal the responses of mammalian herbivores to climatically mediated vegetation changes.
The Siwalik Record of Northern Pakistan.
The Siwalik Group consists of alluvial sediments shed from the southern margin of the Himalayas over much of the Neogene. A well exposed sequence in the Potwar Plateau, south of Islamabad, Pakistan, has been studied by an interdisciplinary team of geologists and paleontologists for >30 years (11). In this area, Siwalik sediments > 4,000-m thick range from 18 to 1 Ma. Detailed lithostratigraphy and geochronology based on densely sampled magnetostratigraphy provide a stable temporal framework for fossil localities within an ancient flood basin tens of kilometers wide. Individual localities, spatially resolved to tens of meters squared, can be assigned with confidence to 100,000-yr intervals. Mammalian remains, documented by >50,000 catalogued specimens, dominate this fossil record. Major groups include common artiodactyls, perissodactyls, and rodents; uncommon primates, carnivores, and proboscideans; and rare creodonts, lagomorphs, aardvarks, and tree shrews (11). Surface collecting and screen washing have yielded substantial samples of large and small mammals, respectively. Although fossil plants are extremely rare from the Miocene deposits of the Potwar Plateau, stable isotopes from paleosol carbonates throughout the sequence provide evidence for vegetation composition on the flood plain. A large shift in carbon and oxygen isotope values during the late Miocene, initially documented in this Potwar Siwalik sequence, records changes in vegetation composition and structure linked to tectonic uplift and onset of the South Asian monsoon system (12–14). Isotopic analysis of mammalian tooth enamel independently documents changes in vegetation and precipitation sources and correlates well with the record from paleosol carbonates (15–17).
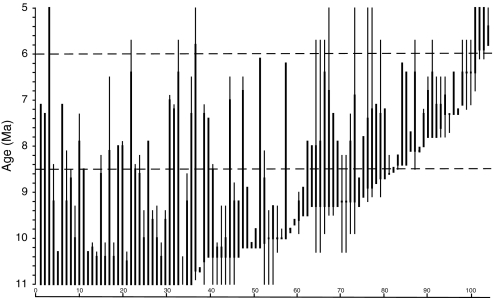
Our analysis focuses on the late Miocene interval from 10.5 to 5.5 Ma, represented by ≈550 fossil localities, ≈18,000 vertebrate fossils, and ≈150 mammalian lineages from 13 mammalian orders. About two-thirds of these lineages are well resolved taxonomically, allowing us to evaluate residence times (local biostratigraphic ranges) and faunal turnover (appearances and disappearances of lineages) at the species level. The residence time of mammalian lineages in the Siwalik record varies from 0.1 myr (the maximum resolution of individual fossil localities) to several million years. Estimated biostratigraphic ranges, based on confidence intervals on observed ranges (11), establish the pattern of faunal turnover. Biogeographic data and phylogenetic analyses indicate that most newly appearing lineages did not have ancestors in older Siwalik faunas; thus, most first appearances represent immigration events. The inferred ranges of 105 lineages [Fig. 1 and supporting information (SI) Table S1] show that, over the late Miocene, standing richness of mammals decreased by more than half (from ≈50 to ≈20 taxonomically resolved species) and that turnover was continuous rather than pulsed. Rarefaction analysis validates the decline in species richness after standardization for interval sample size (Fig. S1). Dietary inferences for herbivores are based on general tooth morphology, tooth-crown height, dental microwear and mesowear, and stable carbon isotopes from enamel. In combination with intensive isotopic sampling, these data provide historical ecological profiles of individual lineages and herbivore trophic structure as flood plain vegetation changed from forest to grassland.
Fig. 1.
Biostratigraphic ranges of 105 taxonomically resolved mammalian lineages from the late Miocene, Siwalik record of the Potwar Plateau. Most artiodactyls, perissodactyls, primates, and rodents are included. Most proboscideans, carnivores, and some small mammals are omitted. Dark lines connect observed first and last occurrences. Thin vertical lines represent 80% confidence intervals, calculated for the majority of lineages. Dashed horizontal lines bracket the interval of major environmental change. Table S1 lists the lineages and age ranges that are the basis for this figure; updated from ref. 11.
The Late Miocene Environmental Record.
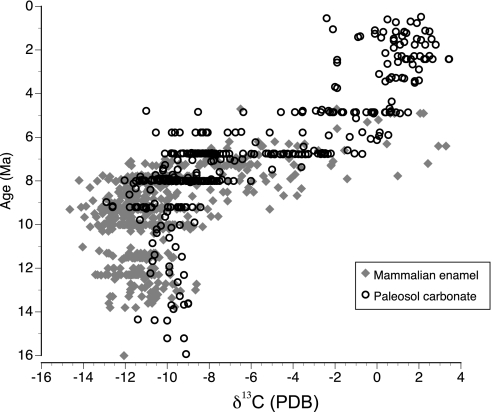
The paleoenvironmental record of this study, inferred from sedimentary facies, paleosol features, and stable isotopes of carbon and oxygen in pedogenic carbonates and mammal teeth, documents substrates, vegetation, and climate over 5 myr. The Miocene flood basin was traversed by large rivers—on the scale of major tributaries to the modern Indus and Ganges—and smaller flood plain channels (18–20). Paleosol sequences of variable maturity indicate the presence of forest, woodland, and grassland vegetation that was stable for decades to thousands of years (12, 14). Although shallow lacustrine sediments imply the presence of year-round water, the high frequency of pedogenic carbonate nodules signifies high seasonal evapotranspiration. C3 plants dominated flood plain vegetation until the late Miocene, according to δ13C values of pedogenic carbonates (Fig. 2). Beginning ≈8.5 Ma, δ13C values became increasingly enriched over time, reaching a new long-term average ≈6.0 Ma (12). Based on calibration of δ13C values with modern vegetation, the C-isotopic shift signifies a transition from C3-dominated to C4-dominated vegetation. C3 plants include trees, most shrubs, and cool-season grasses, whereas C4 plants are primarily warm-season grasses (21).
Fig. 2.
Carbon isotope record from pedogenic carbonates (12, 14) and mammalian tooth enamel (15–17, this study) show a pronounced shift to lighter values starting ≈8.5 Ma. Over the well sampled interval from 9.5 to 7.0 Ma, mammalian δ13C values vary more than do paleosol values, indicating that mammalian herbivores sampled a broader range of habitats than documented by the paleosol record. To facilitate comparison of paleosol and mammal δ13C values with respect to vegetation, all paleosol values were adjusted by −1‰ because the fractionation values (vegetation to palesol carbonate, vegetation to mammalian enamel) differ by this amount.
Enrichment in δ18O from pedogenic carbonates and mammalian teeth, beginning ≈9 Ma, signifies an increase in temperature, a decrease in precipitation, or a change in source of precipitation—any of which can be attributed to the onset of the Indian monsoon system (12). The prevalence of diverse crocodilians as well as the paleolatitude (27–30°N) and low elevation imply that temperatures were well above freezing year round.
Isotopic historical trends were documented for mammalian herbivores and for individual lineages over the late Miocene interval of environmental change. Mammalian δ13C values (n = 570) show a greater range of variation than do those from pedogenic carbonates (Fig. 2). This variation reflects dietary differences among individuals and species as mammals ranged across the sub-Himalayan flood basin and documents the presence of more closed and more open habitats than are represented by paleosol carbonates (17). Isotopic profiles and ecomorphological dietary information for individual lineages were evaluated in relation to appearances and disappearances of species to test the hypothesis that climatically driven vegetation change caused the observed faunal turnover.
Environmental Sorting of Species Through Time.
A model of climatically driven sorting of species predicts that as vegetation habitats shrink, expand, or move across the landscape, herbivorous species reliant on particular plant resources will shift their geographic ranges to remain in their preferred habitats (2). In the Siwalik record, this scenario implies that species that relied on C3 vegetation (forest fruit, browse) should disappear during the transition to C4 vegetation (open grassland), resulting in local extinction of these lineages, whereas immigrant herbivores should consume mixed C3–C4 or pure C4 vegetation. Thus, the pattern of faunal change should involve local extinction of frugivores and browsers and replacement by mixed feeders and grazers. Consumers of C3 vegetation could persist by changing their diets to include C4 vegetation. This option is especially plausible for herbivores that consumed substantial quantities of C3 grasses before the vegetation transition and then switched to C4 grasses. The hypothesis of climatically induced faunal change is falsified if immigrant lineages during or after the vegetation shift are consumers of pure C3 vegetation or if pure C3 consumers persist after the shift to C4 grassland. Other models of faunal change result in different patterns of faunal turnover in the Siwalik record (22).
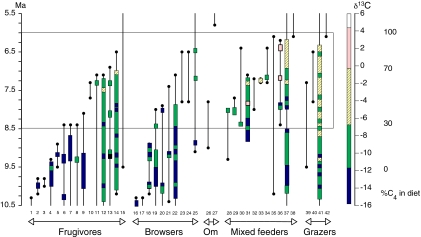
Fig. 3 shows the inferred biostratigraphic ranges of 42 taxonomically well resolved herbivorous lineages (at the species level except for hipparionine equids), including artiodactyls, perissodactyls, rodents, primates, and one proboscidean—taxa that dominate the fossil assemblages in numerical abundance over the analysis interval. This pattern of faunal change is robust to changes in fossil preservation, although the sample sizes from intervals < 7.0 Ma decline (11). Lineages are grouped according to major dietary category, as inferred from dental ecomorphology. The major dietary categories are (i) frugivore (predominantly fruit year-round), (ii) browser (broad leaves of angiosperms), (iii) omnivore (broad-leaved plants and animal material), (iv) mixed feeders (broad leaves and grasses), and (v) grazers (predominantly grasses). Isotopic data for 27 lineages (color-coded in Fig. 3) indicate their diets at different times during their residence. These historical isotopic trends show that some lineages maintained C3-dominated diets, whereas other lineages altered their feeding habits to include varying amounts of C4 vegetation during the transition from C3 to C4 vegetation.
Fig. 3.
Biostratigraphic ranges of 42 herbivorous lineages (at the species level except for hipparionine equids) from the late Miocene analysis interval. Isotopic sampling is indicated by colored vertical bars; if a sampling gap of >0.5 Ma is present, the lineage is represented by multiple bars rather than a continuous bar. Table S2 provides the lineages and age ranges that are the basis for the biostratigraphy.
The interval from 8.5 to 6.0 Ma encompasses the δC13 shift and the major period of faunal turnover. Before 8.5 Ma, most herbivores consumed C3 vegetation from closed forest (δ13C values < −12‰). C3 grasses were also part of the flood plain vegetation. Microwear data for nine artiodactyl lineages categorized as frugivores, browsers, or mixed feeders indicate the consumption of trace amounts (1–3%) of C3 grasses (16). Microwear data for hipparionine equids indicate that they consumed both browse and grass (15), and mesowear data imply that they ate >80% monocots (23). Hipparionines were the earliest large herbivores to use C4 grasses and likely consumed C3 grasses before C4 grasses became common on the landscape. Other mixed feeders may also have consumed substantial amounts of C3 grass before C4 grasses became widespread. At 7.5 Ma, most herbivores consumed C3 plants from woodland and more open habitats, whereas a few incorporated some C4 grass into their diets (δ13C values between −12‰ and −7.5‰). By 6.0 Ma, all herbivores sampled ate a mixed C3–C4 or pure C4 diet (δ13C values > −7.5‰), and paleosol carbonates indicate that C4 vegetation was widespread. Lateral variation in δ13C values from younger paleosols document areas of C3 vegetation that remained on the flood plain in specific depositional environments until at least 4.5 Ma (14).
Over the interval of major change, 15 of 17 lineages that relied on fruit and browse disappeared from the Siwalik record (Fig. 3). These disappearances include a hominoid, two large suids, one peccary, four tragulids, and an anthracothere among the frugivorous species, and a chalicothere, a deinothere, a giraffe, a colobine monkey, a tragulid, and a bovid among the browsers. The lineages with the most depleted δ13C values (<−12‰), signifying forest-canopy resources, disappeared earliest (by 8.0 Ma). Although one frugivorous and two browsing lineages appeared between 8.0 and 7.5 Ma, all three had disappeared by 6.5 Ma. Two of the sampled frugivore lineages (one tragulid, one suid) show isotopic enrichment in geologically younger individuals, signifying that their diets had changed to include some C4 vegetation between 7.5 and 7.0 Ma. These lineages eventually disappeared, however, and by 6.5 Ma, only one frugivorous and one browsing lineage remained in the herbivore faunal subset.
Mixed feeders and grazing herbivores show quite a different pattern. Whereas 11 lineages disappeared between 8.5 and 6.0 Ma, 9 lineages appeared during this interval. Both the disappearing lineages and the appearing lineages include artiodactyls and rodents. Four lineages show enrichment of δ13C values in younger localities, and two of these persisted well beyond the interval of major vegetation change. One bovid lineage (no. 36, see Table S2) changed from a C3 diet at 8.0 Ma to a C4 grass-dominated diet after 7.5 Ma. Hipparionine equids consumed some C4 grass by 8.5 Ma, earlier than any other lineage sampled, but included substantial amounts of C3 plants in their diets throughout the vegetation shift, a pattern consistent with dietary habits inferred for late Neogene equids from other regions (24, 25). For mixed feeders and grazers, new taxa appeared throughout the critical interval, in contrast to the frugivores and browsers.
At the temporal scale of this analysis, herbivore diets closely tracked changes in vegetation, and trophic structure changed via selective removal of forest frugivores and browsers. At 8.5 Ma, frugivores and browsers outnumbered mixed feeders and grazers by two to one; by 6.0 Ma, these proportions were reversed. Overall species richness of herbivorous mammals declined. Among rodents, hypsodonty increased in one long-lasting rhizomyid lineage, and other hypsodont rhizomyid lineages appeared after 7.5 Ma (26). This pattern of faunal turnover matches our model predictions for prolonged climatic forcing of faunal change.
Environment, Ecology, and Evolution.
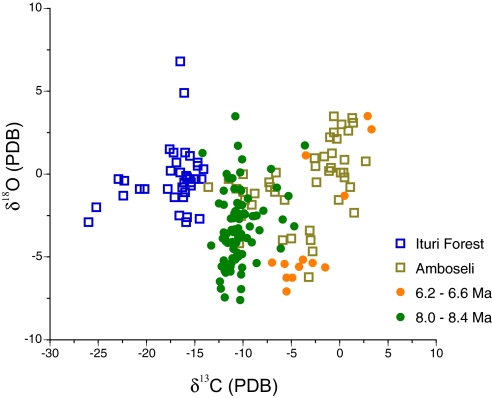
Climatic conditions determine the amount and seasonal timing of primary productivity as well as habitat structure in modern terrestrial ecosystems and thereby the kinds of herbivores that ecosystems can support. In terms of modern environments, this late Miocene record represents a transition from a wet monsoonal forest before 8.5 Ma to dry monsoonal forest at 7.0 Ma to savannah at 6.0 Ma. Isotopic profiles of mammalian faunas document a shift in δ13C values from those characteristic of humid tropical forest and woodland at 8.4–8.0 Ma toward values characteristic of a modern African savannah at 6.2–6.6 Ma (Fig. 4). In the Siwalik record, seasonality of precipitation, as inferred from annual profiles of δ18O in equid teeth, followed a monsoonal pattern with a long dry season each year (27). From 10.0 to 6.3 Ma, changes in δ18O values of equid tooth profiles imply a decline of several hundred mm in annual rainfall, whereas the seasonality of precipitation remained the same. In South Asia today, the ecotone between savannah (C4) and dry monsoon forest (C3) is maintained by small differences in the length of the dry season, grazing pressure, or fire (28, 29). Alluvial grasslands can persist even in areas receiving as much as 4,000 mm of annual rainfall because the soil dries out completely during the dry season (30). Even though such grasslands have high productivity, the highly seasonal rainfall and productivity limit the species richness and abundance of mammalian herbivores in these ecosystems. For the late Miocene record, the vegetation change also represents the transformation of a three-dimensional to a more two-dimensional habitat with a reduced variety of food resources. These climatic and ecological changes were driven by the uplift of the Himalayas and Tibetan Plateau that, in turn, intensified the South Asian monsoon system (13).
Fig. 4.
Isotopic cross-plots (δ18O versus δ13C) of (i) tooth enamel from mammalian herbivores of the Ituri forest, a modern evergreen rainforest in Zaire (32), (ii) tooth enamel from Amboseli National Park, a modern savannah in Kenya (33), and (iii) tooth enamel from Miocene ungulates and proboscideans from two intervals of the Potwar Plateau, Pakistan. δ18O values from (32) were converted from the SMOW standard to PDB (34). To facilitate comparison between modern isotopic data and Miocene data, all modern δ13C values were adjusted by +2.5‰ because of changes in atmospheric composition of δ13C since the late Miocene.
In the Pakistan record, species with older appearances have longer mean residence times than do species with younger appearances (5.2 versus 2.3 myr for lineages of Fig. 1 older and younger, respectively, than 11.0 Ma). During the interval of major environmental change, long-term residents were the first to disappear (Fig. 3), and their trophic habits corresponded to the resources of the disappearing mesic forest. Within this interval, half of the species of mixed feeders have short residence times—consistent with the environmental-sorting model. As monsoon savannah replaced mesic forest, generalist herbivores immigrated and then disappeared as vegetation became more homogeneous. Thus, the Siwalik record does not fit the “seniority rule” scenario, in which transients, or lineages of relatively short duration, are the first to disappear during intervals of environmental stress, as proposed for the mid-Miocene, small-mammal record of Spain (31).
This Miocene record, characterized by cumulative extinction of resident mammals and immigration of new species into the sub-Himalayan ecosystem, over ≈2.5 myr, supports a model of climatically induced environmental sorting of community composition and structure. Both prolonged and punctuated patterns (2) of faunal change belong to this general model of environmental sorting over different intervals of time. Although the particular details of the Siwalik record involve aridification, followed by selective extinction, the capacity for prolonged ecological response to climatic change documented for Miocene mammalian faunas of South Asia may be a general feature of terrestrial ecosystems past, present, and future.
Supplementary Material
Acknowledgments.
We thank our colleagues at the Geological Survey of Pakistan for ongoing support and collaboration, Larry Flynn for information about rodent taxonomy and biostratigraphy, Paul Koch for advice about isotope ecology, and Andrew Hill and Mikael Fortelius for reviews. This work was supported by National Science Foundation Grant EAR-0125663.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805592105/DCSupplemental.
References
- 1.Rosenzweig ML. Species Diversity in Space and Time. Cambridge, UK: Cambridge Univ Press; 1995. [Google Scholar]
- 2.Vrba ES. In: Paleoclimate and Evolution, with Emphasis on Human Origins. Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. New Haven, CT: Yale Univ Press; 1995. pp. 24–45. [Google Scholar]
- 3.FAUNMAP Working Group. Spatial response of mammals to the late Quaternary environmental fluctuations. Science. 1996;272:1601–1606. doi: 10.1126/science.272.5268.1601. [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol System. 2006;37:637–669. [Google Scholar]
- 5.van Dam JA, et al. Long-period astronomical forcing of mammal turnover. Nature. 2006;443:687–691. doi: 10.1038/nature05163. [DOI] [PubMed] [Google Scholar]
- 6.Alroy J, Koch PL, Zachos JC. In: Deep Time: Paleobiology's Perspective. Erwin DH, Wing SL, editors. Lawrence, KS: Paleontol Soc; 2001. pp. 259–288. [Google Scholar]
- 7.Barnosky AD, Carrasco MA. Effects of Oligo-Miocene global climate changes on mammalian species richness in the northwestern quarter of the USA. Evol Ecol Res. 2002;4:811–841. [Google Scholar]
- 8.Barnosky AD. Effects of Quaternary climatic change on speciation in mammals. J Mamm Evol. 2005;12:247–264. [Google Scholar]
- 9.Jernvall J, Fortelius M. Common mammals drive evolutionary increase of hypsodonty in the Neogene. Nature. 2002;417:538–540. doi: 10.1038/417538a. [DOI] [PubMed] [Google Scholar]
- 10.Badgley C, Fox DL. Ecological biogeography of North American mammals: Species density and ecological structure in relation to environmental gradients. J Biogeogr. 2000;27:1437–1467. [Google Scholar]
- 11.Barry JC, et al. Faunal and environmental change in the Late Miocene Siwaliks of Northern Pakistan. Paleobiology. 2002;28:1–71. [Google Scholar]
- 12.Quade J, Cerling TE. Expansion of C4 grasses in the late Miocene of Northern Pakistan: Evidence from stable isotopes in paleosols. Paleogeogr Paleoclimatol Paleoecol. 1995;115:91–116. [Google Scholar]
- 13.Zhisheng A, Kutzbach JE, Prell WL, Porter SC. Evolution of Asian monsoons and phased uplift of the Himalaya–Tibetan Plateau since Late Miocene times. Nature. 2001;411:62–66. doi: 10.1038/35075035. [DOI] [PubMed] [Google Scholar]
- 14.Behrensmeyer AK, et al. The structure and rate of late Miocene expansion of C4 plants: Evidence from lateral variation in stable isotopes in paleosols of the Siwalik Group, northern Pakistan. Geol Soc Am Bull. 2007;119:1486–1505. [Google Scholar]
- 15.Morgan ME, Kingston JD, Marino BD. Carbon isotopic evidence for the emergence of C4 plants in the Neogene from Pakistan and Kenya. Nature. 1994;367:162–165. [Google Scholar]
- 16.Nelson SV. The Extinction of Sivapithecus. Boston: Brill; 2003. [Google Scholar]
- 17.Nelson SV. Isotopic reconstructions of habitat change surrounding the extinction of. Sivapithecus, a Miocene hominoid, in the Siwalik Group of Pakistan. Paleogeogr Paleoclimatol Paleoecol. 2007;243:204–222. [Google Scholar]
- 18.Willis BJ. Evolution of Miocene fluvial systems in the Himalayan foredeep through a two kilometer-thick succession in northern Pakistan. Sediment Geol. 1993;88:77–121. [Google Scholar]
- 19.Khan IA, Bridge JS, Kappelman J, Wilson R. Evolution of Miocene fluvial environments, eastern Potwar Plateau, northern Pakistan. Sedimentology. 1997;44:221–251. [Google Scholar]
- 20.Zaleha MJ. Intra- and extrabasinal controls on fluvial deposition in the Miocene Indo-Gangetic foreland, northern Pakistan. Sedimentology. 1997;44:369–390. [Google Scholar]
- 21.Ehleringer JR, Cerling TE, Helliker BR. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 1997;112:285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- 22.Badgley C, Nelson S, Barry JC, Behrensmeyer AK, Cerling TE. In: Interpreting the Past: Essays on Human, Primate, and Mammal Evolution. Lieberman DE, Smith RH, Kelley J, editors. Boston: Brill; 2005. pp. 29–46. [Google Scholar]
- 23.Belmaker M, Nelson S, Morgan ME, Barry J, Badgley C. Mesowear analysis of ungulates in the middle to late Miocene of the Siwaliks, Pakistan: Dietary and paleoenvironmental implications. J Vertebr Paleontol. 2007;27:46A. [Google Scholar]
- 24.Quade J, Solounias N, Cerling TE. Stable isotopic evidence from paleosol carbonates and fossil teeth in Greece for forest or woodlands over the past 11 Ma. Paleogeogr Paleoclimatol Paleoecol. 1994;108:41–53. [Google Scholar]
- 25.MacFadden BJ, Cerling TE, Harris JM, Prado J. Ancient latitudinal gradients of C3/C4 grasses interpreted from stable isotopes of New World Pleistocen horse (Equus) teeth. Glob Ecol Biogeogr. 1999;8:137–149. [Google Scholar]
- 26.Flynn LJ, Jacobs LL. Effects of changing environments on Siwalik rodent faunas of Northern Pakistan. Paleogeogr Paleoclimatol Paleoecol. 1982;38:129–138. [Google Scholar]
- 27.Nelson SV. Paleoseasonality inferred from equid teeth and intra-tooth isotopic variability. Paleogeogr Paleoclimatol Paleoecol. 2005;222:122–144. [Google Scholar]
- 28.Stott P. Stability and stress in the savanna forests of mainland South-East Asia. J Biogeogr. 1990;17:373–383. [Google Scholar]
- 29.Mariotti A, Peterschmitt E. Forest savanna ecotone dynamics in India as revealed by carbon isotope ratios of soil organic matter. Oecologia. 1994;97:475–480. doi: 10.1007/BF00325885. [DOI] [PubMed] [Google Scholar]
- 30.Yadava PS. Savannas of North-East India. J Biogeogr. 1994;17:385–394. [Google Scholar]
- 31.van der Meulen AJ, Peláez-Campomanes, Levin SA. Age structure, residents, and transients of Miocene rodent communities. Am Nat. 2005;165:108–125. doi: 10.1086/428683. [DOI] [PubMed] [Google Scholar]
- 32.Cerling TE, Hart JA, Hart TB. Stable isotope ecology in the Ituri Forest. Oecologia. 2004;13:5–12. doi: 10.1007/s00442-003-1375-4. [DOI] [PubMed] [Google Scholar]
- 33.Bocherens H, Koch PL, Mariotti A, Geraads D, Jaeger J-J. Isotopic biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene hominid sites. Palaios. 1996;11:306–318. [Google Scholar]
- 34.Hoefs J. Stable Isotope Geochemistry. Berlin: Springer; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.