Abstract
Interfacial zones between tissues provide specialized, transitional junctions central to normal tissue function. Regenerative medicine strategies focused on multiple cell types and/or bi/tri-layered scaffolds do not provide continuously graded interfaces, severely limiting the integration and biological performance of engineered tissue substitutes. Inspired by the bone-soft tissue interface, we describe a biomaterial-mediated gene transfer strategy for spatially regulated genetic modification and differentiation of primary dermal fibroblasts within tissue-engineered constructs. We demonstrate that zonal organization of osteoblastic and fibroblastic cellular phenotypes can be engineered by a simple, one-step seeding of fibroblasts onto scaffolds containing a spatial distribution of retrovirus encoding the osteogenic transcription factor Runx2/Cbfa1. Gradients of immobilized retrovirus, achieved via deposition of controlled poly(l-lysine) densities, resulted in spatial patterns of transcription factor expression, osteoblastic differentiation, and mineralized matrix deposition. Notably, this graded distribution of mineral deposition and mechanical properties was maintained when implanted in vivo in an ectopic site. Development of this facile and robust strategy is significant toward the regeneration of continuous interfacial zones that mimic the cellular and microstructural characteristics of native tissue.
Keywords: fibroblasts, gene therapy, heterogeneous tissue engineering, Runx2, transcription factor gradients
Heterogeneous tissues containing graded, transitional interfaces play a central role in normal organ development and function (1). These interfaces typically consist of multiple cell types and spatially graded matrix components arranged in complex hierarchical structures to fulfill specific functional roles. For example, precise spatial patterning of distinct neuronal cell fates along the dorsoventral axis of the developing vertebrate spinal cord coordinates functional organization of the adult central nervous system (2). Similarly, repeated mosaic patterns of sensory and nonsensory cellular phenotypes within the mammalian cochlea are essential for the development of normal auditory function (3). Within the musculoskeletal system, graded interfaces between bone and soft tissue facilitate transmission of complex mechanical loads across limb joints by minimizing stress concentrations at the junction of two tissue types (4, 5). Conventional soft tissue autografts typically fail at the bony insertion site due to inadequate tissue integration, further highlighting the critical importance of these transitional junctions (6).
Regenerative medicine strategies have been pursued to engineer graded tissue interfaces that restore native tissue architecture and function (7). Current approaches rely on the integration of multiple cell types or growth factor gradients within multilayered 3D scaffolds (8–10). Strategies based on laminated/layered constructs have restricted potential for generating continuous, graded interfaces due to inherent discontinuities across dissimilar materials (11). Furthermore, approaches based on soluble bioactive factors are severely limited by suboptimal delivery vehicles, poor spatiotemporal dosage control, short protein half-life, and the cost-prohibitive supraphysiologic concentrations required to initiate a cellular response (12, 13). Ultimately, efforts to engineer robust protein gradients with precise targeting/delivery to specific cell types in vivo may be prohibited by the complex milieu. Due to these limitations, the development of heterogeneous tissue grafts containing graded/transitional interfacial zones has not been realized.
The present study describes a genetic approach that exploits spatially regulated biomaterial-mediated gene transfer to create continuous tissue gradients. We hypothesized that graded interfaces of differential cell function could be engineered by precisely controlling expression of a tissue-specific transcription factor within biomaterial scaffolds. Using the bone-soft tissue interface as a model system, we generated 3D gradients of immobilized retrovirus encoding the osteogenic transcription factor Runx2/Cbfa1. Runx2 was selected as the biological cue because it plays an essential role in the commitment of mesenchymal stem cells to the osteoblastic lineage during skeletal development (14, 15). Furthermore, recent studies have reported promising results for this factor as a therapeutic transgene in orthopedic tissue engineering applications (16, 17). Importantly, we have previously shown that retroviral overexpression of Runx2 reprograms nonosteoblastic dermal fibroblasts into a mineralizing, osteoblast-like phenotype in vitro and in vivo (18–20). Here we demonstrate that a continuous bone-soft tissue-mimetic interface can be engineered by a simple, one-step seeding of primary dermal fibroblasts onto polymeric scaffolds containing a graded distribution of immobilized Runx2 retrovirus. The concept of controlling expression of a tissue-specific transcription factor to create spatial gradients of differential cell function within 3D matrices may be applicable to the development of interfacial zones for a diverse range of tissue engineering applications.
Results
Biomaterial-Mediated Retroviral Gene Transfer Approach.
As a first step toward creating a heterogeneous interface, we explored the feasibility of genetically modifying fibroblasts in situ within tissue constructs using biomaterial-mediated retroviral gene transfer. Runx2 retrovirus was immobilized onto collagen scaffolds by exploiting the ability of cationic polymers, in this case poly(l-lysine) (PLL), to charge neutralize and aggregate viral particles (21, 22). Collagen scaffolds were uniformly coated with 0.01% PLL and incubated in retroviral supernatant for 4–5 h before seeding with primary dermal fibroblasts. The pTJ66-Runx2 retroviral vector expresses an eGFP selectable marker from an internal ribosomal entry site, allowing for noninvasive analysis of transduction efficiency via flow cytometry (Fig. 1A). eGFP expression was detected in ≥20% of fibroblasts recovered from collagen scaffolds by enzymatic dissociation after 14 days culture in osteogenic growth media, demonstrating sustained and integrated transgene expression from the retroviral vector (Fig. 1B). Quantitative RT-PCR (qRT-PCR) analysis revealed up-regulation of eGFP gene expression on PLL-coated scaffolds incubated in Runx2 retrovirus (PLL+R2RV) compared to uncoated scaffolds incubated in Runx2 retrovirus (R2RV-only), indicating that retrovirus immobilization and the subsequent ability to transduce cells is dependent on coating scaffolds with PLL [supporting information (SI) Fig. S1a]. Immunohistochemical staining for eGFP expression further confirmed these results, because eGFP colocalized with fibroblasts only on scaffolds coated with PLL+R2RV (Fig. S1b). Finally, confocal image analysis of live/dead fluorescence staining (Fig. S1c) and quantification of DNA content (Fig. S1d) indicated that fibroblasts remain viable throughout the 42 day culture period and equivalently colonize collagen scaffolds independent of treatment group. We note that the interior of these constructs remained devoid of cells, probably due to nutrient/waste transport limitations. Taken together, these results indicate that retroviral particles noncovalently immobilized within 3D scaffolds via PLL retain the ability to transduce cells cultured on these matrices.
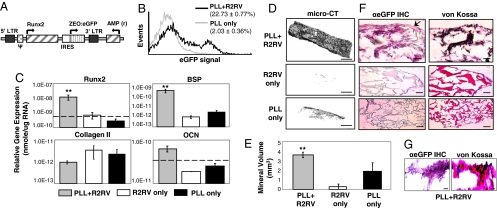
Fig. 1.
Biomaterial-mediated retroviral gene delivery promotes sustained Runx2 transgene expression and osteoblastic differentiation in fibroblasts. (A) The Runx2 retroviral vector expresses the type II Runx2 isoform and a Zeocin:eGFP selectable marker from a single bicistronic mRNA transcript. Runx2 retrovirus (R2RV) was noncovalently immobilized onto fibrous collagen scaffolds coated with PLL. Primary dermal fibroblasts were then seeded onto these scaffolds and cultured in osteogenic media until endpoint assay. (B) Detection of eGFP expression by flow cytometry demonstrated sustained fibroblast transduction at 14 days postseeding. (C) Osteogenic gene expression was up-regulated within constructs uniformly coated with PLL+R2RV compared to R2RV- and PLL-only controls at 7 days postseeding. [Mean + SEM, n = 3; Runx2: ANOVA: P = 0.002, ** vs. R2RV only (P = 0.01) and PLL only (P = 0.002); Bone Sialoprotein: ANOVA: P = 0.000066, ** vs. R2RV only (P = 0.00009) and PLL only (P = 0.00023)]. (D) MicroCT images demonstrated enhanced mineral deposition on scaffolds coated with PLL+R2RV compared to R2RV- and PLL-only controls. (Scale bar: 1 mm.) (E) Quantification of mineral deposition by microCT image analysis after 49 days culture in osteogenic media. [Mean + SEM, n ≥ 4; ANOVA: P < 0.0001; ** vs. R2RV only (P = 0.00018) and PLL only (P = 0.05).] (F and G) Histological analyses demonstrated colocalization of genetically engineered fibroblasts (eGFP, pink, arrow; hematoxylin, blue) and mineral deposits (von Kossa, black), indicating that mineral deposition originated from fibroblasts that were susceptible to biomaterial-mediated viral gene delivery. (Scale bars: F, 500 μm; G, 20 μm.)
Biomaterial-Mediated Runx2 Gene Delivery Promotes Osteogenesis in Fibroblasts.
We next examined the effect of scaffold-mediated Runx2 gene transfer on fibroblast differentiation into an osteoblast-like phenotype. Osteogenic gene expression was quantified by qRT-PCR after 7 days culture in osteogenic differentiation media. Biomaterial-mediated delivery of the Runx2 retroviral vector from PLL-coated scaffolds up-regulated expression of the osteoblastic markers Runx2, osteocalcin, and bone sialoprotein compared to PLL-only and R2RV-only control constructs (Fig. 1C). This response was specific to osteogenic markers, because expression of the chondrocytic marker type II collagen remained unchanged. Mineral deposition was analyzed after 49 days culture in osteogenic differentiation media as a functional marker of osteogenesis. Fibroblasts deposited significant amounts of mineralized matrix on scaffolds coated with PLL+R2RV, whereas R2RV- and PLL-only control scaffolds displayed negligible mineral deposits (Fig. 1 D and E). Furthermore, Fourier-transform infrared spectroscopic analysis of the matrix surrounding cells seeded within PLL+Runx2RV-coated scaffolds revealed the characteristic spectra of a poorly crystalline, carbonate-containing hydroxyapatite consistent with the inorganic phase of bone (data not shown) (19, 20). In contrast, the matrix surrounding untransduced fibroblasts displayed no evidence of such mineral deposition. Immunohistochemical staining for eGFP expression was performed to assess the spatial distribution of transduced fibroblasts and mineral deposits within constructs (Fig. 1 F and G). eGFP-positive cells colocalized with von Kossa-positive mineral deposits, indicating that mineral deposition was primarily mediated by fibroblasts transduced by the immobilized retrovirus.
Histological and microCT analyses of cross-sectional images revealed that cell colonization and mineral deposition patterns were differentially modulated by the virus delivery strategy (Fig. S2). Mineral deposits displayed a dense morphology and were confined to the periphery of scaffolds seeded with fibroblasts transduced by conventional gene transfer techniques. In contrast, biomaterial-mediated transduction resulted in deposition of distinct punctate nodules throughout the construct interior. It is not clear whether the ability to circumvent the formation of the peripheral mineralized shell is a direct cause, or a consequence of, improved mass transport and extended cell viability. Because cells within the construct core eventually die by day 42 with both approaches, we speculate that biomaterial-mediated gene transfer enables extended cellular lifespan for a short window of time that is sufficient for mineral deposition within the scaffold interior. These results are important because the clinical application of current tissue engineering strategies to critical-sized bone defects is significantly limited by the formation of a mineralized shell around the scaffold periphery and, consequently, cell necrosis within the inner construct core (23). Taken together, these results indicate that biomaterial-mediated retroviral gene delivery is a feasible strategy for the genetic modification and differentiation of fibroblasts into a mineralizing osteoblastic phenotype within 3D matrices.
Precise Spatial Control over 3D PLL Gradient.
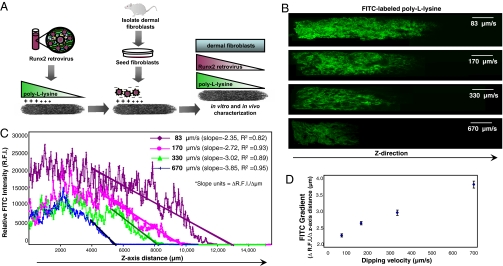
We next postulated that spatial patterns of retroviral transduction, Runx2 expression, and osteogenic differentiation could be generated within tissue constructs by creating gradients of PLL coating density before incubation in retroviral supernatant and cell seeding (Fig. 2A). Toward this end, we first performed experiments to demonstrate precise spatial control over PLL deposition within 3D matrices. PLL gradients were generated by dipping scaffolds into a solution of FITC-labeled PLL at a controlled rate using a motorized dip coater (60–700 μm/s dipping speed range). This approach is commonly used in industrial coating applications to vary coating density/thickness according to the Landau-Levich law (24). Confocal microscopy image analysis demonstrated that coating density depends on incubation/residence time in the coating solution and, therefore, the dipping speed (Fig. 2B). Quantification of FITC intensity along the direction of PLL coating (z-axis) confirmed that differential PLL gradient slopes can be engineered by varying scaffold dipping rate (Fig. 2 C and D). Importantly, the PLL coating density can be spatially controlled by varying the coating speed, residence time, and concentration, providing a wide range of parameters to efficiently optimize these coatings.
Fig. 2.
Precise spatial control over 3D PLL gradient. (A) Schematic representation of scaffold-mediated gene delivery strategy implemented to create a spatial distribution of Runx2 retrovirus within 3D matrices. The proximal portion of collagen scaffolds was coated with PLL before incubation in retroviral supernatant and cell seeding. (B) PLL gradients were generated by dipping collagen scaffolds into a PLL solution at a controlled rate using a motorized dip coater (60–700 μm/s dipping speed range). FITC-labeled PLL was used to facilitate gradient visualization by confocal microscopy. (Scale bar: 2 mm.) (C and D) Quantification of FITC intensity demonstrated that the steepness/slope of PLL density within collagen scaffolds depends on dipping speed as described by the Landau-Levich law.
Spatially Regulated Genetic Modification of Fibroblasts in 3D Matrices.
PLL gradients were exploited to spatially regulate R2RV immobilization and cell transduction within 3D matrices (Fig. 3A). The proximal portion (left side) of collagen scaffolds was coated with PLL at a dipping speed of 170 μm/s before incubation in retroviral supernatant and cell seeding. Generation of a PLL gradient was confirmed by confocal microscopy image analysis of FITC-labeled PLL (Fig. 3B). High magnification confocal microscopy images along the z-axis of tissue-engineered construct shown in Fig. 3B revealed high, medium, and low PLL densities controlled by incubation/residence time in the coating solution (Fig. 3E). DAPI-stained fibroblast nuclei were uniformly distributed throughout 3D scaffolds independent of PLL coating (Fig. 3C). Immobilized retroviral particles were visualized by incubating scaffolds containing a PLL gradient in red fluorescent protein (RFP)-labeled retrovirus (RFP-RV). Although individual retroviral particles are below the resolution of light microscopy, the resulting fluorescence from these particles can be tracked via confocal microscopy. RFP-RV colocalized with FITC-labeled PLL and DAPI-stained fibroblast nuclei (Fig. S3). Moreover, the intensity (density) of RFP-RV correlated with PLL density, confirming PLL-mediated immobilization of retrovirus within collagen scaffolds (Fig. 3F).
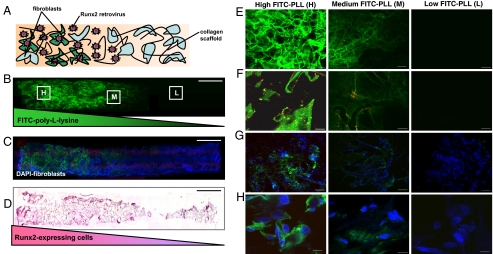
Fig. 3.
Spatially regulated genetic modification of fibroblasts within 3D matrices. (A) Schematic representation of a fibroblast-seeded construct containing spatial patterns of noncovalently immobilized retrovirus. A spatial distribution of Runx2 retrovirus (R2RV) was created by partially coating the proximal portion (left side) of collagen scaffolds with PLL at a dipping speed of 170 μm/s before incubation in retroviral supernatant and cell seeding. (B and C) Confocal microscopy images demonstrating a graded distribution of FITC-labeled PLL (green) (B) and FITC-labeled PLL gradient colocalized with uniformly distributed cell nuclei (DAPI, blue) (C). (Scale bar: 2 mm.) (D) Immunohistochemical staining for eGFP (pink) counterstained with hematoxylin (blue) revealed elevated eGFP expression on the proximal scaffold portion coated with PLL+R2RV. (Scale bar: 2 mm.) (E–H) High magnification confocal microscopy images moving lengthwise down the z-axis of construct shown in (B) at high (H), medium (M), and low (L) PLL densities. (E) FITC-labeled-PLL density is controlled by incubation/residence time in coating solution according to dipping speed. (Scale bar: 100 μm.) (F) Red fluorescent protein-labeled retrovirus (RFP-RV) colocalizes with FITC-PLL, confirming PLL-mediated immobilization of retrovirus. (Scale bars: 10 μm.) (G and H) Spatial control over retroviral transduction was assessed by coating scaffolds with a gradient of unlabeled PLL and unlabeled R2RV before cell seeding. High levels of eGFP expression (green) in DAPI-stained cells (blue) indicated efficient retroviral transduction in construct region containing high PLL density (H). (Scale bars: G, 100 μm; H, 10 μm.)
Spatial control over biomaterial-mediated retroviral gene transfer was assessed by coating scaffolds with a gradient of unlabeled PLL followed by incubation in eGFP retrovirus. Fibroblasts were uniformly seeded on these scaffolds and eGFP expression was investigated at 5 days postseeding via confocal microscopy. High levels of eGFP expression were evident in scaffold regions containing high PLL density (Figs. 3 G and H), indicating efficient in situ retroviral transduction. These results were further confirmed by immunohistochemical staining for eGFP expression, which revealed the presence of a graded distribution of genetically modified, Runx2-expressing cells (Fig. 3D). Taken together, these results demonstrate spatially regulated retrovirus immobilization, fibroblast transduction, and Runx2 expression by precise control over deposition of PLL within 3D matrices.
Runx2-Induced Zonal Organization of Osteoblastic and Fibroblastic Phenotypes in Vitro and in Vivo.
Tissue constructs containing a graded distribution of Runx2-expressing cells were investigated for their potential to organize osteoblastic and fibroblastic cell fates into distinct 3D patterns. Mineral deposition within constructs containing a spatial distribution of Runx2-expressing cells was evaluated with microCT image analysis after 42 days in vitro culture in osteogenic differentiation media. Importantly, PLL-coated scaffolds containing a spatial distribution of immobilized retrovirus (PLL+R2RV) showed zonal organization of both mineral deposition and nonmineralized, fibroblastic extracellular matrix (Fig. 4A). This mineral gradient was not detected in PLL-only and R2RV-only control samples. We attribute the differences in magnitude of mineral deposition shown in Fig. 1 vs. Fig. 4 to differences in the PLL coating (static 30 min) vs. dipping at a controlled rate (5–10 sec). Confocal microscopy image analysis of live/dead fluorescent staining confirmed that cells were uniformly distributed and viable at the scaffold periphery, indicating that the spatial distribution of mineral was not due to differences in cell numbers (Fig. 4B). These functional results were further confirmed by qRT-PCR analysis, which revealed significant up-regulation of osteoblastic genes on the proximal, PLL+R2RV-coated portion of collagen scaffolds, whereas cells seeded on the distal scaffold portion incubated in retrovirus alone (no PLL coating) expressed background levels of these osteoblastic markers (data not shown).
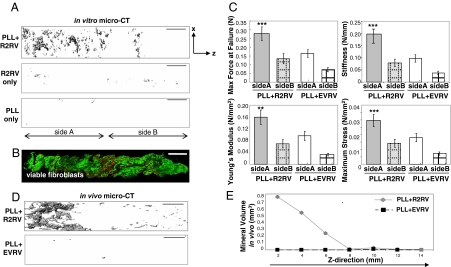
Fig. 4.
Runx2-induced zonal organization of osteoblastic and fibroblastic phenotypes. (A) MicroCT image analysis demonstrated spatial patterning of both mineral deposition and nonmineralized, fibroblastic extracellular matrix within constructs containing a graded distribution of PLL+R2RV after 42 days in vitro culture. (Scale bar: 2 mm.) (B) Confocal microscopy image of live (green)/dead (red) fluorescently stained cells showing uniform fibroblast distribution across the periphery of a PLL+R2RV coated sample. (Scale bar: 2 mm.) (C) Tensile testing at a strain rate of 0.2 mm/s indicated that both structural and material mechanical properties were significantly enhanced on the proximal, PLL-coated portion (side A) of R2RV-coated scaffolds compared to control specimens. (See statistics in SI Methods.) (D) Representative microCT images of explanted constructs after 14 days in vivo in a s.c., ectopic site confirming spatially patterned mineral deposition in constructs containing a graded distribution of Runx2-expressing cells. (Scale bar: 2 mm.) (E) Quantification of mineral volume in 2-mm segments moving lengthwise down the z-axis of representative constructs with microCT image analysis.
The biomechanical properties of these heterogeneous constructs were characterized by uniaxial tensile testing at a strain rate of 0.2 mm/s (Fig. 4C). Construct stiffness (0.20 ± 0.038 N/mm) and maximum force at failure (0.28 ± 0.04 N) were significantly enhanced on the proximal, PLL-coated portion (side A) of R2RV-treated scaffolds compared to control specimens. Furthermore, the material properties of PLL+R2RV-coated constructs (Young's Modulus 0.16 ± 0.024 N/mm2 and maximum stress 0.03 ± 0.004 N/mm2) were also significantly up-regulated compared to control samples. Taken together, these analyses demonstrate increases in both the structural and material mechanical properties within the mineral-containing portion of heterogeneous bone-soft tissue constructs.
Finally, engineered scaffolds were implanted into an ectopic, s.c. site to assess the capacity of these constructs to maintain the Runx2-induced spatial distribution of mineral deposition in vivo in the absence of osteoinductive cues and cell types typically present in orthotopic defects. MicroCT image analysis revealed spatial patterning of mineral deposition within PLL-coated constructs containing a graded distribution of R2RV after 14 days in vivo (Fig. 4D). The magnitude of mineral volume fraction was highest on the proximal portion of the scaffold and decreased continuously along the length of the construct toward the distal end (Fig. 4E). Negligible mineral deposits were detected on control constructs coated with PLL and incubated in empty vector (no Runx2 insert) retrovirus. Furthermore, cell-free scaffolds containing immobilized R2RV did not result in any mineral deposition in vivo, indicating that the transplanted donor cells are required for mineral deposition (data not shown). Collectively, these results demonstrate that spatial control over retroviral transduction and Runx2 expression results in zonal organization of osteoblastic and fibroblastic phenotypes within 3D matrices.
Discussion
The development of transitional interfacial zones between tissues represents a significant challenge in current tissue engineering and regenerative medicine strategies (1). Using the bone-soft tissue interface as a model system, we describe a biomaterial-mediated gene delivery approach for spatially regulated expression of the osteogenic transcription factor Runx2 within tissue-engineered constructs. We first demonstrate that biomaterial-mediated retroviral gene transfer is a feasible strategy for the genetic modification and differentiation of fibroblasts into a mineralizing osteoblastic phenotype within a 3D culture environment. A spatial distribution of R2RV was created by noncovalently immobilizing retroviral particles to scaffolds regions containing a PLL gradient. Precise control over PLL density was achieved by varying the coating solution incubation/residence time via scaffold dipping speed. These 3D retroviral gradients resulted in spatially regulated genetic modification of fibroblasts and, consequently, zonal organization of osteoblastic and fibroblastic cellular phenotypes in vitro and in vivo. Taken together, these results indicate that a continuous, graded bone-soft tissue-mimetic interface can be developed by a simple, one step seeding of fibroblasts into polymeric scaffolds containing a gradient of the Runx2 retroviral vector. This work is fundamentally different from current heterogeneous tissue engineering strategies (7, 8) because it requires only a single cell type to generate a continuous, graded interface.
The ultimate goal of tissue engineering is the regeneration of complex, higher-order grafting templates that mimic the cellular and microstructural characteristics of native tissue. In the present study, we developed graded interfaces that display several important attributes of native interfacial tissue zones, namely a spatially patterned distribution of differential cell types (osteoblasts/fibroblasts) and matrix components (mineral). Importantly, we did not observe the intermediate fibrocartilage zone that is typically present within the bone-ligament enthesis (5), suggesting that these engineered constructs fall short of precisely recapitulating all anatomical features of native tissue gradients. We postulate that the assembly of increasingly complex tissue structures will be enabled by the interplay of these continuous interfaces with the in vivo milieu after implantation into the appropriate anatomical site. Furthermore, the use of alternative cell types (e.g., ligament fibroblasts, mesenchymal stem cells) and bioreactors for applied mechanical loading in vitro should also enhance higher-order tissue organization. Ultimately, the degree to which a tissue engineered substitute must recapitulate native tissue architecture to induce functional integration and healing must be defined for each regenerative medicine application. Future work involving in vivo implantation of our gradient-containing tissue constructs into an orthotopic tissue repair model should yield valuable insights into this important question.
Understanding the mechanism(s) by which density gradients of immobilized Runx2 retrovirus promote the organization of cell fates into specific 3D patterns may provide insight into strategies to precisely engineer complex tissues. One explanation for our results is that spatially graded patterns of immobilized retrovirus lead to a gradient in transduction efficiency, whereby a decreasing percentage of cells are genetically engineered in proportion to the density of immobilized retrovirus. This model is based on the assumption that all transduced cells are reprogrammed into an osteogenic lineage, whereas untransduced cells remain fibroblasts. In support of this hypothesis, we show a decreasing percentage of Runx2/eGFP-expressing cells along the gradient of viral particles (Fig. 3). These eGFP-positive cells colocalized with von Kossa-positive mineral deposits (Fig. 1), indicating that mineral deposition was primarily mediated by genetically engineered fibroblasts. Alternatively, these data do not rule out the possibility that cells on the scaffold regions containing high levels of immobilized retrovirus receive a higher number of viral copies (i.e., “viral integrations”) per cell. In this scenario, a threshold of Runx2 protein levels is required to reprogram fibroblasts into a bone cell phenotype. Support for this explanation comes from our previous studies demonstrating that a small fraction of fibroblasts overexpressing Runx2/eGFP did not deposit mineral in vivo (19). Validation of this model requires a correlation between the number of viral integrations per cell and the propensity of each individual cell to form mineral. It is likely that a combination of both factors play a role in establishing tissue gradients.
We expect this biomaterial-based gene therapy strategy to be broadly applicable to a wide range of heterogeneous biological tissues. Various gene delivery modalities, including adeno- and adeno-associated viral vectors, as well as naked plasmid DNA, may be considered for applications where transient transgene expression is sufficient to induce the desired cellular response. Furthermore, alternative immobilization schemes (covalent vs. noncovalent) may be considered for their impact on gene carrier stability, transduction efficiency, and particle release in vivo (25). Therapeutic transgenes encoding tissue-specific transcription factors and/or soluble growth factors with demonstrated efficacy in conventional ex vivo gene therapy approaches could be combined with this biomaterial-based approach. Overall, the optimal combination of gene transfer vector/modality, therapeutic factor(s), and cell source necessary to produce the desired tissue gradient will be dependent on the particular application.
A retroviral vector was selected in the present study based on previous work demonstrating that constitutive Runx2 transgene expression is necessary to induce functional reprogramming of nonosteogenic cells toward a mineralizing osteoblastic phenotype (16, 26). Retrovirus-based strategies have been extensively characterized in human clinical trials and offer low immunogenicity, high transduction efficiency, and sustained transgene expression (24, 25). Despite these advantages, retroviral vectors are limited by their lack of specificity for target cell-types and their potential for insertional mutagenesis due to random integration into the host cell genome. Notably, we have shown that cationic polymers can also charge neutralize and aggregate lentiviral particles (J.M.L.D., unpublished observations), suggesting that the virus immobilization strategy will be amenable to vectors with a safer, more clinically viable gene delivery route.
Heterogenous tissues were developed in the present study with a cell-based approach. However, these results do not rule out the possibility that cell-free scaffolds containing retroviral gradients will generate tissue interfaces when exposed to host cells and biochemical cues present in an orthotopic defect. In support of this concept, previous reports have demonstrated transient, substrate-mediated genetic modification of host cells in vivo via biomaterial scaffolds containing naked plasmid DNA (27, 28). More recently, allogenic bone grafts coated with immobilized adeno-associated virus have shown utility in bone healing (29). Pursuit of both cellular and cell-free strategies in parallel will be important because no single approach is likely to be universally appropriate for all tissue healing applications.
In summary, we demonstrate that a continuous, graded interface of osteoblastic and fibroblastic tissue can be developed by seeding a homogeneous population of fibroblasts into polymeric scaffolds containing a graded distribution of the Runx2 retroviral vector. Overall, these results are significant toward the development of autologous grafting templates containing transitional interfacial zones for enhanced tissue integration and biological function.
Methods
Primary fibroblasts were harvested from 8- to 16-week-old male Wistar rats by enzymatic digestion of the dermal skin layer and cultured as described in SI Methods. Retroviral vectors (pTJ66-Runx2, pTJ66-eGFP, and MLV-Gag-RFP) were packaged by transient transfection of helper-virus free ΦNX amphotropic producer cells with plasmid DNA as described in SI Methods and elsewhere (16–20). Collagen scaffolds were coated with a 0.01% PLL solution prior to retrovirus immobilization and cell seeding. Procedures to generate uniform and gradient coated constructs are detailed in SI Methods. Cell-seeded constructs were cultured in vitro in osteogenic differentiation media or s.c. implanted in vivo according to protocols described in SI Methods. Characterization of cell distribution/viability and the full osteogenic differentiation program within tissue constructs was assessed by a wide range of endpoint assays as detailed in SI Methods and elsewhere (16–20).
Supplementary Material
Acknowledgments.
We thank C. Gersbach for helpful discussions and A. Lin for technical assistance with microCT image analysis and mechanical testing. This work was supported by the National Institutes of Health Grant R01-EB003364, the Georgia Tech/Emory Engineering Research Center on the Engineering of Living Tissues (National Science Foundation Grant EEC-9731643), and a National Science Foundation Graduate Research Fellowship (to J.E.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801988105/DCSupplemental.
References
- 1.Mikos AG, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulloa F, Briscoe, J Morphogens and the control of cell proliferation and patterning in the spinal cord (2007) Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- 3.Montcouquiol M, Kelley MW. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci. 2003;23:9469–9478. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 5.Woo SL, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech. 2006;39:1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:225–229. doi: 10.1177/036354658701500306. [DOI] [PubMed] [Google Scholar]
- 7.Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148–159. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 8.Wang IN, et al. Role of osteoblast-fibroblast interactions in the formation of the ligament-to-bone interface. J Orthop Res. 2007;25:1609–1620. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 9.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of BFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Chen RR, Silva EA, Yuen WW, Mooney D J. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 11.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497–3508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 12.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 13.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 14.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 15.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 16.Gersbach CA, Le Doux JM, Guldberg RE, Garcia AJ. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006;13:873–882. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 17.Byers BA, Guldberg RE, Hutmacher DW, Garcia AJ. Effects of Runx2 genetic engineering and in vitro maturation of tissue-engineered constructs on the repair of critical size bone defects. J Biomed Mater Res A. 2005;76:646–655. doi: 10.1002/jbm.a.30549. [DOI] [PubMed] [Google Scholar]
- 18.Phillips JE, Gersbach CA, Wojtowicz AM, Garcia AJ. Glucocorticoid-induced osteogenesis is negatively regulated by Runx2/Cbfa1 serine phosphorylation. J Cell Sci. 2006;119:581–591. doi: 10.1242/jcs.02758. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JE, Guldberg RE, Garcia AJ. Dermal fibroblasts genetically modified to express Runx2/Cbfa1 as a mineralizing cell source for bone tissue engineering. Tissue Eng. 2007;13:2029–2040. doi: 10.1089/ten.2006.0041. [DOI] [PubMed] [Google Scholar]
- 20.Phillips JE, Hutmacher DW, Guldberg RE, Garcia AJ. Mineralization capacity of Runx2/Cbfa1-genetically engineered fibroblasts is scaffold dependent. Biomaterials. 2006;27:5535–5545. doi: 10.1016/j.biomaterials.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Davis HE, Rosinski M, Morgan JR, Yarmush ML. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys J. 2004;86:1234–1242. doi: 10.1016/S0006-3495(04)74197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Doux JM, Landazuri N, Yarmush ML, Morgan JR. Complexation of retrovirus with cationic and anionic polymers increases the efficiency of gene transfer. Hum Gene Ther. 2001;12:1611–1621. doi: 10.1089/10430340152528110. [DOI] [PubMed] [Google Scholar]
- 23.Cartmell SH, Porter BD, Garcia AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 24.Maleki M, Reyssat E, Quere D, Golestanian R. On the Landau-Levich transition. Langmuir. 2007;23:10116–10122. doi: 10.1021/la700822y. [DOI] [PubMed] [Google Scholar]
- 25.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, et al. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 18:705–715. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 28.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.