Abstract
Targeting androgens/androgen receptor (AR) functions via androgen deprivation therapy (ADT) remains the standard treatment for prostate cancer. However, most tumors eventually recur despite ADT. Here we demonstrate that the prostate AR may function as both a suppressor and a proliferator to suppress or promote prostate cancer metastasis. Results from orthotopically recombining stromal WPMY1 cells with epithelial PC3 prostate cancer cells in mice demonstrated that restoring AR in epithelial PC3 cells or knockdown of AR in stromal WPMY1 cells suppressed prostate cancer metastasis. Knockdown of the AR in epithelial CWR22rv1 prostate cancer cells also resulted in increased cell invasion in vitro and in vivo. Restoring AR in PC3 cells (PC3-AR9) results in decreased invasion in bone lesion assays and in vivo mouse models. Mice lacking the prostate epithelial AR have increased apoptosis in epithelial luminal cells and increased proliferation in epithelial basal cells. The consequences of these two contrasting results led to the expansion of CK5/CK8-positive intermediate cells, and mice developed larger and more invasive metastatic tumors in lymph nodes and died earlier than wild-type littermates. Mechanistic dissection suggested that androgens/AR might directly or indirectly modulate metastasis-related genes and suppression of TGFβ1 signals results in the partial inhibition of AR-mediated metastasis. Collectively, our understanding of these opposing roles of prostatic AR may revolutionize the way we combat prostate cancer, and allow the development of new and better therapies by targeting only the proliferative role of AR.
Keywords: androgen deprivation therapy, testosterone, TGFβ1, metastasis
Early studies suggested that the prostatic epithelial androgen receptor (AR), when activated by androgens, increased cellular proliferation (1, 2). Clinical studies also pointed out that androgen deprivation therapy (ADT) with suppression of androgens/AR functions, is an effective treatment for most prostate cancer patients (3, 4). However, most prostate tumors regrow after 12–18 months of continuous ADT (1–4). The detailed mechanisms of why suppression of androgens/AR ultimately fails and cancers recur as a more aggressive type and metastasize remain unclear.
The conventional concept of the AR role in prostate cancer is to promote cancer progression, and positive AR staining can be found in many prostate tumors even at the later stages. In addition to androgens, other factors could also affect AR activity, such as (a) AR mutations or amplification, (b) changes in AR and AR coregulators interactions, or (c) growth factors/kinases signal pathways that activate AR activity at the castration level of androgen (1–4). However, why patients receiving ADT tended to have an earlier development of more aggressive types of cancer and whether AR has a differential role in different prostatic cells and/or in different prostate cancer stages remain unclear.
Here, we report the generation of a mouse cancer model lacking the AR only in its prostatic epithelium (pes-ARKO-TRAMP), which develops prostate cancer spontaneously with an intact immune system. Notably, through AR gain- and loss-of-function in epithelial–stromal cell coculture and coimplantation experiments, we demonstrated that the AR could function in epithelial basal intermediate cells as a tumor suppressor to suppress prostate cancer metastasis, in epithelial luminal cells as a surviving factor, and in stromal cells as a proliferator to stimulate cancer progression. These contrasting data challenge the currently used ADT that systematically suppresses androgen actions, and thus reduces both proliferative and suppressor functions of AR. Our results suggest the need for better therapies that only target the proliferative function of AR.
Results
AR Is a Proliferator in Stroma to Promote Prostate Cancer Progression and a Suppressor in Epithelium to Suppress Prostate Cancer Cell Invasion.
PC3-v cells vs. PC3-AR9 cells.
To dissect how the AR influences prostate cancer metastasis, we stably transfected an AR cDNA whose expression is regulated by a natural AR promoter (5) into AR-negative prostate cancer PC3 cells (designated PC3-AR9). Unlike other cell models in which the AR is overexpressed with strong viral promoters (6), leading to an unnatural build-up of AR, these PC3-AR9 cells express a normal quantity of functional AR and are activated by dihydrotestosterone (DHT) (Fig. 1 A and B). Using the Boyden chamber invasion assay, we found PC3-AR9 cells to be significantly less invasive than the parental PC3 cells stably transfected with the vector only (designated PC3-v) (Fig. 1C).
Fig. 1.
Epithelial AR suppresses and stromal AR stimulates prostate cancer cell invasion. (A) AR protein expression in PC3 cells transfected with AR cDNA under the control of a natural proximal AR promoter region (PC3-AR7, PC3-AR8, and PC3-AR9), strong SV40 promoter (PC3-AR2), or vector only (PC3-v). (B) 1 nM DHT treatment increased AR transactivation of (ARE)4-Luc activity in PC3-AR9 cells. (C) The invasion of PC3-AR9 was decreased as compared to PC3-v with 1 nM DHT using Matrigel-coated Boyden chambers. (D–F) Two-chamber cell recombination assays showed stromal AR stimulated, whereas epithelial AR suppressed, prostate cancer cell invasion. (D) PC3-v or PC3-AR9 cells on the upper layer of the Boyden chamber were cocultured with WPMY1-v or WPMY1-ARsi cells on the lower layer of the chamber. (E) Invasion of PC3-v or PC3-AR9 cells was significantly higher when cocultured with WPMY1-v cells than when cultured with WPMY1-ARsi cells. (F) The quantitative data from experiments (n = 3) *, P < 0.05, **, P < 0.01. (G) Genetic knockdown of AR resulted in increased invasion ability in human prostate cancer CWR22rv1 cells. The alleles of the AR gene in CWR22rv1-AR+/+ cells were genetically disrupted by homologous recombination strategy to produce CWR22rv1-AR+/− cells. Western blot analysis indicated that expression of AR is knocked down in the presence or absence of 1 nM DHT in CWR22rv1-AR+/− cells compared with CWR22rv1-AR+/+ cells (Upper). AR transactivation is diminished in CWR22rv1-AR+/− compared with CWR22rv1-AR+/+ in the presence of 1 nM DHT (Lower Left). The cell invasion (n = 5) increased in CWR22rv1-AR+/− compared with CWR22rv1-AR+/+ in the Boyden chamber assay (Lower Right). *P < 0.05 between the two cell lines. (H) knockdown of AR with siRNA in CWR22rv1-AR+/+cells increased cell invasion, and knock-in of the AR in CWR22rv1-AR+/− cells decreased cell invasion using Boyden chamber assays (n = 5). * Indicates a significant difference between the two transfectants with P < 0.05.
WPMY1-v cells vs. WPMY1-ARsi cells.
The above results, which contradict the classical concept that the prostatic AR functions as a proliferator for prostate cancer progression, prompted us to coculture epithelial PC3-v cells with human prostatic stromal WPMY1 (7) cells to verify the stromal AR roles in prostate cancer cell invasion. Early reports demonstrated that the functional AR expressed in WPMY1 cells promoted androgen-dependent gene expression (8). We knocked down endogenous AR expression in WPMY1 cells with stably transfected AR-siRNA (designated WPMY1-ARsi) and cocultured these cells with PC3-v cells on different layers of the Boyden chamber (Fig. 1D) for the cell invasion assay. The result suggested that knockdown of the stromal AR in WPMY1-ARsi cells resulted in suppression of epithelial PC3-v cell invasion (Fig. 1 E and F). In contrast, from the Boyden chamber coculture of either PC3-v or PC3-AR9 cells with WPMY1 cells transfected with the vector (WPMY1-v), our results indicated that addition of AR in PC3-AR9 prostate cancer epithelial cells results in suppression of epithelial cell invasion (Fig. 1 E and F), and coculture of PC3-AR9 cells with WPMY1-ARsi cells further suppressed cell invasion (Fig. 1 E and F). These coculture cell invasion data are consistent with the results of Fig. 1C and suggest that the epithelial AR may function as a suppressor of cell invasion and the stromal AR may function as a stimulating factor in prostate cancer cell invasion.
CWR22rv1-AR+/+ cells vs. CWR22rv1-AR+/− cells.
We then used a homologous gene recombination strategy to knockdown the AR in human CWR22rv1 cells isolated from a prostate tumor growing despite ADT (9) (CWR22rv1-AR+/−). CWR22rv1-AR+/− cells expressed much less AR with negligible AR transactivation compared to the parental CWR22rv1-AR+/+ cells (Fig. 1G). Results of the invasion assay indicated that knockdown of the AR in CWR22rv1 (CWR22rv1-AR+/−) cells increases their invasive ability as compared to parental CWR22rv1-AR+/+ cells (Fig. 1G Lower). In a different approach using AR-siRNA (10) to knockdown AR in CWR22rv1-AR+/+ (CWR22rv1-AR+/+-ARsi) cells, we found similar results, with enhanced invasive ability as compared to parental CWR22rv1-AR+/+ cells transfected with scramble RNA; adding functional AR in CWR22rv1-AR+/−-hAR cells resulted in decreased cell invasion as compared to CWR22rv1-AR+/−-V cells (Fig. 1H).
Together, based on four different approaches (knock-in of AR, knockdown of AR with genetic recombination, knockdown of AR with siRNA, and epithelium–stroma coculture system) with different prostate cancer cells, results shown in Fig. 1 all demonstrated that epithelial AR could function as a suppressor, and the stromal AR may function as a promoter for prostate cancer cell invasion.
Addition of Functional AR in PC3 Cells (PC3-AR9) Results in Decreased Invasion in Bone Lesion Assay and in Vivo Mouse Models.
As PC3 cells were isolated from a bone metastasis, we examined the influence of the AR on PC3-AR9 cell invasion by measuring osteoclastogenesis in a bone-wafer absorption assay (11). We cocultured PC3-v or PC3-AR9 cells with bone cells, from newborn rat bone marrow, onto bone wafers and quantified osteoclast formation. Compared to PC3-v cells, the PC3-AR9 cells on bone wafers had decreased numbers of osteolytic lesions (pitted areas) (Fig. 2A). To evaluate invasion in vivo, we injected cells into the tibia of nude mice (12). PC3-v tumors grew more aggressively and more invasively (Fig. 2 B and C) than PC3-AR9 tumors as determined by x-ray analysis. Collectively, these data from knock-in of the functional human AR suggest that loss of the AR signaling in prostate epithelial cells promotes invasion both in vitro and in vivo.
Fig. 2.
Adding functional AR to PC3 cells (PC3-AR9) decreased prostate cancer cell invasion in bone lesion test and in preclinical animal models. (A) PC3-AR9 cells formed fewer osteolytic lesions than PC3-v cells. Osteoclasts and osteoclast precursors (OC) were cultured with PC3-v and PC3-AR9 cells on cortical bone wafers. After 10 days, the bone wafers were scraped, dried, and stained with the OC cell indicator tartrate-resistant acid phosphatase (TRAP). The extent of bone resorption with PC3-AR9 cells decreased compared to PC3-v cells as shown by measurement of the area of osteoclast lacunae on the bone wafers. OC alone and OC with parathyroid hormone (PTH) were used as the negative and positive control, respectively. Data were from three independent experiments and are presented as mean ± SD *P < 0.05, **P < 0.01. (B, C) PC3-AR9 cells had less bone invasion compared to PC3-v cells. Effects of intratibial injection of PC3-v and PC3-AR9 cells in nude mice. PC3-v cells developed larger osteolytic lesions than PC3-AR9 cells at 6–8 wks (B, representative x-ray radiograph shown), and larger and more invasive tumors as measured by dial caliper at week 12 (C, arrows and quantitated tumor volume, Lower). Data are shown as mean ± SD; *, P < 0.05; **, P < 0.01. (D) PC3-AR9 cells generated smaller metastatic PLN tumors compared to those generated by PC3-v cells. PC3-v and PC3-AR9 cells (5 × 105) suspended in 100 μl Matrigel were inoculated into the anterior prostate of 16-wk-old nude mice. Twelve weeks after injection, large PC3 prostate tumors developed (not shown). To visualize PLN-metastatic tumors, we removed prostates containing primary tumors. Note that larger PLN-metastatic tumors (shown by gross and H&E staining, Left) were developed in mice inoculated with PC3-v tumors (arrows). The tumor volume was quantitated (right panel, n = 5). (E) PC3-AR9 cells recombined with WPMY1-v or WPMY1-ARsi cells generated smaller metastatic tumors than their control PC3-v recombined groups (arrows). PC3-v or PC3-AR9 cells (5 × 105), combined with WPMY1-v or WPMY1-ARsi cells, were suspended in 100 μl of Matrigel and inoculated into the anterior prostate of 16-wk-old nude mice. Twelve weeks after injection, the tumors developed (not shown), and the sizes of PLN metastatic tumors were compared via gross appearance and histology (H&E). The tumor volume was quantitated (Right, n = 5).
We also tested the roles of AR in a metastatic assay in an in vivo mouse prostate cancer model. PC3-v or PC3-AR9 cells were orthotopically inoculated into the anterior prostate of nude mice. Consistent with the above findings, mice inoculated with PC3-v cells developed bigger metastatic tumors in pelvic lymph nodes (PLN) than mice inoculated with PC3-AR9 cells (Fig. 2D). Similar results also occurred when we replaced PC3/PC3-AR9 cells with CWR22rv1-AR+/−/CWR22rv1-AR+/+ cells, in which knockdown of AR in CWR22rv1-AR+/− cells led to the development of bigger metastatic tumors in lymph nodes (data not shown).
We also inoculated different combinations of epithelial cancer cells (PC3-v and PC3-AR9) and stromal cells (WPMY1-v and WPMY1-ARsi) into the anterior prostate of nude mice. These in vivo results are consistent with the in vitro data shown in Fig. 1, and demonstrate that mice inoculated with recombinants, with either restoration of the epithelial AR (PC3-AR9 cells) or knockdown of the stromal AR (WPMY1-ARsi cells), developed smaller metastatic tumors in lymph nodes (Fig. 2E).
Together, using either knockdown or knock-in of the AR in various human prostate cells, our in vitro cell invasion results and in vivo mouse tumor data consistently demonstrated that the epithelial AR may suppress, and the stromal AR may promote, prostate cancer metastasis.
Generation of pes-ARKO-TRAMP Mice Lacking AR in Epithelial Luminal and Basal Cells.
All of the above data were generated from human prostate cancer cells that were already transformed. We were interested in using a mouse model that underwent carcinogenesis spontaneously with an intact immune system. We first mated female floxAR (C57BL/6) mice with TRAMP (FVB) mice (13), to generate floxAR-TRAMP (C57BL/6-FVB) mice. We then crossed these mice with Pb-Cre (C57BL/6) male mice (14) to generate pes-ARKO-TRAMP (C57BL/6-FVB) mice that lack the AR only in the prostatic epithelium (15). This pes-ARKO-TRAMP mouse was further bred with ROSA26R-β-Gal mouse (16), and from their offspring we found probasin-cre expressed in all of the epithelial luminal cells and some epithelial basal cells [supporting information (SI) Fig. S1 A and B, n = 3]. We confirmed that AR was knocked down in epithelial cells, but not in stromal cells, of pes-ARKO-TRAMP mice using (a) laser capture microdissection (LCM)-separating epithelial and stromal cells and (b) prostate immunohistochemical staining with the antibody specific to the AR C-terminal region, (Fig. S1 C–E). Other urogenital organs from both wild-type AR-TRAMP (Wt-TRAMP) and pes-ARKO-TRAMP mice developed normally (Fig. S1F).
Increased Apoptosis in Epithelial Luminal Cells and Increased Proliferation in Epithelial Basal Cells of pes-ARKO-TRAMP Mice.
Recent studies suggested that prostate cell growth develops from AR negative (AR−) to AR positive (AR+) cells via prostate stem cells (AR−), to basal cells (AR−), to basal-intermediate cells (mix of AR− and AR+), to luminal cells (AR+) (17). Using double staining of the luminal cell marker CK8 red-fluorescence (18) and the apoptotic marker TUNEL green-fluorescence detection (Fig. 3A), we found a significantly higher level of apoptosis in CK8-positive luminal cells in 16-wk-old pes-ARKO-TRAMP mice compared to those of Wt-TRAMP littermates. In contrast, using double staining of basal cell marker CK5 red-fluorescence (18) and a proliferation marker (Ki67 staining or BrdU incorporation as green-fluorescence) (Fig. 3B), we found a significantly higher proliferation rate in CK5-positive basal cells in 16-wk-old pes-ARKO-TRAMP mice compared to those of Wt-TRAMP littermates. These data suggest that the epithelial AR plays contrasting roles, promoting the survival of epithelial luminal cells and suppressing the proliferation/expansion of epithelial basal cells. Furthermore, the increased apoptosis may lead to a breakdown of the epithelial barrier and facilitate the invasion of tumor cells. Results from other animal models of prostate tumors, via knockout of NKX3.1 and p27, also found increased proliferation and increased apoptosis during prostatic intraepithelial neoplasia (PIN) development (19). Similar observations also occurred in liver cancer (20) and breast cancer (21).
Fig. 3.
Loss of epithelial AR in pes-ARKO-TRAMP leads to higher proliferation in basal cells and higher apoptosis in luminal cells with increased basal intermediate cells. (A) Using the TUNEL assay, the apoptosis signals in the luminal epithelial cells from 16-wk-old pes-ARKO-TRAMP prostatic epithelium were higher than those from Wt-TRAMP (arrowheads). CK8 immunostaining (red) was used to identify the luminal epithelial cells (Upper). Quantitative results showed the differences were 18% vs. 2% (Lower). (B) The growth rates of prostate epithelium were demonstrated by Ki67 immunostaining (Top) and BrdU incorporation (Middle) in 16-wk-old Wt-TRAMP vs. pes-ARKO-TRAMP. The mice were i.p. injected with BrdU (10 μg/g body weight) every 6 h, and killed 24 h later. Paraffin-fixed tissue sections were stained by the BrdU detecting kit (Zymed Laboratories). The results from double immunofluorescent staining of BrdU (green) and CK5 (red) indicated that the higher proliferative prostate cells belong to the CK5 positive-basal cells in 16 wk-old pes-ARKO-TRAMP (Bottom with overlapped image, yellow color). The quantitative data were shown (n = 3). (C) Loss of the AR in the epithelium of pes-ARKO-TRAMP led to the expansion of the basal intermediate cell populations (yellow), with increased CK5/CK8-positive signals in prostates of 16-wk-old pes-ARKO-TRAMP compared to Wt-TRAMP mice (Upper). The quantitative data are shown (Lower, n = 3). (D) Increased CD44-positive cells in primary tumors of 24-wk-old pes-ARKO-TRAMP compared to Wt-TRAMP littermates (n = 3).
Increased Basal–Intermediate Cells in pes-ARKO-TRAMP Mice.
Loss of epithelial AR in pes-ARKO-TRAMP mice resulted in increased apoptosis in epithelial luminal cells and increased proliferation in epithelial basal cells. The consequences of these two contrasting results lead to the expansion of CK5/CK8-positive basal–intermediate cells in pes-ARKO-TRAMP mice (Fig. 3C, n = 6), which is further confirmed by the increase of CD44-positive cells in the primary tumors of 24-wk-old pes-ARKO-TRAMP mice compared to Wt-TRAMP mice. Our observation (Fig. 3D, n = 6) is consistent with another report that CD44+ prostate cancer cells are highly tumorigenic and metastatic (22). These results suggest that knockout of AR in epithelium resulted in the cell population changes, with expansion of epithelial basal-intermediate–like cells and decrease of epithelial luminal cells in pes-ARKO-TRAMP prostates. This is in agreement with a recent report showing that after ADT in prostate cancer patients, the percentage of CK5-positive basal–intermediate cells increased significantly (from 29% to 75%) (18).
pes-ARKO-TRAMP Mice Develop More Aggressive and Invasive Metastatic Tumors.
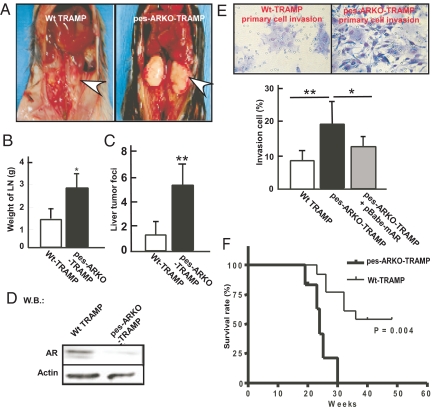
Increased apoptosis in epithelial luminal cells and increased proliferation in epithelial basal cells in pes-ARKO-TRAMP mice also led to increased size of metastatic tumors in PLN in 24-wk-old pes-ARKO-TRAMP compared to Wt-TRAMP mice (P < 0.05; 3.0 vs. 1.7 mg, n = 7 for each genotype) (Fig. 4 A and B). In addition, more prostate cancer metastatic foci were observed within the livers of pes-ARKO-TRAMP mice (n = 6) than those of Wt-TRAMP (n = 6) (Fig. 4C). Western blotting analysis confirmed loss of the AR within PLN metastatic tumors in pes-ARKO-TRAMP mice (Fig. 4D).
Fig. 4.
pes-ARKO-TRAMP mice develop more aggressive and invasive tumors than Wt-TRAMP littermates. (A) PLN metastases are significantly larger in 24-wk-old pes-ARKO-TRAMP mice compared to Wt-TRAMP mice. (B) Weights of PLN metastases isolated from 24-wk-old pes-ARKO-TRAMP were greater than those of Wt-TRAMP mice (n = 7 mice in each group). (C) The number of liver tumor foci was increased in pes-ARKO-TRAMP mice compared to Wt-TRAMP mice (n = 6 mice in each group). (D) Western blot analysis of AR protein in PLN tumor, from 24-wk-old Wt-TRAMP or pes-ARKO-TRAMP mice. (E) Higher invasiveness of primary cultured PLN tumor cells from pes-ARKO-TRAMP mice as compared to those from Wt-TRAMP mice using Boyden chamber invasion assays (Upper). Adding functional AR by retrovirus infection into primary-cultured pes-ARKO-TRAMP tumor cells results in suppression of invasion (Lower). The purity and originality of primary cultured PLN tumor cells was confirmed by the expression of pan-CK epithelial cell marker (data not shown). Data were presented as mean ± SD (n = 5); *, P < 0.05; **, P < 0.01. (F) survival rate was decreased in pes-ARKO-TRAMP (C57BL/6 × TRAMP-FVB, n = 10) as compared with Wt-TRAMP (C57BL/6 x TRAMP-FVB, n = 16).
Using invasion assay (23) of primary cultured PLN tumor cells isolated from both genotypes of TRAMP mice, we found that the primary culture PLN tumor cells from pes-ARKO-TRAMP mice (n = 5) were more invasive than those from Wt-TRAMP mice (n = 5) (Fig. 4E). Importantly, restoring a functional AR by infecting retrovirus-AR reduced the invasiveness of PLN tumor cells from pes-ARKO-TRAMP mice (Fig. 4E). These results (Fig. 4 A–E) showed that loss of the prostatic epithelial AR leads to the development of more invasive and metastatic prostate cancers and that gain of AR function reverses these characteristics. Consequently, increased tumor invasiveness and metastases lead to lower survival rates in pes-ARKO-TRAMP mice (n = 20) compared with Wt-TRAMP littermates (n = 30) (Fig. 4F).
Human Clinical Data from Prostate Cancer Patients.
Recent clinical data from 254 prostate cancer patients found that ADT treatment resulted in the promotion of metastatic prostate tumors (24). We also evaluated AR expression in primary (97 cases) and metastatic (28 cases) prostate tumors and observed a significant difference between AR expression in primary tumors (91.75%) and metastatic tumors (67.86%), (P < 0.01) (Fig. S2). These in vivo clinical data are consistent with a recent study (25) that used tissue arrays from prostate cancer patients who received radical prostatectomy, in which it was concluded that AR expression was significantly decreased in metastatic prostate cancer as compared to primary prostate cancer or normal prostate (mean 1.30 vs. 3.49, P < 0.01). Together, these clinical findings all support the suppressor roles of AR in metastasis, as we demonstrated above in human prostate cancer cells and various mouse prostate tumor models.
Molecular Mechanisms by Which AR can Function as a Suppressor of Prostate Metastasis.
It is now well accepted that few, if any, solid cancers rely on a single pathway/mechanism for tumor progression and metastasis. For example, breast cancer may use at least 6 different factors to regulate its progression (26). We also hypothesize that the AR may modulate prostate cancer metastasis via multiple pathways. Therefore, using DNA microarray, we evaluated the differential gene expression of CWR22rv1-AR+/+ and CWR22rv1-AR+/− cells. Among these genes up-regulated or down-regulated by knockdown of AR, we focused on those that have been linked to prostate cancer metastasis and confirmed the results using Q-PCR. The results (Table 1) suggest that the relative mRNA expression of those metastasis/invasion-related genes correlate well with the metastatic status in PLN tumors from Wt-TRAMP and pes-ARKO-TRAMP mice as well as xenografted tumors from PC3-v vs. PC3-AR9 (Table 1). The above data support the concept that AR can function as a suppressor of prostate metastatic tumor invasion. Further mechanistic dissection, (Fig. S3 and Fig. S4) suggests that the AR might use multiple mechanisms, including modulation of TGFβ1 signals, to modulate those metastasis/invasion-related genes. Suppression of TGFβ1 signals could then partially reverse the AR-mediated suppression of metastasis.
Table 1.
Expression profiles of prostate metastasis/invasion related genes in pelvic lymph node tumor (PLN) of pes-ARKO-TRAMP mice and PC3 xenograft tumors compared with PLN of Wt-TRAMP mice and PC-3AR9 xenograft tumors, respectively
| Genes | pes-ARKO-TRAMP(PLN) | PC3 (xenograft tumors) | ||||||
|---|---|---|---|---|---|---|---|---|
| NEP | −5.9 | ± 0.2 | ** | −3.7 | ± 0.3 | ** | ||
| Cox-2 | ↑ | 3.3 | ± 0.2 | ** | ↑ | 6.2 | ± 0.1 | * |
| P27 | −4.6 | ± 0.3 | ** | −2.5 | ± 0.4 | * | ||
| MMP-2 | 1.2 | ± 0.1 | N.S. | 0.5 | ± 0.5 | N.S. | ||
| MMP-9 | ↑ | 6.8 | ± 0.4 | * | ↑ | 8.5 | ± 0.2 | ** |
| IGF-2 | ↑ | 3.5 | ± 0.3 | * | ↑ | 8.1 | ± 0.3 | ** |
| IL-6 | ↑ | 3.5 | ± 0.1 | ** | ↑ | 3.9 | ± 0.5 | ** |
| TNFα | ↑ | 3.6 | ± 0.4 | * | ↑ | 4.5 | ± 0.9 | ** |
| RKIP | −2.9 | ± 0.4 | ** | −2.6 | ± 0.4 | ** | ||
Data are presented as mean ± SD;
*, P < 0.05;
**, P < 0.01. N.S., no significant difference.
Impact to current clinical treatment and development of new therapy to battle prostate cancer.
Results from the above studies all point out that AR has multiple and distinct functions: as a proliferator in stromal cells and a survival factor in epithelial luminal cells to promote cancer progression, or a metastatic suppressor in epithelial basal cells to inhibit tumor metastasis. These results are in stark contradiction to the accepted role of the prostatic AR to function only as a proliferator. Therefore, the conclusions drawn from these data may influence current clinical prostate cancer therapy. Based on our findings, systemic targeting of androgens/AR signals via ADT may result in either suppression or promotion of prostate cancer, depending on which role of AR (proliferator vs. suppressor) is more dominant at a particular stage of prostate cancer progression (27). Indeed, clinicians also face similar case decisions in which some prostate cancer patients do respond well when treated with androgens instead of antiandrogens (Nicholas Vogelzang, personal communication).
Therefore, the ideal therapeutic approach(es) could be to target the proliferative function or suppressive function of AR separately, which could be achieved by development of specific vehicles that carry androgens only to those prostate cells with AR suppressive function, or carry antiandrogens only to those prostate cells with AR proliferative function. Alternatively, a drug might be developed to specifically target the AR suppressor with its unique associated coregulators, or the proliferative AR with its unique associated coregulators. Before such an ideal therapy can be developed, perhaps a combination of anti-androgens/AR signals with antimetastasis signals, such as antagonists for Akt, Cox-2, or MMP-9 signals (28), could be applied to battle prostate cancer.
Methods
Please see SI Text for the following detailed methods: (i) Cell culture, plasmids, and reagents; (ii) construction of retroviral vector with human AR cDNA and AR-siRNA; (iii) generation of pes-ARKO-TRAMP mice; (iv) in vitro bone-wafer resorption and osteoclastogenesis assays; (v) BrdU incorporation assay; (vi) TUNEL assay; (vii) immunohistochemistry stainings of Ki67, CK5, CK8, and CD44 on mouse prostate tumors; (viii) immunofluorescence stainings of CK5 and CK8 on mouse prostate tumors; (ix) isolation of primary culture prostate PLN tumor cells for invasion assay; (x) laser capture microdissection to separate the prostate stromal and epithelail cells; and (xi) statistical analyses of results.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants CA122840 and CA127300, and the George H. Whipple Professorship Endowment.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804700105/DCSupplemental.
References
- 1.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto H, Messing EM, Chang C. Does androgen deprivation improve treatment outcomes in patients with low-risk and intermediate-risk prostate cancer? Nat Clin Pract Oncol. 2005;2:236–237. doi: 10.1038/ncponc0168. [DOI] [PubMed] [Google Scholar]
- 4.Fluchter SH, Weiser R, Gamper C. The role of hormonal treatment in prostate cancer. Recent Results Cancer Res. 2007;175:211–237. doi: 10.1007/978-3-540-40901-4_13. [DOI] [PubMed] [Google Scholar]
- 5.Mizokami A, Yeh SY, Chang C. Identification of 3′,5′-cyclic adenosine monophosphate response element and other cis-acting elements in the human androgen receptor gene promoter. Mol Endocrinol. 1994;8:77–88. doi: 10.1210/mend.8.1.8152432. [DOI] [PubMed] [Google Scholar]
- 6.Yuan S, et al. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- 7.Webber MM, et al. A human prostatic stromal myofibroblast cell line WPMY-1: A model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis. 1999;20:1185–1192. doi: 10.1093/carcin/20.7.1185. [DOI] [PubMed] [Google Scholar]
- 8.Heitzer MD, DeFranco DB. Hic-5/ARA55, a LIM domain-containing nuclear receptor coactivator expressed in prostate stromal cells. Cancer Res. 2006;66:7326–7333. doi: 10.1158/0008-5472.CAN-05-2379. [DOI] [PubMed] [Google Scholar]
- 9.Nagabhushan M, et al. CWR22: The first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–3046. [PubMed] [Google Scholar]
- 10.Yeh S, et al. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med. 2003;198:1899–1908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goater JJ, O'Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–173. doi: 10.1016/S0736-0266(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 12.Corey E, et al. Establishment and characterization of osseous prostate cancer models: Intra-tibial injection of human prostate cancer cells. Prostate. 2002;52:20–33. doi: 10.1002/pros.10091. [DOI] [PubMed] [Google Scholar]
- 13.Gingrich JR, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 14.Wu X, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 15.Wu CT, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA. 2007;04:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles' heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 18.Van Leenders GJ, Aalders TW, Hulsbergen-van de Kaa CA, Ruiter DJ, Schalken JA. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol. 2001;195:563–570. doi: 10.1002/path.993. [DOI] [PubMed] [Google Scholar]
- 19.Gary B, et al. Interaction of Nkx3.1 and p27kip1 in prostate tumor initiation. Am J Path. 2004;164:1607–1614. doi: 10.1016/S0002-9440(10)63719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasl-Kraupp B, et al. Inherent increase of apoptosis in liver tumors: Implications for carcinogenesis and tumor regression. Hepatology. 2003;25:906–912. doi: 10.1002/hep.510250420. [DOI] [PubMed] [Google Scholar]
- 21.Vakkala M, Lähteenmäki MK, Raunio H, Pääkkö P, Soini Y. Apoptosis during breast carcinoma progression. Clin Cancer Res. 1999;5:319–324. [PubMed] [Google Scholar]
- 22.Patrawala L, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 23.Pilatus U, et al. Imaging prostate cancer invasion with multi-nuclear magnetic resonance methods: The metabolic Boyden chamber. Neoplasia. 2000;2:273–279. doi: 10.1038/sj.neo.7900089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleeberger W, et al. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199–9206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, et al. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: Cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–934. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Easton DF, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447 doi: 10.1038/nature05887. 087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Y, et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier state. Proc Natl Acad Sci USA. 2008;105:12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto H, Altuwaijri S, Cai Y, Messing EM, Chang C. Inhibition of the Akt, cyclooxygenase-2, and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: Potential therapeutic approaches for prostate cancer. Mol Carcinog. 2005;44:1–10. doi: 10.1002/mc.20121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.