Abstract
To differentiate roles of androgen receptor (AR) in prostate stromal and epithelial cells, we have generated inducible-(ind)ARKO-TRAMP and prostate epithelial-specific ARKO TRAMP (pes-ARKO-TRAMP) mouse models, in which the AR was knocked down in both prostate epithelium and stroma or was knocked out in the prostate epithelium, respectively. We found that loss of AR in both mouse models resulted in poorly differentiated primary tumors with expanded intermediate cell populations. Interestingly, knockdown of both epithelial and stromal AR in ind-ARKO-TRAMP mice at earlier stages resulted in smaller primary prostate tumors with lower proliferation rates, and knockout of AR in pes-ARKO-TRAMP mice resulted in larger primary prostate tumors with higher proliferation rates. The differential proliferation rates, yet with similarly expanded intermediate cell populations, indicated that the prostate stromal AR might play a more dominant role than the epithelial AR to promote primary tumor proliferation at an early stage of tumor. Tissue recombination of human prostate stromal cell lines (WPMY1-v or WPMY1-ARsi) with human prostate cancer epithelial cell lines (PC3-v or PC3-AR9) further demonstrated that the AR might function as a suppressor in epithelial cells and a proliferator in stromal cells in the primary prostate tumors. The dual roles of the AR in prostate epithelium and stroma may require us to reevaluate the target and timing of androgen-deprivation therapy for prostate cancer patients and may suggest a need to develop new drugs to selectively target stromal AR in the primary prostate tumors at earlier stages.
Keywords: androgen deprivation therapy, testosterone, TRAMP
Early studies documented that prostate epithelial cell differentiation, proliferation, and apoptosis are regulated by androgen action through the prostatic stromal androgen receptor (AR) (1, 2). In contrast, the prostatic epithelial AR might play little role as a result concluded from the mouse renal capsule–tissue recombination of normal prostatic stroma with testicular feminization syndrome (Tfm) epithelium (3), in which the prostate develops and grows normally despite having a nonfunctional epithelial AR. These contrasting roles between the stromal AR and epithelial AR suggest the essential role of the stromal AR during initial prostate development (1–3). However, the epithelial AR is required for the expression of some prostatic secretory proteins (4), and prostatic epithelium induces smooth muscle differentiation of the stroma (4). It was proposed that continuous reciprocal stromal–epithelial interaction enables mature prostate to maintain cellular homeostasis (5). Wu et al. (6) reported that specific ablation of the AR in mouse prostatic epithelium resulted in apoptosis of epithelial luminal cells and increased proliferation of epithelial basal cells in the ventral prostate, leading to enlargement of the gland. These dual roles of AR in stromal vs. epithelial cells may explain why the mature prostate maintains homeostasis without active proliferation in milieu rich in androgens.
The proliferation-stimulating role of AR is at the center of the premise for androgen deprivation therapy (ADT) for treating prostate cancer (7). ADT with either surgical or medical castration usually results in a response rate of 70–80%, with ≈12–33 months duration of progression-free survival (8). However, after an average of 24 months, the tumor almost always recurs and no longer responds to ADT (9), even though the expression of AR in the prostate cancer remains unchanged (10) or is slightly increased (11). Interestingly, cell sorting of these ADT-refractory tumors found that the prostatic epithelial basal cell marker, cytokeratin 5 (CK5), increased from 29% to 75% (12, 13). The detailed mechanisms of how androgen/AR signals are altered and why cell populations changed after ADT remain unclear.
Using inducible-(ind)ARKO-TRAMP and prostate epithelial-specific ARKO TRAMP (pes-ARKO-TRAMP) mice, we found that the prostate stromal AR might play a more dominant role than the epithelial AR to promote primary tumor proliferation at an early stage of tumor progression. This unexpected result may help us to better understand the nearly invariable ultimate failure of ADT in achieving long-term tumor control and encourage us to develop new approaches that selectively (or preferentially) targets the stromal AR's proliferating role at earlier stages to treat prostate cancer.
Results and Discussion
AR Functions as a Suppressor and a Proliferator in Primary Prostate Tumor Growth in Nude Mice with Coinoculated Epithelial and Stromal Cell Xenografts.
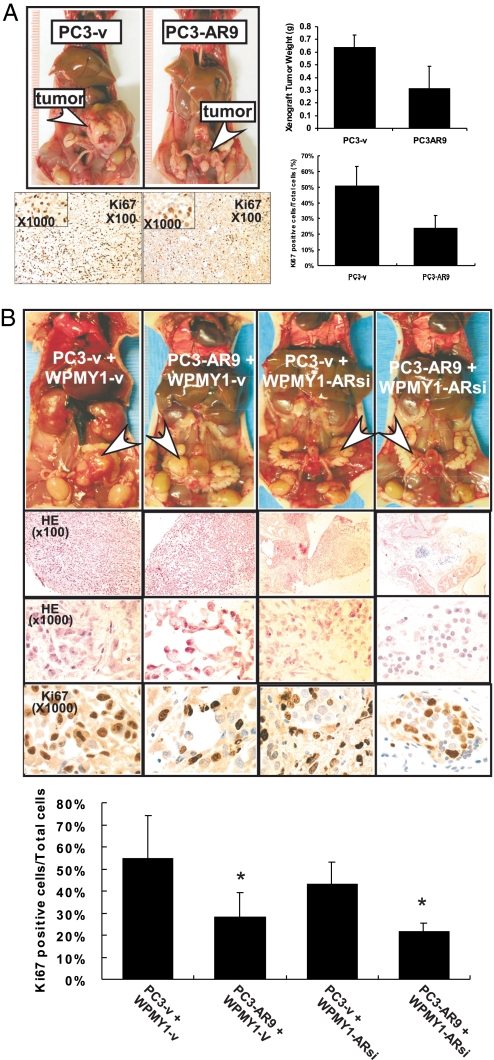
To study AR's roles in primary prostate tumor growth, we stably transfected functional human AR cDNA that was driven by the human AR's natural promoter (14, 15) into human prostate cancer PC3 (CK5/CK8-positive) cells (designated PC3-AR9). We then orthotopically inoculated PC3-AR9 cells and parental PC3 cells that were stably transfected with vector only (designated PC3-v) into the anterior prostates of nude mice. We found significantly larger primary prostate tumors 12 wk after inoculation with PC3-v cells compared with PC3-AR9 cells (Fig. 1A Upper). Assaying the proliferation marker Ki67, we also found higher proliferating rates in the primary tumors of PC3-v cells than in those of PC3-AR9 cells (Fig. 1A Lower). These results suggest that addition of a functional AR in PC3-AR9 cells may result in the suppression of primary prostate tumor growth.
Fig. 1.
Knockin of AR in prostate cancer PC3-AR9 cells suppressed tumor growth, and knockdown of AR in the prostate stromal WPMY1-ARsi cells suppressed the recombined epithelial PC3 tumor growth using in vivo orthotopic tumor implantation strategy. (A) Orthotopically implanted PC3-AR9 cells generated smaller tumors than tumors generated by PC3-v at 12 wk after implantation (arrows). Tumor cell proliferation signals were determined by Ki67 staining. (B) PC3-v or PC3-AR9 were recombined with WPMY1-v or WPMY1-ARsi cells for orthotopic implantation in anterior prostates of male nude mice. PC3-v cells recombined with WPMY1-v generated larger tumors than those from PC3-v recombined with WPMY1-ARsi cells (arrows). Twelve weeks after implantation, tumors were harvested to analyze the size, histology (by H&E staining) and proliferation (by Ki67 staining; quantitative results were attached). The differences are described in the text. Data are presented as mean ± SD; *P < 0.05.
These in vivo PC3-AR9 cell growth results are contrary to the classic concept that the AR functions as a proliferator in primary prostate tumor growth. We decided to further confirm these findings by in vivo orthotopic implantation of malignant prostatic epithelial and nonmalignant prostatic stromal cells. We coinoculated PC3-v or PC3-AR9 with stromal WPMY1-v cells that express the functional AR (16) and orthotopically implanted those stromal/epithelial cells into the anterior prostates of nude mice. The results were consistent with Fig. 1A showing addition of the functional AR in PC3-AR9 cells results in smaller primary prostate tumors after 12 wk (Fig. 1B).
We then stably transfected AR-siRNA that can effectively knockdown endogenous AR (17, 18) into WPMY1 cells (designated WPMY1-ARsi). These WPMY1-ARsi cells were orthotopically coinoculated with either PC3-v or PC3-AR9 cells into the anterior prostates of nude mice. The results (Fig. 1B) showed that knockdown of the AR in stromal WPMY1-ARsi cells resulted in the suppression of primary prostate tumor growth (PC3-v + WPMY1-v vs. PC3-v + WPMY1-ARsi) and knockin of AR in PC3-AR9 cells resulted in the suppression of primary prostate tumor growth (PC3-v + WPMY1-v vs. PC3-AR9 + WPMY1-v). We performed H&E staining, which demonstrates that PC3-v + WPMY1-v primary prostate tumors are larger and more poorly differentiated tumors compared with PC3-AR9 + WPMY1-v tumors. The latter form lumen-like structures (Fig. 1B, H&E), which can be due to the expression of AR in epithelial PC3 cells. We confirmed this phenotypic observation in a cell growth assay by staining with the proliferation marker Ki67 and found that the AR suppressed proliferation in the primary prostate tumors developing from these PC3-AR9 xenografts (Fig. 1B). Together, we clearly demonstrated that the AR could function as either a suppressor or a proliferator depending on its location for primary prostate tumor growth. Because of the expression of AR in PC3-AR9, as driven by the AR natural promoter, in vitro growth of PC3-AR9 is slightly increased in the presence of 1 nM 5α-dihydrotestosterone (15). However, with PC3-v cells, we found that in vivo orthotopic-implanted PC3-AR9 tumors form smaller primary tumors (Fig. 1). The possible explanation for these different in vivo and in vitro findings could be that the in vivo observation is a relatively long-term condition, and primary tumor growth could be influenced by the prostate cell microenvironment and stroma-derived factors, whereas in vitro growth is under a simplified, two-dimension, nonphysiological condition. Our results suggest that the long-term comparison of in vivo tumor cell growth may lead to more accurate assessment than the short term in vitro growth assay.
Generation and Confirmation of ind-ARKO-TRAMP Mice with Time Point-Controlled Knockdown of AR in Both the Prostate Stroma and Epithelium and in pes-ARKO-TRAMP Mice That Lack the AR Only in Prostatic Epithelium.
Because the above in vivo data were generated from orthotopically coimplanted human prostate epithelial tumor (CK5/CK8-positive PC3 cells) and human stromal (WPMY1-v) cell lines in nude mouse, we developed a different strategy using mice that can spontaneously develop prostate tumor as another in vivo animal model to confirm our findings. We first generated pes-ARKO mice that lack the AR only in the prostatic epithelium and demonstrated that loss of the epithelial AR resulted in increased prostatic epithelial cell proliferation (6). We then used floxAR mice (19) to generate pes-ARKO-TRAMP mice that spontaneously developed prostate tumors lacking AR in tumor epithelial cells [supporting information (SI) Fig. S1]. We also generated ind-ARKO-TRAMP mice in which prostatic AR could be knocked down in both the epithelium and stroma (Figs. S1 and S2)
The ind-ARKO-TRAMP mice can be induced by pI-pC to delete the floxed AR gene in whole body, including prostate epithelium and stroma. The rationale for establishing ind-ARKO-TRAMP mouse is to mimic the condition in human prostate cancer patients treated with ADT. This ind-ARKO-TRAMP mouse has the reduced AR expression in prostate epithelium and stroma (Fig. S2) and reduced serum androgen levels. The advantage of using this model is that AR knockout could be controlled at given time points by pI-pC injections, for example, at 4 wk (before tumor initiation), 12 wk (PIN stage), or 20 wk (tumor progression). More importantly, it allows us to compare the effects of reduced AR in both prostate epithelium and stroma vs. reduced epithelial AR only in pes-ARKO-TRAMP mice.
Serum Testosterone, Prostate Size, and Cell Population Changes in pes-ARKO-TRAMP and ind-ARKO-TRAMP Mice.
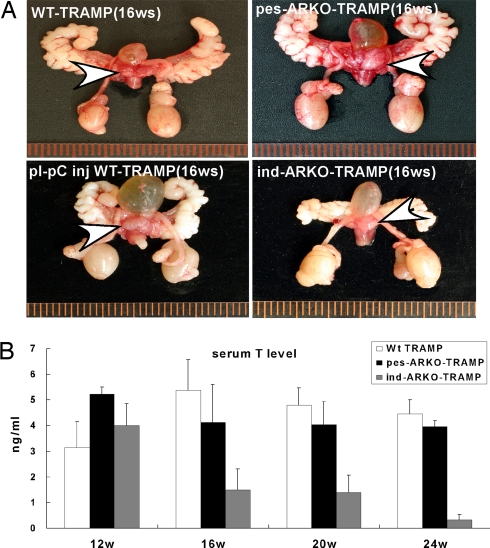
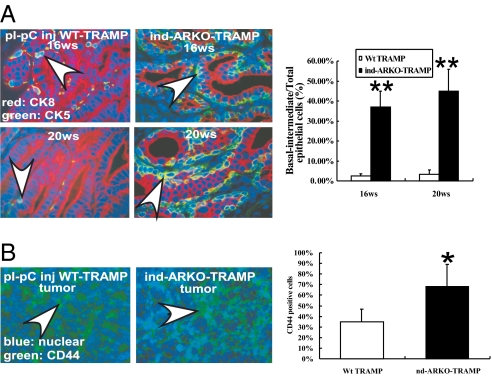
Except for the larger primary prostate tumors found in pes-ARKO-TRAMP mice, we found the reproductive organs are similar between pes-ARKO-TRAMP mice and their WT TRAMP male littermates (Fig. 2A). In contrast, ind-ARKO-TRAMP mice had smaller reproductive organs and prostates as compared with their WT TRAMP littermates (Fig. 2A). Serum testosterone remained similar between pes-ARKO-TRAMP mice and their WT TRAMP littermates. However, serum testosterone was reduced from 16 to 24 wk in ind-ARKO-TRAMP mice that were injected with pI-pC at 12 wk old (Fig. 2B). The observation of larger primary prostate tumors with similar serum testosterone in pes-ARKO-TRAMP mice, as compared with WT TRAMP mice, suggests that serum testosterone may not be a good marker to predict primary prostate tumor growth. Early epidemiological studies also indicated there is little linkage between serum testosterone and prostate cancer risk (20). Moreover, several other studies found that lower serum testosterone levels were associated with more advanced and poorly differentiated tumors (21, 22). Furthermore, among the three types of prostatic epithelial cells (basal cells, intermediate cells, and secretory luminal cells), knockout of the epithelial AR results in the loss of most of the secretory luminal cells (23). In contrast, CK5/CK8 double-positive intermediate cells were increased in both pes-ARKO-TRAMP (23) and ind-ARKO-TRAMP mice (Fig. 3A). Similarly, there was an increased CD44-positive basal intermediate cells in prostate tumors of 16-wk-old pes-ARKO-TRAMP (23) and ind-ARKO-TRAMP (Fig. 3B) compared with their WT TRAMP mice. A previous study has indicated that CD44+ prostate cancer cells purified from human prostate cancer xenografts have high tumorigenic and metastatic potentials (24).
Fig. 2.
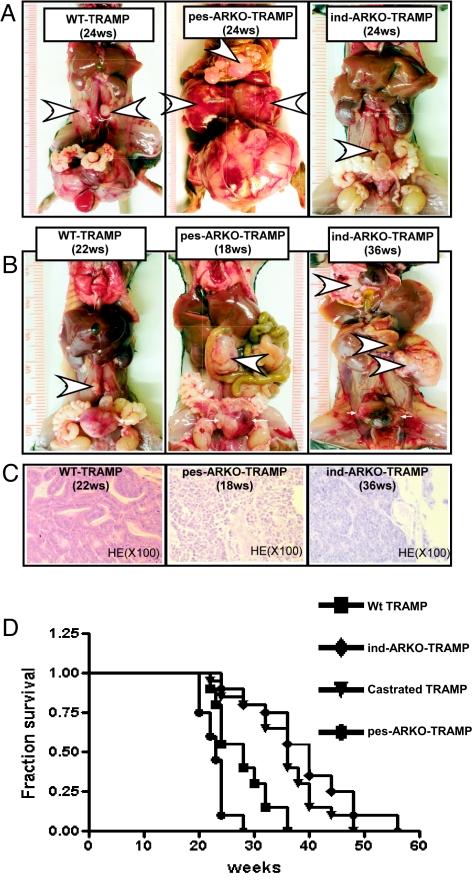
AR knockout led to changes of prostate tumor growth in TRAMP mice. (A) We observed general visual changes of the reproductive organs among 16-wk-old pes-ARKO-TRAMP, WT TRAMP mice, pI-pC ind-ARKO-TRAMP, and pI-pC injected WT TRAMP. pes-ARKO-TRAMP mice had enlarged prostates (arrows) compared with WT TRAMP mice, without alteration of other reproductive organs. Ind-ARKO-TRAMP with pI-pC induction of AR knockdown at 12-wk-old had significantly smaller reproductive organs, including prostates, seminal vesicles, and testes. (B) Serum testosterone (T) levels were detected sequentially at 12 wk (before pI-pC injection), 16, 20, and 24 wk. The serum T levels remained unchanged in pes-ARKO-TRAMP and were significantly reduced in ind-ARKO-TRAMP mice.
Fig. 3.
Inducing AR knockdown in ind-ARKO-TRAMP mice led to changed cell populations in prostate tumor. (A) We observed more intermediate cell-like populations (arrows indicate yellow color, overlapped by green and red signals) in ind-ARKO-TRAMP mice after pI-pC injection at 12-wk-old, mice compared with pI-pC injected WT TRAMP mice by double immunofluorescence staining of CK5 (green) and CK8 (red) of ventral prostate tumors. (B) ind-ARKO-TRAMP tumors expressed higher CD44-positive cells (arrows) compared with pI-pC injected WT TRAMP tumors. Data are presented as mean ± SD; *P < 0.05; **P < 0.01.
Interestingly, because the AR can regulate the expression of PB promoter-derived SV 40 T antigen, this argues that ARKO-TRAMP mouse may have reduced expression of SV 40 T antigen and thus reduce the tumorigenicity or tumor progression. However, we observed the faster primary tumor growth in pes-ARKO-TRAMP mice as compared with the littermate WT TRAMP mice, suggesting the faster growth of primary tumor in pes-ARKO-TRAMP mice may be little influenced by the reduction of SV 40 T antigen expression in prostate. It is possible that the initial SV 40 T antigen expression, before knockout of AR, is sufficient to promote the tumor initiation and continuing progression.
To date, there is still a lack of a perfect mouse model to mimic perfectly the human prostate cancer. For example, the Pten-KO mouse model has almost 100% CD44 positive prostate cancer cells, yet there are only limited CD44-positive cells in human prostate cancer. Moreover, several reports have documented that Pten could modulate AR function directly or indirectly (via PI3K), and loss of the Pten could consequently change the AR function, which would confound interpretation of the data. Furthermore, we have a parallel study to evaluate tumor growth in 16-wk-old TRAMP mice under ADT conditions via inducing AR knockdown in both stroma and epithelium (ind-ARKO-TRAMP) that results in the decreased tumor volumes with increased apoptosis. These data are consistent with clinical studies demonstrating that ADT, which antagonizes both stromal and epithelial AR, reduces tumor volume and increases apoptosis. Because the PB promoter was used to drive SV 40 T antigen and cre expression in pes-ARKO-TRAMP mice, both SV 40 T antigen and cre transgenes were expected to express in the same cell.
Increased vs. Decreased Primary Prostate Tumor Growth in pes-ARKO-TRAMP and ind-ARKO-TRAMP Mice.
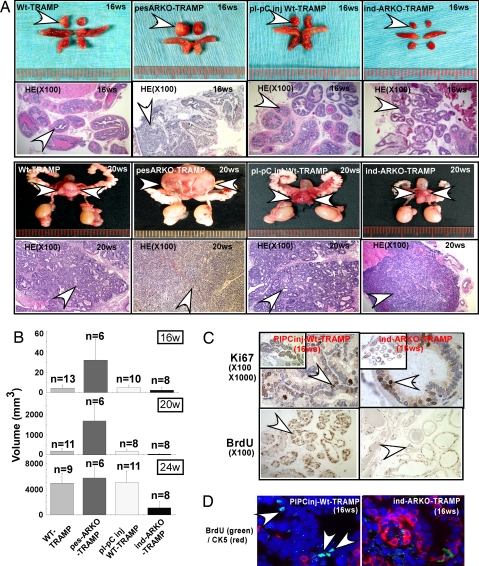
We found larger primary prostate tumors in 16-, 20-, and 24-wk-old pes-ARKO-TRAMP mice than in their WT TRAMP littermates. In contrast, in 16-wk-old ind-ARKO-TRAMP mice, we found smaller primary prostate tumors than in their WT littermates after injection with pI-pC at 12 wk old. (Fig. 4 A and B). With H&E staining, we also observed that primary prostate tumors in pes-ARKO-TRAMP mice were poorly differentiated as compared with their WT littermates at the age of 16 or 20 wk (Fig. 4A, H&E), suggesting primary tumors in pes-ARKO-TRAMP mice may have more aggressive behavior as compared with tumors in their WT TRAMP littermates. Notably, the smaller primary prostate tumors found in ind-ARKO-TRAMP mice were also poorly differentiated as compared with tumors in their WT TRAMP littermates (Fig. 4A), further confirming that the expanded population of intermediate-like tumor cells could result in poorly differentiated tumors in these mice.
Fig. 4.
AR-negative role in the growth of epithelium tumor was dominated by AR stroma function, which may positively stimulate epithelium proliferation. (A) The gross visual observation of the ventral prostates at 16 (lane 1) and 20 wk (lane 3). pes-ARKO-TRAMP mice generated larger tumors (arrowheads) than WT TRAMP mice, whereas ind-ARKO-TRAMP generated much smaller tumors than their WT TRAMP littermate mice. Histological analysis of different lobes of prostates at 16 and 20 wk (Lower). pes-ARKO-TRAMP and ind-ARKO-TRAMP tumor are more poorly differentiated than WT controls (loose). (B) We killed mice at different time points of 16, 20, and 24 wk and measured tumor size differences. The animal number of each group is indicated. (C) We demonstrated tumor growth differences by Ki67 IHC staining (Upper) and by BrdU incorporation (Lower) in 16-wk-old prostates. We i.p. injected mice with BrdU every 6 h 4 times and killed mice 24 h later. Tissue sections were stained by the BrdU-detecting kit (Zymed). (D) Double immunofluorescent staining of BrdU (green) and CK5 (red) on mouse prostate cancers. BrdU proliferation signals are reduced with CK5-positive cells and are increased in ind-ARKO-TRAMP mice (arrows indicate prostate tumor cells with positive nuclear BrdU green fluroscence staining). Although ind-ARKO-TRAMP also had a higher percentage of CK5 positive cells, the proliferation in their prostate was still low as compared with WT TRAMP mice.
To correlate the increased tumor size with proliferating rates in the primary prostate tumors, we assayed proliferation rates via Ki67 and BrdU stainings. We found substantially higher Ki67 staining and higher BrdU incorporation rates in primary prostate tumors of pes-ARKO-TRAMP mice than those in their WT TRAMP littermates (23). In contrast, we found less Ki67 staining and BrdU incorporation rates in primary prostate tumors of ind-ARKO-TRAMP mice compared with those in their WT TRAMP littermates (Fig. 4C). With double staining of proliferation marker BrdU and basal cell marker CK5, we confirmed the cells with higher proliferation rates are basal-intermediate cells in primary prostate tumors of pes-ARKO-TRAMP mice (23) and lower proliferation rates in those basal-intermediate cells in tumors from ind-ARKO-TRAMP mice than those from their WT TRAMP littermates (Fig. 4D). Interestingly, although basal-intermediate cell populations expanded in tumors of pes-ARKO-TRAMP and ind-ARKO-TRAMP mice, the proliferation rates of these basal-intermediate cells were higher in primary prostate tumors from pes-ARKO-TRAMP mice, indicating prostate stromal AR may play a more dominant stimulating role in primary prostate tumor growth. Together, we found larger and more aggressive primary prostate tumors with higher proliferation rates in pes-ARKO-TRAMP mice and smaller primary prostate tumors with lower proliferation rates in ind-ARKO-TRAMP mice as compared with those in littermate WT TRAMP mice.
Ind-ARKO-TRAMP Mice Develop Less Aggressive and Invasive Metastatic Tumors.
We also compared metastatic tumor size in 24-wk-old pes-ARKO-TRAMP mice, WT TRAMP mice (with or without injection of pI-pC at 12 wk old), and ind-ARKO-TRAMP mice (injected with pI-pC at 12 wk old), our results indicated pes-ARKO-TRAMP mice developed larger and histologically more aggressive metastatic tumors in lymph nodes as compared with their WT littermates (Fig. 5A). In contrast, knockdown of the AR in both epithelial and stromal cells in ind-ARKO-TRAMP mice led to smaller and less-aggressive metastatic tumors in lymph nodes as compared with their WT TRAMP littermates injected with pI-pC (Fig. 5A).
Fig. 5.
Tumor metastasis is delayed in ind-ARKO-TRAMP mice. (A) Twenty-four-week-old ind-ARKO-TRAMP mice, with decreased (50–60%) AR expression in both prostate epithelium and stroma, developed smaller and less-aggressive metastatic tumors (arrowheads) compared with tumors from the WT TRAMP littermates. The size of metastatic tumors among different groups followed the sequence: pes-ARKO-TRAMP > WT TRAMP (with and without pI-pC)> ind-ARKO-TRAMP. The WT TRAMP mice with or without injection of only pl-pC developed similar sizes of metastatic tumors; only data of WT TRAMP mice without injection are shown. (B) Differences in tumor malignancies were demonstrated by comparing metastases from TRAMP mice with different AR-status when the original tumors reached to 1-cm diameter in different mouse groups. At the age of ≈22 wk, WT TRAMP mice developed 1-cm-diameter-size tumors, and those tumors were well differentiated with small pelvic lymph node metastases. As early as 18 wk, pes-ARKO-TRAMP tumors reached the similar size, and the mice had much larger tumor metastases to lymph nodes and multiple organs. It took 36 wk for the ind-ARKO-TRAMP to form the 1-cm-diameter tumors, which invaded into the seminal vesicle (lower arrowhead) and migrated to the liver (upper arrowhead). (C) Histological analysis of tumor sections. H&E staining showed WT TRAMP primary tumors were better differentiated than pes-ARKO-TRAMP and ind-ARKO-TRAMP tumors. (D) Survival rates were statistically different among WT TRAMP, castrated TRAMP, ind-ARKO-TRAMP, and pes-ARKO-TRAMP mice.
Because prostate tumors developed at different rates between pes-ARKO-TRAMP mice and ind-ARKO-TRAMP mice, we took another approach to compare the development of primary tumors between these two different ARKO mice. We found pes-ARKO-TRAMP mice required 18 wk to develop a primary prostate tumor with size near 1 cm in diameter. However, it took 36 wk for ind-ARKO-TRAMP to develop similarly sized primary prostate tumors (Fig. 5B). Moreover, histological analysis of prostate primary tumors of similar size from pes-ARKO-TRAMP at 18 wk old and ind-ARKO TRAMP mice at 36 wk old demonstrated more aggressive appearing primary tumors in pes-ARKO-TRAMP mice (Fig. 5C). Furthermore, pes-ARKO-TRAMP mouse tumors metastasized to pelvic lymph nodes, and ind-ARKO-TRAMP mouse tumors metastasized to lung, kidney, and liver when primary tumors grew to 1 cm in diameter (Fig. 5B).
The larger and more-aggressive primary and metastatic tumors in pes-ARKO-TRAMP mice may then lead to earlier death than in WT TRAMP littermates (Fig. 5D). In contrast, the smaller and less-proliferative primary and metastatic prostate tumors in ind-ARKO-TRAMP mice may result in longer lives compared with their WT TRAMP littermates (Fig. 5D).
These results suggest that stromal AR functions as a proliferator and may play a dominant role for the primary prostate tumor growth. Other studies also demonstrate that stromal cells play vital roles in prostate carcinogenesis and metastasis. For example, alteration of stromal TGFβ signals have been demonstrated to play a key initiating role in prostate carcinogenesis in mice lacking the stromal TGFβ receptor II (25). Stromal cells isolated from cancerous tissues also elicited irreversible malignant transformation of the human epithelia and that TGFβ stimulates this cell transformation (26). Finally, bone marrow stroma cells have been demonstrated to establish a prometastatic niche “soil” in which circulating metastatic prostate cancer cells can preferentially grow (27, 28). Together, these data suggest that the stromal cells may be able to promote prostate primary tumor growth and migration to distant tissues and stromal AR play critical roles in those processes.
Impact to Current Clinical Treatment of Prostate Cancer.
The conclusions drawn from above data may influence clinical prostate cancer therapy. Based on our findings, we believe the ideal therapeutic approach to battle androgen sensitive prostate tumors would be to target stromal AR at earlier stages, perhaps via a stromal-specific delivery system that delivers AR-siRNA or a compound, such as ASC-J9 (29), to suppress or degrade the AR in stromal cells only. Unfortunately, no such stromal-specific delivery system has been developed. However, because of the unique surface antigens expressed by prostatic stroma, such an approach may be possible in the future. Nevertheless, even if we can target only the whole AR as in the ind-ARKO-TRAMP mouse model, we may still be able to battle prostate cancer with better timing. Based on our ind-ARKO-TRAMP mouse model that targeted the AR at different times, we found the earlier targeting of the AR via knockdown of AR (at 4 wk) results in a much better suppression of primary prostate tumor growth than that occurred with later targeting (at 20 wk) (Table 1). The current practice of surgical or medical castration with or without antiandrogen treatment targets androgen/AR functions in both stromal and epithelial cells, which may be the reason why tumors of prostate cancer patients were suppressed at the beginning of treatment and then have a recurrence after ADT treatment (30). Therefore, it will be interesting to see whether selectively targeting the stromal AR at earlier stages can become a better strategy to battle prostate cancer.
Table 1.
Induction of ARKO on ind-ARKO-TRAMP mice at early age (4 wk) significantly reduced turmorigenesis and progression, whereas at a later age of 20 wk failed to block tumor progression
| Age, wk | WT TRAMP (n = 40) |
Induced ARKO at 4 wk (n = 40) |
Induced ARKO at 20 wk (n = 30) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival | Tumor | Lymph node | Survival | Tumor | Lymph node | Survival | Tumor | Lymph node | |
| 20 | 10/10 | 6.6 ± 4.5 | 1.6 ± 0.8 | 10/10 | 1.5 ± 0.5* | 0 ± 0* | — | — | — |
| 24 | 10/10 | 20.5 ± 3.8 | 4.6 ± 3.3 | 10/10 | 4.6 ± 2.1* | 1.5 ± 0.5* | 10/10 | 19.9 ± 5.1 | 3.8 ± 3.1 |
| 28 | 6/10 | 25.5 ± 1.3 | 5.7 ± 0.9 | 10/10 | 6.9 ± 4.5* | 2.3 ± 1.5* | 7/10 | 20.9 ± 8.6 | 4.4 ± 3.6 |
| 32 | 2/10 | 26.7 ± 4.7 | 11.0 ± 1.0 | 10/10 | 8.8 ± 5.3* | 2.9 ± 1.5* | 3/10 | 27.7 ± 2.1 | 6.7 ± 5.3 |
Diameter of primary prostatic tumor or lymph node metastatic tumor; data are presented as mean ± SD mm.
*P < 0.05.
Methods
Establishment of Stable Transfected WPMY1-ARsi Cells.
We constructed a siRNA into retroviral pSuperior vector to target human AR mRNA sequence 5′-gtggccgccagcaaggggctg-3′ (1530–1550). This pSuperior ARsiRNA was used to infect human stromal WPMY1 cells to establish WPMY1-ARsi cells.
Orthotopic Implantation of Prostate Cancer Cells in Nude Mice.
We coinoculated PC3 and PC3-AR9 cells with WPMY1-v or WPMY1-ARsi cells directly into the anterior prostate of athymic nude mice. After anesthesia, the abdomens of 8-wk-old athymic nude mice were surgically opened in sterile environments. PC3-v coinuculated or PC3-AR9 cells (5 × 106) with 5 × 106 WPMY1-v or WPMY1-ARsi cells suspended in 50 μl of Matrigel were injected into one lobe of anterior prostate by 25-gauge needle, and the abdomens were closed by silk sutures. Mice were killed 12 wk later, and xenograft tumors and metastatic tumors were fixed and embedded in paraffin for further analyses.
Generation of ind-ARKO-TRAMP Mice.
To generate ind-ARKO-TRAMP mice, we first mated TRAMP (FVB) transgenic mice with flox AR mice (C57BL/6), to generate TRAMP-floxAR female mice. Then we interbred TRAMP-floxAR female mice with Mx-Cre male mice (C57BL/6-FVB, Jackson Laboratory) to generate the Mx-cre-floxedAR-TRAMP male mice. After genotype confirmation, we induced the ARKO in Mx-cre-floxAR-TRAMP male mice (T-Ag positive, floxAR positive, and Mx-Cre positive) mice to knockout AR by i.p. injection of 300 μl of pI-pC (Sigma-Aldrich) solution (1 mg/ml water) six times at 48-h intervals at the age of 4, 12, and 20 wk. Littermate TRAMP mice (T-Ag positive, floxAR negative, and Mx-Cre positive) mice were also pI-pC injected as controls.
Other Methods in SI Text.
Additional methods included in SI Text are as follows: (i) cell culture, plasmids, and reagents; (ii) light microscopy procedures; (iii) RNA extraction, RT-PCR, and real-time RT-PCR; (iv) immunohistochemistry; (v) BrdU incorporation assay; (vi) TUNEL assay; (vii) laser-capture microdissection to obtain selected prostate cells; and (viii) statistics.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants CA122840 and CA127300, and the George H. Whipple Professorship Endowment.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804701105/DCSupplemental.
References
- 1.Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol. 1976;47:137–194. doi: 10.1016/s0074-7696(08)60088-1. [DOI] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Cunha GR, Chung LW. Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 4.Donjacour AA, Cunha GR. The effect of androgen deprivation on branching morphogenesis in the mouse prostate. Dev Biol. 1988;128:1–14. doi: 10.1016/0012-1606(88)90260-6. [DOI] [PubMed] [Google Scholar]
- 5.Hayward SW, et al. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63:131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu CT, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 8.Bruchovsky N. In: Cancer Medicine. 3rd Ed. Holland JF, et al., editors. Philadelphia: Lea and Febiger; 1993. pp. 884–896. [Google Scholar]
- 9.Eisenberger MA, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 10.Mostaghel EA, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 11.Visakorpi T, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 12.van Leenders GJ, Aalders TW, Hulsbergen-van de Kaa CA, Ruiter DJ, Schalken JA. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol. 2001;195:563–570. doi: 10.1002/path.993. [DOI] [PubMed] [Google Scholar]
- 13.Bruchovsky N, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–2282. [PubMed] [Google Scholar]
- 14.Mizokami A, Yeh SY, Chang C. Identification of 3′,5′-cyclic adenosine monophosphate response element and other cis-acting elements in the human androgen receptor gene promoter. Mol Endocrinol. 1994;8:77–88. doi: 10.1210/mend.8.1.8152432. [DOI] [PubMed] [Google Scholar]
- 15.Altuwaijri S, et al. Expression of human AR cDNA driven by its own promoter results in mild promotion, but not suppression, of growth in human prostate cancer PC-3 cells. Asian J Androl. 2007;9:181–188. doi: 10.1111/j.1745-7262.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 16.Webber MM, et al. A human prostatic stromal myofibroblast cell line WPMY-1: A model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis. 1999;20:1185–1192. doi: 10.1093/carcin/20.7.1185. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto H, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 18.Yeh S, et al. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med. 2003;198:1899–1908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh S, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgentaler A. Testosterone and prostate cancer: An historical perspective on a modern myth. Eur Urol. 2006;50:935–939. doi: 10.1016/j.eururo.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Schatzl G, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 22.Isom-Batz G, et al. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173:1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu J, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am J Pathol. 2004;165:1543–1556. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 26.Hayward SW, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- 27.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi C, et al. Bi-directional interactions of bone marrow mesenchymal stem cells with human prostate cancer cells. Abstract in AUA. 2007 [Google Scholar]
- 29.Yang Z, et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 30.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.