Abstract
The virulent properties of the common human and livestock pathogen Clostridium perfringens are attributable to a formidable battery of toxins. Among these are a number of large and highly modular carbohydrate-active enzymes, including the μ-toxin and sialidases, whose catalytic properties are consistent with degradation of the mucosal layer of the human gut, glycosaminoglycans, and other cellular glycans found throughout the body. The conservation of noncatalytic ancillary modules among these enzymes suggests they make significant contributions to the overall functionality of the toxins. Here, we describe the structural basis of an ultra-tight interaction (Ka = 1.44 × 1011 M−1) between the X82 and dockerin modules, which are found throughout numerous C. perfringens carbohydrate-active enzymes. Extensive hydrogen-bonding and van der Waals contacts between the X82 and dockerin modules give rise to the observed high affinity. The μ-toxin dockerin module in this complex is positioned ≈180° relative to the orientation of the dockerin modules on the cohesin module surface within cellulolytic complexes. These observations represent a unique property of these clostridial toxins whereby they can associate into large, noncovalent multitoxin complexes that allow potentiation of the activities of the individual toxins by combining complementary toxin specificities.
Keywords: enzyme complexes, glycoside hydrolases, protein–protein interaction
Clostridium perfringens is a ubiquitous, Gram-positive, spore-forming bacterium that is a major cause of gastroenteritis and gas gangrene in humans (1). This organism is a prolific producer of toxins, which are responsible for its virulence, and is classified into five biotypes (A–E) on the basis of strain-specific production of major toxins (2). Biotype A encompasses the myonecrotic or “flesh-eating” strains of C. perfringens and mainly produces the major α-toxin, which has phospholipase C activity (1, 3). These strains are also notable for their genomic content of numerous open-reading frames that encode large and highly modular carbohydrate-active enzymes (4, 5), which are primarily glycoside hydrolases (6). Their apparent role in infection involves the degradation of complex glycans, including the mucosal layer of the human gut, which comprises a group of highly hydrated glycoproteins, glycosaminoglycans, found throughout the body, and other cellular glycans. The μ-toxin (hyaluronidase) (7) and “large” sialidases (NanI and NanJ) (8) are known toxins that fall into this class of enzyme and are thought to contribute to virulence through varying mechanisms, such as the uncapping of receptors for other toxins and/or destruction of tissue (1). The carbohydrate-active toxins are among the largest and most modular enzymes produced by C. perfringens (Fig. 1).
Fig. 1.
Modular architecture of Clostridium perfringens X82- and Doc-containing glycoside hydrolases. The FIVAR, Doc, and X82 modules used in this study are shaded in orange, light blue, and green, respectively. GHXX, glycoside hydrolase catalytic domains (shaded gray) where XX represents the family number; CBM32, family 32 carbohydrate-binding module; UNK, modules having unknown function and little sequence identity with other protein modules; F, FIVAR modules; Doc, dockerin-like modules; CBM40, family 40 carbohydrate-binding module; X82, cohesin-like family 82 X-module; FN3, fibronectin III-like module; CBM50, family 50 carbohydrate-binding module; ConA-like, Con A-like module; BIG_2, group 2 bacterial Ig-like domain.
The extensive modularity and the reoccurring appearance of various ancillary modules within these carbohydrate-active toxins suggest that these nonatalytic modules play a role in their function. In this context, carbohydrate-binding modules (CBM) have been found in many of these enzymes and shown to mediate their attachment to host glycan targets (9, 10). The μ-toxin contains a dockerin (Doc)-like sequence at its C terminus, which comprises two conserved calcium-binding EF-hand loop sequences (11, 12). Doc modules have been extensively characterized in cellulolytic bacteria, where they mediate the enzyme assembly and cell-surface attachment of the cellulose-degrading complex termed the cellulosome through interactions with its cognate binding partner, a cohesin module (12–17). Recent structural characterization of the X82 module from CpGH84C (NagJ), a C. perfringens homolog of the μ-toxin, indicated that this module displays structural homology to the cellulolytic cohesin modules (18), leading to speculation that carbohydrate-active toxins can form complexes through X82–Doc interactions.
To assess the potential number of such complexes, we identified putative X82 and Doc modules from five and four C. perfringens glycoside hydrolases, respectively, and characterized their binding properties, including determining the 1.6 Å-resolution complex crystal structure of the CpGH84C X82 module and the found-in-various architectures (FIVAR)-Doc modular pair from the μ-toxin, and the 1.7 Å-resolution crystal structure of the isolated X82 module from the NanJ sialidase toxin. The ultra-high-affinity X82–Doc interaction results from extensive intermodular contacts between both Doc helices and the 9a-8-3-6-5 face of the X82 module. The relationship to the cellulolytic cohesin-Doc interactions and the implications of X82–Doc-mediated toxin complexes in C. perfringens pathogenesis are discussed.
Results
Identification and Interaction of C. perfringens X82 and Dockerin Modules.
In addition to the CpGH84C X82 module (CpGH84CX82) (18), through bioinformatic analyses we have identified putative X82 modules in four other C. perfringens glycoside hydrolases, including and the “large-sialidase”-toxin NanJ (8), [Fig. 1 and supporting information (SI) Fig. S1A]. Modules possessing two EF-hand calcium-binding loops that are similar in sequence to Doc modules, the biological ligands of cohesin modules in cellulolytic bacteria (12), occur in four C. perfringens carbohydrate-active enzymes (Fig. 1 and Fig. S1B), including the μ-toxin (7, 11). These observations led us to hypothesize that C. perfringens X82 and Doc modules, and by extension their associated enzymes, interact to form noncovalent complexes, in a manner similar to that seen in cellulolytic anaerobic bacteria (12, 14, 17). Such C. perfringens-associated complexes would allow the individual enzymes to function in concert to efficiently and rapidly degrade their target substrates, which include the mucosal layer of the human gut, carbohydrates associated with the extracellular matrix, and cell surface components within the infected tissue.
The binding ability and specificity of the five C. perfringens X82 modules and four C. perfringens Doc modules were qualitatively assessed in an ELISA-based assay (Fig. 2A) (19). Interactions involving three (μ-toxin, CpGH2, CpGH95) of the four Doc modules and three (NanJ, CpGH31, CpGH84C, CpGH20) of the five X82 modules were identified. No binding was observed for the X82 modules from the family 3 and family 31 glycoside hydrolases (CpGH3 and CpGH31, respectively) and the Doc module from a family 31 glycoside hydrolase (CpGH31). For a more quantitative analysis of representative interacting C. perfringens Doc-X82 pairs, we selected the isolated CpGH84C (CpGH84CX82) and NanJ (NanJX82) X82 modules, and a tandem derivative comprising the third FIVAR module and the Doc module (FIVAR-Doc) from the μ-toxin for isothermal titration calorimetry (ITC) and differential scanning calorimetry (DSC) studies (Fig. 2 B–D and Fig. S2). Although the interactions were too strong to accurately determine association constants (Ka) by ITC (>109 M−1), both indicated 1:1 binding stoichiometries. The changes in enthalpy (ΔH) for the CpGH84CX82–FIVAR-Doc interaction could be accurately established at several different temperatures allowing the determination of the change in heat capacity (ΔCp) to be assessed at 143.5 ± 11.1 cal·mol−1·K−1. The Ka value for this interaction was determined by DSC to be 2.05 × 109 M−1 at 88.9°C or 1.44 × 1011 M−1 at 37°C, when the experimentally determined ΔCp was used with the integrated form of the van't Hoff equation to extrapolate to physiological temperature.
Fig. 2.
The ultrahigh affinity of the C. perfringens X82–Dockerin interaction. (A) ELISA-based binding specificities. X82 modules from family 3 (CpGH3), 20 (CpGH20), 31 (CpGH31), and 84 (CpGH84C) glycoside hydrolases and the “large” sialidase (NanJ) fused to a CBM were probed with Doc modules from the μ-toxin, and family 2 (CpGH2), 31 (CpGH31), and 95 (CpGH95) glycoside hydrolases, which were fused to a G. stearothermophilus xylanase T6. (+) and (−) indicate detectable and no detectable binding, respectively. (B) Isothermal titration calorimetric analysis of the CpGH84CX82-μ-toxin FIVAR-Doc interaction at 30°C. (Upper) Raw heat measurements. (Lower) Integrated heats after correction for heats of dilution as determined from the heats of ligand additions at the excess of saturation. The curves represent the best fit to a single-site model. (C) Temperature dependence of ΔH for the CpGH84CX82-μ-toxin FIVAR-Doc interaction. ΔH values were determined at 30°C, 35°C, and 37°C. (D) Differential scanning calorimetric denaturation profiles of 171 μM CpGH84CX82 (X82), 198 μM μ-toxin FIVAR-Doc (FivarDoc), and 18.2 μM CpGH84C-FIVAR-Doc complex. All samples contained 5 mM CaCl2.
Structural Insights into C. perfringens X82–Dockerin-Mediated Enzyme Complexes.
To explore the molecular basis of the ultra-high-affinity X82–FIVAR-Doc interaction, we examined the high-resolution x-ray crystal structure of the 1:1 CpGH84CX82–FIVAR-Doc complex (Fig. 3), which was determined at 1.6 Å with Rwork and Rfree values of 20.5 and 26.4, respectively. CpGH84CX82 (Fig. 3, green) in complex with FIVAR-Doc has the expected β-sandwich structure formed by nine strands in a jellyroll topology, with the two faces of the sandwich comprising strands 9a, 8, 3, 6, 5 (9a-8-3-6-5 face) and 9b, 1, 2, 7, 4 (9b-1–2-7–4 face) (18). Comparison of the CpGH84CX82 structure in the complex with isolated CpGH84CX82 (18) [backbone root mean square distance (rmsd) of 1.3 Å] illustrates that, similar to the cellulolytic cohesin modules (13, 16), the C. perfringens X82 modules undergo only a very slight conformational rearrangement on binding a Doc module.
Fig. 3.
Structure of a C. perfringens X82–Doc complex. Ribbon representation of the CpGH84CX82-μ-toxin FIVAR-Doc complex with CpGH84CX82 depicted in green, the FIVAR shown in orange, and the Doc module in light blue. The N- and C-termini are colored labeled accordingly. The calcium ions are depicted as purple spheres.
The third FIVAR module (residues 1498–1577; Fig. 3, orange) of the μ-toxin FIVAR-Doc protein fragment comprises three helices (I: Lys-1499-Glu-1515; II: Gly-1528-Phe-1544; III: Glu-1551-Leu-1571) arranged in a left-handed three-helix bundle. The C-terminal module (residues 1578–1628; Fig. 3, light blue) has a typical Doc fold with two antiparallel F-hand motifs separated by a 9-residue linker. The two F-hand motifs each possess a canonical EF-hand calcium-binding loop (I: Asp-1579-Asp-1590; II: Asp-1607-Glu-1618) that binds calcium in a pentagonal bipyramidal configuration, and an exiting helix (IV: Asp-1587-Asn-1597; V: Asp-1615-Asn-1628). The two helices form an exposed and flush face that constitutes the X82 recognition surface.
An interface exists between the FIVAR and Doc modules of the μ-toxin. Residues Phe-1520, His-1526, Lys-1527, Arg-1567, Ser-1570, Leu-1571, and Ile-1573 of the FIVAR module contact Leu-1608, Asn-1609, Lys-1610, Tyr-1617, Phe-1621, Ile-1622, and His-1624 of the Doc module, which gives the modular pair an extended prolate conformation.
C. perfringens X82–Dockerin Complex Interface.
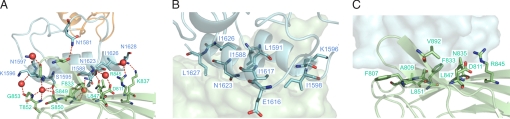
The X82–Doc interface involves both helices of the Doc module and the 9a-8-3-6-5 face of the X82 module, and comprises 3,974 Å2 of total occluded surface area. The ultrahigh affinity of this noncovalent interaction is derived from an extensive hydrogen-bonding network and nonpolar interactions between residues of the X82 and Doc modules (Fig. 4 and Table S1).
Fig. 4.
C. perfringens X82–Doc intermolecular contacts. (A) CpGH84CX82-μ-toxin Doc interface hydrogen-bonding network, with hydrogen-bond contacts shown as red dashed lines and water molecules as red spheres. (B) Ribbon representation of μ-toxin Doc, displaying residues involved in intermolecular van der Waals contacts as stick modules on the CpGH84CX82 surface. (C) Ribbon representation of CpGH84CX82, displaying residues involved in intermolecular van der Waals contacts as stick modules on the μ-toxin Doc surface. CpGH84CX82 and μ-toxin Doc are colored green and light blue, respectively. Residues are labeled in one-letter code, and numbered and colored accordingly.
To assess the conservation of molecular determinants associated with Doc recognition among the C. perfringens X82 modules, we also determined the x-ray crystal structure of the isolated NanJX82 module at 1.7 Å with Rwork and Rfree values of 21.6 and 25.7, respectively (residues 939–1081; Fig. 5A, salmon). Despite sharing only ≈33% amino acid sequence identity, comparison of the NanJX82 and CpGH84CX82 structures reveals very similar overall folds (backbone rmsd of 1.2 Å) with a well conserved Doc-binding surface (Fig. 5B), which explains the observed high affinity of NanJX82 for FIVAR-Doc (Fig. S2). Indeed, the binding-interface residues are well conserved among the three C. perfringens X82 modules that were demonstrated to be functional (Fig. S1). In contrast, these residues are poorly conserved in the CpGH3 and CpGH31 X82 modules, explaining their lack of affinity for the tested Doc modules. Likewise, the interface residues are highly conserved among the functional Doc modules, but not in the CpGH31 Doc module (Fig. S1). A functional role for these latter X82 and Doc modules remains to be determined.
Fig. 5.
Structural homology and interface residue conservation displayed by the C. perfringens X82 modules. (A) Backbone overlay (N, Cα, C′ atoms) of CpGH84CX82 (green) from the CpGH84CX82-μ-toxin FIVAR-Doc complex structure and isolated NanJX82 (salmon). (B) Overlay of the 9a-8-3-6-5 X82 faces and from CpGH84CX82 (green) and NanJX82 (salmon) depicting the Doc recognition residues of CpGH84CX82 and the analogous residues in NanJX82 as stick models. Residues are identified by one-letter code, and colored and numbered accordingly.
Discussion
The repeating appearance of various ancillary modules, such as CBMs, X82, and Doc modules, within C. perfringens toxins and associated carbohydrate-active enzymes suggests that they contribute important functionalities to the overall effectiveness of these enzymes. Our identification of several Doc- and X82-containing C. perfringens enzymes, including the μ-toxin and NanJ sialidase toxin, indicate that these modules fall into such a class of protein modules. Further, the biochemical, biophysical, and structural characterization of the C. perfringens X82–Doc interaction presented here suggests a conserved role of these protein modules in mediating enzyme complex formation via their ultra-high affinity interaction.
Doc modules are best known for their role in the cellulosome, a multienzyme complex produced by anaerobic bacteria to efficiently and synergistically degrade cellulose-based biomass (12, 14, 17). These calcium-binding modules interact with their cognate cohesin modules, allowing the integration of their covalently attached catalytic domains onto the cellulosome scaffold (type-I interaction) and cell-surface attachment (type-II interaction). Although they possess similar sequences, the cellulolytic Doc modules involved in these two processes (type-I vs. type-II) display a high degree of binding specificity, which arises from the number of nonpolar contacts and the extent of interaction between helix I of the Doc modules and their respective cohesin modules (13, 16). The C. perfringens X82–Doc interaction is reminiscent of the C. thermocellum type-II interaction, with substantial nonpolar character, and both Doc helices making direct contacts the X82 9a-8-3-6-5 face (13). However, the orientation of the μ-toxin Doc module on the surface of the X82 module is rotated ≈180° from the Doc position in the C. thermocellum native type-I (16) and type-II complexes (13) (Fig. 6). This orientation is similar to that of a C. thermocellum type- I Doc mutant, which was generated to illustrate how the internal sequential symmetry of the type-I Doc would allow for plasticity in cohesin binding (15). The μ-toxin Doc does not display this type of symmetry; thus we do not expect it, or the other C. perfringens Doc modules, to display a similar dual mode of X82 recognition. The structural similarity to the cellulolytic cohesin modules displayed by the C. perfringens X82 modules (18), in conjunction with their ability to interact with Doc modules with ultrahigh affinity, lends itself to their reclassification as cohesin modules.
Fig. 6.
Variation of Doc orientations in clostridial complexes. Ribbon representations of (A) C. perfringens μ-toxin Doc (light blue) on CpGH84CX82 (light green); (B) native C. thermocellum type-I Doc (red) on type-I cohesin (yellow) (16); (C) C. thermocellum Ser45Ala/Thr46Ala type-I Doc mutant (red) on type-I cohesin (yellow) (36); (D) C. thermocellum type-II Doc (emerald green) on type-II cohesin (slate blue) (13). The helices within the two F-hand motifs of the Doc modules are identified as α1 and α2, respectively.
The predicted catalytic targets (eukaryotic glycans and/or glycosaminoglycans) of the X82- and Doc-containing C. perfringens glycoside hydrolases, which include the μ-toxin and NanJ sialidase, and their secretion to the extracellular milieu suggest that these enzymes are important components in the host–pathogen interaction. Recent structural and functional characterization of CBMs associated with the NanJ sialidase (9) and CpGH95 (20) indicates that these modules target their respective catalytic domains to their eukaryotic carbohydrate targets, further supporting a role of these carbohydrate-active enzymes in the host–pathogen relationship. It therefore appears that this complex arsenal of C. perfringens glycoside hydrolases contributes to the bacterium's virulence through tissue/glycan destruction to promote spread, potentiating the activity of the major toxins and providing a source of nutrition for the bacterium.
The generally accepted dogma associated to C. perfringens toxins and related glycoside hydrolases involves the secretion of soluble enzymes, which attach to the bacterial cell wall through a sortase-mediated process (21) or are free to diffuse and degrade host glycans as individual entities. Given that eukaryotic carbohydrates comprise a heterogeneous compilation of monosaccharide moieties that are densely packed within the extracellular matrix and on host cell surfaces, the expedient tissue destruction displayed by C. perfringens must require not only substantial production and secretion of these carbohydrate-active enzymes, but also their coordinated effort to degrade the complex carbohydrates. The extensive modularity of these enzymes beyond the catalytic domains and the reoccurring appearance of various protein modules suggest important functional roles. Because several of these large, multimodular enzymes contain additional uncharacterized modules, and because no binding partners for the putative CpGH31 Doc and cohesin modules have been identified, there exists the possibility of higher order enzyme complexes being formed. What our present findings clearly suggest is the formation of bimolecular glycoside hydrolase complexes, where these enzymes can act simultaneously and in concert, via the ultra-high-affinity X82 (cohesin)-Doc interaction, to impart their pathogenic effects. Such a synergistic action between glycoside hydrolases demands that these enzymes attack the same carbohydrate. Complexation facilitates the optimization of the enzymes such that they can attack a common complex carbohydrate and affords the opportunity of binding multiple substrates by incorporating multiple CBMs with varying specificities into a single complex, a scenario that could also enhance substrate binding through an avidity effect. Our biochemical structural characterization of C. perfringens X82-Doc interactions represents an example of noncellulosomal Doc-mediated enzyme complexes, and provides a rationale for the expeditious rate of tissue damage associated with C. perfringens infections.
Materials and Methods
Cloning.
The cloning of the C-terminal FIVAR-Doc modular pair fragment from the μ-toxin and the X82 module of CpGH84C (CpGH84CX82) were performed as previously described (11, 22). FIVAR-Doc and CpGH84CX82 methionine mutant constructs were generated, in which Leu-1533 and Val-1535 of the former and Leu-784 and Leu-856 of the latter were each mutated to methionine residues for the generation of a seleno-L-methionine (SeMet)-containing complex. The methionine mutations were engineered by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and a modified PCR protocol as previously reported (23). Subcloning of the FIVAR-Doc mutant construct made use of a single set of forward and reverse primers, whereas the generation of the CpGH84CX82 methionine-encoding mutant required a unique set of primers for each mutation (see Table S2). The respective methionine mutant-encoding plasmids were transformed into Escherichia coli XL1-Blue, isolated by using a QIAprep Spin Miniprep kit (Qiagen), and sequenced to assess mutagenesis.
The gene fragment encoding NanJX82 was PCR amplified from the genomic DNA of C. perfringens ATCC 13124 using a set of forward and reverse oligonucleotide primers (see Table S2). The amplified product was digested with NdeI and XhoI restriction endonucleases and ligated to NdeI and XhoI digested pET28a (Novagen). The resulting plasmid, encoding for NanJX82 fused to an N-terminal His6-tag via a thrombin protease cleavage site, was transformed into E. coli BL21(DE3) for recombinant production.
Protein Production and Purification.
Native FIVAR-Doc and CpGH84CX82 recombinant protein derivatives were expressed and purified as described previously (11, 22). Expression and purification of SeMet-labeled FIVAR-Doc and CpGH84CX82 was also performed in a manner similar to that previously described for the uniform 13C/15N-labeling of FIVAR-Doc and CpGH84CX82 (11, 22), with the exception that in the current study the expression plasmids encoding the FIVAR-Doc and CpGH84CX82 methionine mutants were transformed into the auxotrophic E. coli strain DL41(DE3) and the medium was supplemented with 50 mg SeMet rather than 1 g of 15N-NH4Cl and 2 g of 13C-glucose (Cambridge Isotope Laboratories). Recombinant expression, refolding, and purification of the SeMet-FIVAR-Doc and SeMet-CpGH84CX82 mutant derivatives were performed as described for the native protein fragments.
Formation of the FIVAR-Doc–CpGH84CX82 complex involved the combination of purified CpGH84CX82 and FIVAR-Doc at a molar ratio of 1.3:1 in 5 mM Hepes, pH 7.5; 50 mM NaCl; and 5 mM CaCl2. The 1:1 FIVAR-Doc–CpGH84CX82 complex was purified from excess CpGH84CX82 by application on a Hi-Load 16/60 Superdex 75 size exclusion column (Amersham Pharmacia Biosciences) equilibrated with 5 mM Hepes, pH 7.5; 50 mM NaCl; and 5 mM CaCl2 and eluted in 2-ml fractions using the same buffer. Fractions containing the complex were identified by SDS/PAGE, pooled, and concentrated by using a Millipore Amicon 10-kDa centrifugal device to a final concentration of 50 mg/ml and stored at 4°C.
Expression and purification of recombinant NanJX82 was performed similar to the method described previously for CpGH84CX82 (22). Purified NanJX82 was concentrated and buffer exchanged into 25 mM Tris·HCl, pH 7.5; 50 mM NaCl; and 5 mM CaCl2, using an Amicon stirred ultra-filtration unit (Amicon) with a 5-kDa molecular weight cutoff membrane (MWCO; Filtron). Purity was assessed by SDS/PAGE, and the concentration determined by UV absorbance (280 nm) with a calculated molar extinction coefficient of 4,470 M−1/cm−1.
ELISA Assays.
The regions encoding the X82 modules from the genes Cpe1234 (NagJ), Cpe1364, Cpe0553 (NanJ), and Cpe0266 and the regions encoding the putative Doc modules from Cpe0191 (μ-toxin), Cpe1266, Cpe1875, and Cpe1046 were amplified from the C. perfringens strain 13 genomic DNA with engineered XhoI and BamHI, and BamHI and KpnI restriction sites, respectively. The X82-encoding fragments were cloned into a CBM3a-fusion construct, and the Doc-encoding fragments were cloned into a His-tagged heat stable xylanase (Xyn)-fusion derivative as previously described (19). The resultant fusion proteins were recombinantly expressed in E. coli and purified on amorphous cellulose (CBM-containing protein derivatives) or by metal affinity chromatography (His-tagged protein derivatives), and the ELISA-based experiments were carried out similar to the method previously described (19), with incubation steps of 1 h at 37°C for each step. The ELISA plate was coated with 100 ng of CBM-X82 and assayed with 100 μl of each of the Xyn-Doc derivatives (0–50 ng/ml). The final colorimetric assay was developed for 1 min. All assays were done in duplicate.
Calorimetry.
The FIVAR-Doc, CpGH84CX82, and NanJX82 protein samples for calorimetry, prepared in 25 mM Tris·HCl (pH 7.5), 50 mM NaCl, and 5 mM CaCl2, were filtered and degassed at 21°C.
The heat capacity measurements of 171 μM CpGH84CX82, 198 μM FIVAR-Doc, and 18.2 μM FIVAR-Doc–CpGH84CX82 complex were performed from 20°C to 110°C with a scan rate of 45°C per hour by using a VP-DSC calorimeter from MicroCal. The thermodynamic parameters of the single-unfolding transitions (Tm, ΔCp, and ΔH) were calculated with Origin 5.0 software (MicroCal). The binding association constant (Ka) for the interaction was calculated as previously described (i.e., the melting temperature of the complex is higher than the melting temperatures of the individual components, taking into account the temperature shift of both transitions) (24).
Titration of FIVAR-Doc (75–150 μM) into CpGH84CX82 (10 μM) or NanJX82 (5 μM) was performed by using a VP-ITC titration calorimeter from Microcal. Twenty-five to 50 injections of 5–10 μl were made, with 240 s of equilibration between injections. Titrations were done in triplicate at the following temperatures: 30°C, 35°C, and 37°C. The changes in enthalpy (ΔH) at these temperatures were determined manually from the total integrated heat generated in the experiment (corrected for heats of dilution), normalized to the amount of complex formed. Fitting a bimolecular binding model to the data gave ΔH values that were in excellent agreement with the determined values (not shown). A change in heat capacity (ΔCp) was determined to be −143.5 ± 11.10 cal·mol−1·K−1 as calculated from the temperature dependence of ΔH. Using ΔCp and assuming its temperature independence, we extrapolated ΔH to 88.9°C, the temperature at which the association constant was determined. Using the ΔCp, ΔH (at 88.9°C), and Ka (at 88.9°C) as references, we determined the association constant at 37°C with the integrated form of the van't Hoff equation (25).
Crystallization.
Using the vapor diffusion hanging drop method at 21°C, we obtained crystals of the native FIVAR-Doc–CpGH84CX82 complex at 25 mg/ml in 20% (wt/vol) PEG 2000; 0.1 M sodium acetate, pH 4.5; and 0.2 M ammonium sulfate. Crystals of the SeMet-containing complex were obtained at 25 mg/ml in 19% (wt/vol) PEG 1500 and 0.1 M sodium acetate (pH 4.5).
NanJX82 was treated overnight at room temperature with thrombin and separated from the cleaved hexa-histidine tag by size exclusion chromatography using a Sephacryl S200 column (GE Biosciences). Pure fractions were concentrated in a 10-ml stirred ultra-filtration Amicon device using a 5-kDa MWCO membrane to 15 mg/ml for crystallization. Crystals of native NanJX82 were obtained in 20% (wt/vol) PEG 4000; 0.2 M ammonium acetate; and 0.1 M sodium acetate, pH 4.6 by using the hanging drop vapor diffusion method at 18°C.
Data Collection and Refinement.
Data for the native (wild-type, unlabeled) and SeMet-containing (mutant) FIVAR-Doc–CpGH84CX82 complex crystals were collected at the National Synchrotron Light Source (NSLS). The crystals were flash-cooled in a cryostream of N2 gas at 100 K by using well solutions supplemented with 20% (vol/vol) glycerol (native) and 20% (vol/vol) PEG 400 (SeMet) as cryoprotectant. Processing of the FIVAR-Doc–CpGH84CX82 diffraction data was performed with HKL2000 (26). Diffraction data for the selenium substituted FIVAR-Doc–CpGH84CX82 mutant were collected on beamline X6A at a wavelength optimized for f′′ at the selenium edge. This structure was solved by SAD using SOLVE (27) to determine selenium substructure (five atoms were found in the asymmetric unit) and RESOLVE (27) for density modification and initial structure tracing. This initial model was used as input into ARP/wARP (28) with the native FIVAR-Doc–CpGH84CX82 diffraction data collected on beamline X8C. ARP/wARP was able to build a virtually complete model that was completed by manual building.
Diffraction data for native NanJX82 were collected on a home source Rigaku R-AXIS 4++ area detector coupled to a MM-002 x-ray generator with Osmic “blue” optics and an Oxford Cryostream 700. These crystals were flash-cooled in the cryo-stream at 113 K after protection with 20% (vol/vol) ethylene glycol. The NanJX82 diffraction data were processed with Crystal Clear/d*TREK (29). This structure was solved by molecular replacement by using the CpGH84CX82 coordinates as a search model and MOLREP (30) to find the single molecule in the asymmetric unit. After minor truncation and manual correction of the initial model, ARP/wARP was able to build a virtually complete model that required minimal alteration.
All computing was done with CCP4 (31) unless otherwise stated. Manual graphical model building was performed with COOT (32), and refinements were done with REFMAC (33). Water molecules were added to all models by using the REFMAC implementation of ARP/wARP and were inspected visually before deposition. In all data sets, 5% of the observations were flagged as “free” (34) and used to monitor refinement procedures. The structure comprises residues 774–906 of CpGH84C, residues 1498–1628 of the μ-toxin, 315 water molecules, two Ca2+ ions, and a chloride ion. All final model statistics are given in Table S3. All structural representations were generated in PyMOL (35).
Supplementary Material
Acknowledgments.
We thank Kim Munro (Protein Function Discovery Facility, Queen's University) for technical assistance and Dr. Yoav Barak (Weizmann Institute) for help with the ELISA studies. We thank the staff members of beamline X6A at the National Synchrotron Light Source for beam time and assistance. This work was supported by Canadian Institutes of Health Research operating grants (to S.P.S. and A.B.B.) and an Israel Science Foundation grant (to E.A.B.). J.J.A. was supported by a Natural Sciences and Engineering Research Council of Canada Doctoral Scholarship. K.G. is a University of Victoria Graduate Fellowship awardee. E.A.B. holds The Maynard I. and Elaine Wishner Chair of Bio-organic Chemistry. A.B.B. is a Canada Research Chair in Molecular Interactions and a Michael Smith Foundation for Health Research Scholar. S.P.S. is a Canadian Institutes of Health Research New Investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org [PDB ID codes codes 2OZN (CpGH84CX82-FIVAR-Doc complex) and 2VO8 (NanJX82)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0803154105/DCSupplemental.
References
- 1.Rood JI, Cole ST. Molecular genetics and pathogenesis of. Clostridium perfringens. Microbiol Rev. 1991;55:621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petit L, Gibert M, Popoff MR. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/s0966-842x(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 3.McDonel JL. Clostridium perfringens toxins (type A, B, C, D, E) Pharmacol Ther. 1980;10:617–655. doi: 10.1016/0163-7258(80)90031-5. [DOI] [PubMed] [Google Scholar]
- 4.Myers GS, et al. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 2006;16:1031–1040. doi: 10.1101/gr.5238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu T, et al. Complete genome sequence of. Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci USA. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol. 1997;7:637–644. doi: 10.1016/s0959-440x(97)80072-3. [DOI] [PubMed] [Google Scholar]
- 7.Canard B, Garnier T, Saint-Joanis B, Cole ST. Molecular genetic analysis of the nagH gene encoding a hyaluronidase of Clostridium perfringens. Mol Gen Genet. 1994;243:215–224. doi: 10.1007/BF00280319. [DOI] [PubMed] [Google Scholar]
- 8.Traving C, Schauer R, Roggentin P. Gene structure of the ‘large’ sialidase isoenzyme from Clostridium perfringens A99 and its relationship with other clostridial nanH proteins. Glycoconj J. 1994;11:141–151. doi: 10.1007/BF00731154. [DOI] [PubMed] [Google Scholar]
- 9.Boraston AB, Ficko-Blean E, Healey M. Carbohydrate recognition by a large sialidase toxin from Clostridium perfringens. Biochemistry. 2007;46:11352–11360. doi: 10.1021/bi701317g. [DOI] [PubMed] [Google Scholar]
- 10.Ficko-Blean E, Boraston AB. The interaction of carbohydrate-binding module from a Clostridium perfringens N-acetyl-beta-hexosaminidase with its carbohydrate receptor. J Biol Chem. 2006;281:37748–37757. doi: 10.1074/jbc.M606126200. [DOI] [PubMed] [Google Scholar]
- 11.Chitayat S, Adams JJ, Smith SP. NMR assignment of backbone and side chain resonances for a dockerin-containing C-terminal fragment from the μ-toxin of Clostridium perfringens. Biomol NMR Assign. 2007;1:13–15. doi: 10.1007/s12104-007-9002-7. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert HJ. Cellulosomes: Microbial nanomachines that display plasticity in quaternary structure. Mol Microbiol. 2007;63:1568–1576. doi: 10.1111/j.1365-2958.2007.05640.x. [DOI] [PubMed] [Google Scholar]
- 13.Adams JJ, Pal G, Jia Z, Smith SP. Mechanism of bacterial cell-surface attachment revealed by the structure of cellulosomal type II cohesin-dockerin complex. Proc Natl Acad Sci USA. 2006;103:305–310. doi: 10.1073/pnas.0507109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayer EA, Belaich JP, Shoham Y, Lamed R. The cellulosomes: Multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol. 2004;58:521–554. doi: 10.1146/annurev.micro.57.030502.091022. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho AL, et al. Evidence for a dual binding mode of dockerin modules to cohesins. Proc Natl Acad Sci USA. 2007;104:3089–3094. doi: 10.1073/pnas.0611173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho AL, et al. Cellulosome assembly revealed by the crystal structure of the cohesin-dockerin complex. Proc Natl Acad Sci USA. 2003;100:13809–13814. doi: 10.1073/pnas.1936124100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi RH, Kosugi A. Cellulosomes: Plant-cell-wall-degrading enzyme complexes. Nat Rev Microbiol. 2004;2:541–551. doi: 10.1038/nrmicro925. [DOI] [PubMed] [Google Scholar]
- 18.Chitayat S, et al. Three-dimensional structure of a putative non-cellulosomal cohesin module from a. Clostridium perfringens family 84 glycoside hydrolase. J Mol Biol. 2007;375:20–28. doi: 10.1016/j.jmb.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Barak Y, et al. Matching fusion protein systems for affinity analysis of two interacting families of proteins: The cohesin-dockerin interaction. J Mol Recognit. 2005;18:491–501. doi: 10.1002/jmr.749. [DOI] [PubMed] [Google Scholar]
- 20.Gregg KJ, Finn R, Abbott DW, Boraston AB. Divergent modes of glycan recognition by a new family of carbohydrate-binding modules. J Biol Chem. 2008;283:12604–12613. doi: 10.1074/jbc.M709865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitayat S, et al. NMR assignment of backbone and side chain resonances for a putative protein-protein interaction module from a family 84 glycoside hydrolase of. Clostridium perfringens. Biomol NMR Assign. 2007;1:7–9. doi: 10.1007/s12104-007-9001-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Malcolm BA. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. BioTechniques. 1999;26:680–682. doi: 10.2144/99264st03. [DOI] [PubMed] [Google Scholar]
- 24.Brandts JF, Lin LN. Study of strong to ultra-tight protein interactions using differential scanning calorimetry. Biochemistry. 1990;29:6927–6940. doi: 10.1021/bi00481a024. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Sturtevant JM. Significant discrepancies between van't Hoff and calorimetric enthalpies. II. Protein Sci. 1995;4:2559–2561. doi: 10.1002/pro.5560041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 27.Terwilliger TC. SOLVE and RESOLVE: Automated structure solution and density modification. Methods Enzymol. 2003;374:22–37. doi: 10.1016/S0076-6879(03)74002-6. [DOI] [PubMed] [Google Scholar]
- 28.Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nat Struct Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 29.Pflugrath JW. The finer things in X-ray diffraction data collection. Acta Crystallogr D Biol Crystallogr. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 30.Vagin A, Teplyakov A. An approach to multi-copy search in molecular replacement. Acta Crystallogr D Biol Crystallogr. 2000;56:1622–1624. doi: 10.1107/s0907444900013780. [DOI] [PubMed] [Google Scholar]
- 31.Collaborative Computational Project N, author. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 32.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 33.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 34.Brunger AT. Free R value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature. 1992;355:472–475. doi: 10.1038/355472a0. [DOI] [PubMed] [Google Scholar]
- 35.DeLano W. The PyMOL Molecular Graphics System. Palo Alto, CA: DeLano Scientific; 2002. [Google Scholar]
- 36.Carvalho AL, et al. Insights into the structural determinants of cohesin-dockerin specificity revealed by the crystal structure of the type II cohesin from Clostridium thermocellum SdbA. J Mol Biol. 2005;349:909–915. doi: 10.1016/j.jmb.2005.04.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.