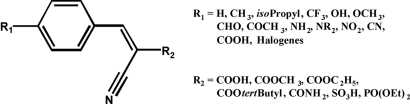Abstract
Matrix-assisted laser desorption ionization (MALDI) has become an enabling technology for the fields of protein mass spectrometry (MS) and proteomics. Despite its widespread use, for example, in protein identification via peptide mass fingerprinting, a comprehensive model for the generation of free gas-phase ions has not yet been developed. All matrices in use today, such as α-cyano-4-hydroxycinnamic acid (CHCA), have been found empirically and stem from the early days of MALDI. By systematic and targeted variation of the functional groups of the α-cyanocinnamic acid core unit, 4-chloro-α-cyanocinnamic acid (Cl-CCA) was selected and synthesized, and it exhibited outstanding matrix properties. Key features are a substantial increase in sensitivity and a considerably enhanced peptide recovery in proteomic analyses because of a much more uniform response to peptides of different basicity. Using Cl-CCA as a matrix for a 1 fmol bovine serum albumin (BSA) in-solution digest, the sequence coverage is raised to 48%, compared with 4% for CHCA. For a gel band containing 25 fmol of BSA, unambiguous protein identification becomes possible with Cl-CCA. These findings also imply ion formation via a chemical ionization mechanism with proton transfer from a reactive protonated matrix species to the peptide analytes. The considerable increase in performance promises to have a strong impact on future analytical applications of MALDI, because current sensitivity limits are overcome and more comprehensive analyses come into reach.
Keywords: ionization mechanism, proton transfer
Matrix-assisted laser desorption ionization (MALDI) (1–3) relies on a cocrystallization of the analytes of interest with an excess of small organic compounds, the matrix, which is accomplished by either simply drying ≈ 1-μl droplet of a solution containing matrix and analyte (dried-droplet preparation) (2, 3) or by depositing a droplet of the analyte solution onto a prespotted matrix layer (surface preparation) (4). The matrix exhibits a strong absorption at the laser wavelength used (typically 337 nm or 355 nm in the UV), which, after irradiation with a short pulse of laser light, leads to the ablation of a shallow surface region into the vacuum of the mass spectrometer, the release of intact gaseous matrix and analyte molecules, and their partial ionization. Since its introduction in the 1980s, MALDI has found widespread use in the mass spectrometric analysis of biological macromolecules but recently also has been used increasingly for small-molecule analysis (5, 6).
However, the main field of application of MALDI mass spectrometry (MS) today is protein identification in proteomic schemes by using the peptide mass fingerprint (PMF) approach (7), often supplemented by MALDI-time-of-flight/time-of-flight (TOF/TOF) analyses. α-Cyano-4-hydroxycinnamic acid (CHCA) (8) as a matrix (in positive-ion mode) allows the researcher to identify proteins via MS or MS/MS analysis of their proteolytic peptide products (typically generated by trypsin) and by using protein or DNA sequence databases and dedicated search engines. This is accomplished directly from bands or spots of one- or two-dimensional (1D or 2D) sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE) without laborious sample cleanup by harnessing the relatively high tolerance of the MALDI technique for ubiquitous contaminants like salts and buffers. The conceptual simplicity of the technique lends itself to robotic sample preparation and to high-throughput analyses, in particular when combined with MALDI-TOF-type instruments. Detection limits strongly depend on the protein, but even in easy cases they are reached at the ≈50 fmol level. On the one hand, mass spectrometric instruments have constantly evolved over the last 15 years. However, despite numerous efforts to overcome apparent limitations of MALDI, such as the use of ionic liquids for spot heterogeneity (9) and matrix-free methods to avoid problems in the analysis of low mass analytes because of intense interfering matrix signals (10), CHCA used in dried-droplet or surface preparation mode still provides the “gold standard” for analytical proteomics by MALDI-MS. A major drawback of CHCA-MALDI is its pronounced preference for strongly basic arginine-containing peptides (11) and the concomitant suppression of small acidic peptides, which limits the sequence coverage and thus impedes the identification of scarce proteins.
Results and Discussion
The rationale of the approach followed here was to synthesize various matrix candidates by modifying the α-cyanocinnamic acid (CCA) core unit (Fig. 1) in the R1- and R2-positions. Each compound was inspected with regard to its mass spectrum and to its performance in a peptide mass fingerprinting experiment.
Fig. 1.
CCA core and different substituents of the synthesized CHCA derivatives.
The results were as follows: (i) The free carboxylic function is essential for matrix performance in MALDI. It is hypothesized that this function is crucial for peptide incorporation into the matrix crystal lattice via ion-pair formation with positively charged peptide functional groups. (ii) All CCA derivatives exhibiting good matrix properties in the positive-ion mode show abundant ion signals of the protonated matrix. This abundance of pronated matrix ion signals points out their crucial role as chemical ionization reagents in the MALDI process via proton transfer to the analyte and to the important role of the gas-phase proton-affinity.
As a consequence of the above results, 4-chloro-α-cyanocinnamic acid (Cl-CCA) was synthesized because of its suspected lower proton affinity, and, indeed, a dramatic qualitative and quantitative improvement in MALDI performance was revealed.
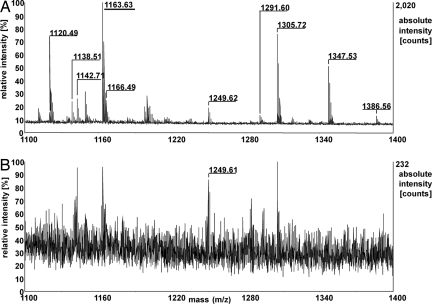
The strong enhancement in MALDI performance is depicted for a bovine serum albumin (BSA) in-solution trypsin digest, which is a widely used reference sample. Fig. 2 shows the section of the m/z range 1,100–1,400 Da [the complete spectrum from 500 to 3,500 Da is shown in supporting information (SI) Fig. S1] for a total sample load of 1 fmol of BSA for Cl-CCA and CHCA (Fig. S1). Absolute peptide-ion intensities and signal-to-noise (S/N) ratios increase strongly when using Cl-CCA instead of CHCA; ion intensities increase by a factor of 2–75, and S/N ratios increase by an average factor of 22 for common peptides, with the only exception being the peptide RHPEYAVSVLLR. Besides the increase in ion intensities, the mass spectra change qualitatively by the appearance of substantially more peptide signals, many of which represent small and/or acidic peptides, including the C-terminal peptide LVVSTQTALA [MW = 1,001.6 Da, isoelectric point (IEP) = 5.52; IEP values were calculated with the Compute pI/Mw tool without consideration of posttranslational modifications (www.expasy.ch/tools/pi_tool.html)] (see Fig. S1 and Table S1). For three independent experiments for each matrix applying stringent selection criteria (internal calibration and restriction to consistently observed signals within a mass tolerance of ± 20 ppm for CHCA and ± 10 ppm for Cl-CCA), only seven tryptic peptides were detected for the CHCA matrix as opposed to 34 tryptic peptides for the Cl-CCA matrix (without consideration of cystines). The sequence coverage for the 1 fmol sample was thus raised to 48% compared with 12% for CHCA [Table 1 (total number of detected peptides and sequence coverage) and Tables S1 and S2 (detected peptides with Cl-CCA and CHCA, respectively)]. For a sample containing 10 fmol of BSA digest, the sequence coverage was 40% in the case of CHCA, compared with 75% for Cl-CCA.
Fig. 2.
MALDI mass spectra of a tryptic in-solution digest of BSA using Cl-CCA (A) or CHCA (B) as a matrix. Mass numbers of assigned BSA peptides are given only for those signals occurring in three independent mass spectra within ± 20 ppm; m/z section 1,100–1,400 Da; total sample load, 1 fmol.
Table 1.
Detectable peptides and sequence coverage referring to unprocessed BSA
| Matrix | Min 1 of 3 spectra error <80 ppm, peptides (% coverage) | Min 2 of 3 spectra error <40 ppm, peptides (% coverage) | All 3 spectra error <30 ppm, peptides (% coverage) | All 3 spectra error <20 ppm, peptides (% coverage) | All 3 spectra error <15 ppm, peptides (% coverage) | All 3 spectra error <10 ppm, peptides (% coverage) |
|---|---|---|---|---|---|---|
| Cl-CCA | 52 (63) | 47 (59) | 40 (52) | 40 (52) | 40 (52) | 34 (48) |
| CHCA | 15 (23) | 10 (15) | 7 (12) | 7 (12) | 4 (7) | 2 (4) |
Because quantitative data on the preference for arginine peptides or the suppression of lysine-containing and acidic peptides cannot be deduced from a BSA digest, this issue was addressed in a further experiment. Three synthesized “tandem” peptides with the amino acid sequences ITITPNRITITPNK (I), ITITPNRITITPNV (II), and ITITPNKITITPNV (III) were each subjected to trypsin digestion, which resulted in three binary mixtures of equimolar concentration of the two cleavage peptides. Fig. 3 shows a comparison of the MALDI mass spectra obtained for peptide (II) directly out of the reaction vial after dilution to 1 μM, using Cl-CCA (Fig. 3A) and CHCA (Fig. 3B) matrices. Although the signal of the peptide ITITPNV is at the detection limit (S/N ratio of 2) in the case of CHCA, its relative abundance is 34% compared with the ITITPNR signal for the Cl-CCA matrix (see Fig. 3 magnifications). Summarizing all three experiments, the signal-intensity ratio ITITPNR:ITITPNK:ITITPNV amounts to 100:8.4:0.22 for CHCA and to 100:56:34 for the Cl-CCA matrix.
Fig. 3.
MALDI mass spectra obtained of a tryptic in-solution digest of ITITPNRITITPNV using Cl-CCA (A) or CHCA (B) as a matrix. Total peptide load: 1 pmol; magnifications by a factor of 3 (A) and 460 (B) of the quasimolecular ion region of [ITITPNV+H]+ are shown
The much lower discrimination effects for peptides were also corroborated in the analysis of phosphopeptides. An in-solution trypsin digestion of β-casein (containing <10% α-casein) served as an example. Samples of digested protein (50 fmol) were spotted with CHCA and Cl-CCA respectively. Analyzing the sum spectra of five independent mass spectra consisting of 500 shots each, only two phosphorylated α-/β-casein peptides with an average intensity of 57 absolute counts and an average S/N ratio of 15 (monoisotopic peptide signals) could be detected using CHCA. In the case of Cl-CCA, eight phosphorylated α-/β-casein peptides were detected with an average intensity of 975 absolute counts and an average S/N ratio of 131 (monoisotopic peptide signals) (Table S3 and Fig. S2).
In a further set of experiments, the two matrices, CHCA and Cl-CCA, were inspected with regard to their dynamic range of peptide detection by adding an increasing molar excess (100- to 10,000-fold) of the strongly basic peptide bradykinin (RPPGFSPFR) to a 10 fmol/μl BSA digest. Again, Cl-CCA proved to be highly superior, because even at a 1,000-fold excess of bradykinin, 30 BSA peptides with an average S/N ratio of 78 could be detected. For the identical sample, only seven BSA peptides were detected with CHCA, with an average S/N ratio of only 30 (Fig. S3).
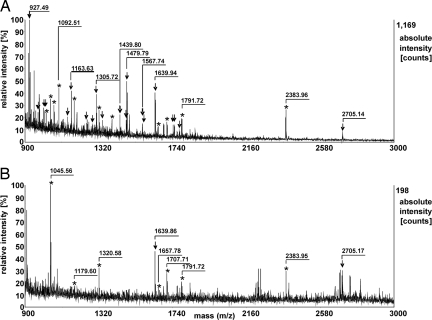
Finally, the strong superiority of the Cl-CCA matrix was also demonstrated for PMFs from a tryptic in-gel digestion of BSA out of a 25-fmol BSA 1D-SDS-PAGE band. Fifty percent of the sample was spotted for MS measurements for each matrix. Fig. 4 shows the mass spectra obtained with Cl-CCA (Fig. 4A) and CHCA (Fig. 4B). Within the same stringent criteria as applied to the in-solution digests, no BSA peptides were detected after internal calibration with CHCA in the mass range from 500 to 3,000 Da within an error of ± 30 ppm. In the case of Cl-CCA, 17 tryptic BSA peptides were detected under identical conditions, which resulted in a sequence coverage of 25% [Table 2 (total number of detected peptides and sequence coverage), Fig S4 (mass spectra using CHCA or Cl-CCA), and Table S4 (detected peptides and keratines with CHCA and Cl-CCA, respectively)]. Peptide mass fingerprinting by using the MOWSE scoring algorithm (12) and Mascot as a search engine (database, SwissProt 54.5; organism, other mammals; peptide mass tolerance, 50 ppm) still allowed for confident protein identification with a significant Mascot Score of 77 (significance threshold: 67) and 48% sequence coverage for Cl-CCA; however, CHCA identification of BSA failed. With respect to sequence coverage, the simple and fast PMF protocol using MALDI and the Cl-CCA matrix yielded comparable results to those reported for a nano-high-performance liquid chromatography/electrospray ionization scheme (13) and, thus, promises to extend the working range and depth of analysis in future proteomic studies.
Fig. 4.
MALDI mass spectra from a tryptic in-gel digest of BSA (25 fmol loaded onto the gel) using Cl-CCA (A) or CHCA (B) as a matrix. BSA peptides are marked with arrows, and keratin and trypsin self-digestion peptides are marked with asterisks.
Table 2.
Detectable peptides and sequence coverage referring to unprocessed BSA
| Matrix | Min 1 of 3 spectra error <80 ppm, peptides (% coverage) | Min 2 of 3 spectra error <40 ppm, peptides (% coverage) | All 3 spectra error <30 ppm, peptides (% coverage) | All 3 spectra error <20 ppm, peptides (% coverage) | All 3 spectra error <15 ppm, peptides (% coverage) | All 3 spectra error <10 ppm, peptides (% coverage) |
|---|---|---|---|---|---|---|
| Cl-CCA | 35 (46) | 24 (35) | 17 (25) | 14 (22) | 9 (14) | 5 (7) |
| CHCA | 6 (14) | – (0) | – (0) | – (0) | – (0) | – (0) |
The effects detected—a strong enhancement in signal intensities, a much more uniform response to peptides irrespective of their amino acid composition, and the enhanced dynamic range—can be explained by a strongly enhanced analyte ion yield achieved with the Cl-CCA matrix. Even though the final ion yield is the result of a complex interplay of analyte incorporation into the matrix crystals, ionization and cluster decay processes, ion stability, and, thus, survival, a chemical ionization process occurring in the ablated material plume forms the most widely used ionization model. Within this approach, it becomes possible to describe the analyte ion yield and phenomena such as matrix and analyte suppression, analyte concentration effects, and the formation of multiply charged analyte ions (2, 14–16). It is assumed that analyte neutrals are ionized by proton transfer from protonated matrix ions, and thus, the role of matrix proton affinity (PA) was investigated (17). PA values have been determined for all common matrices; indeed, all well working matrices exhibit relatively low PA values, with 841 kJ/mol for CHCA being the lowest. Using Cook's kinetic method (18, 19), we determined a PA of 832 kJ/mol for Cl-CCA by investigating the fragmentation of the CHCA–H+–Cl-CCA mixed dimer and applying the literature value for CHCA of 841 kJ/mol (17). The lower PA of Cl-CCA can be rationalized as follows: For both CHCA and Cl-CCA, the proton will be located either at the carboxylic or at the cyano group. In both cases, the positive charge of [CHCA+H]+ can be delocalized over the whole conjugated Π-electron system promoted by the electron-donating effect of the 4-OH group. This stabilization of the protonated matrix molecule results in a larger PA. The charge delocalization is much less pronounced in the case of a 4-Cl functional group. This is substantiated by density functional theory (DFT) electron-density calculations with the hybrid B3LYP method and the 6–311G** basis set for the protonated Cl-CCA and CHCA molecules (Fig. S5) exhibiting a loss of net positive charge in the para position for Cl-CCA because of the strong electron-withdrawing chloro substituent. A further experiment visualized the differences between CHCA and Cl-CCA: Both matrices were mixed in different ratios; even at a mixing ratio of CHCA:Cl-CCA of 1:9, the mass spectrum is clearly dominated by CHCA signals ([CHCA+H]+:[Cl-CCA+H]+ = 75) (see Fig. S6). The higher acidity of the [Cl-CCA+H]+ ion species, or the lower PA of Cl-CCA, respectively, results in an efficient proton transfer to CHCA, resulting in low [Cl-CCA+H]+ and high [CHCA+H]+ ion intensities. It is thus hypothesized that a more efficient proton transfer from [Cl-CCA+H]+ matrix ions to analytes because of the lower matrix PA also accounts for the enhanced protonation of low basicity analytes and therefore effectuates a more uniform protonation within a peptide mixture.
To a first view, it may appear surprising that already a small reduction in the PA can account for the strong effects observed. PAs of the basic functional groups of peptides are substantially larger than those of all matrices in use today for peptide or protein analysis. Therefore, a strong preference for protonated peptide analyte ions would result for chemical reactions under chemical equilibrium. Minor differences in matrix PAs are indeed expected to vanish over a longer time scale. However, for the first few tens of nanoseconds, while the solid matrix-analyte crystals are explosively ablated and expanding into the vacuum of the mass spectrometer, numerous collisions between analyte and matrix neutrals and ions occur. Peptides will not be protonated directly at their most basic site(s) yielding a low-energy stable product, but most probably at low-PA amide bonds. Numerous additional collisions of an initially ionized peptide with matrix neutrals exhibit a certain probability for the reneutralization by the backward reaction. The final ion yield is thus determined both by proton transfer ionization efficiency and by analyte ion survival, and, therefore, the difference of ≈9 kJ/mol does have a large effect.
Attempts to further decrease the PA of the matrix by more or stronger electron-withdrawing substituents failed because of the induced hypsochromic shifts and the reduction of the absorption at the wavelength of 337 nm used for MALDI. Also, the absorption spectrum of Cl-CCA exhibits a strong hypsochromic shift compared with the one of CHCA; therefore, the shown strong increase in performance is only achievable in 337-nm instruments (CHCA: ε(337 nm) = 17.600, ε(355 nm) = 7.100; Cl-CCA: ε(337 nm) = 3.580, ε(355 nm) = 70) (Fig. S7). The replacement of the carboxylic group by sulfonic or phosphonic acids failed for the same reason. Vice versa, the introduction of additional functional groups to yield a bathochromic shift was not successful, because in these cases an undesired stabilization of the protonated matrix species and thus enhancement of the PA was promoted at the same time. Moreover, a second important property of a high-performance matrix lies in the formation of compact crystals, which is not predictable. This allows one to use low matrix concentrations and results in the accumulation of analytes in these matrix crystals. Already minor chemical changes, such as the addition of a methyl group to the aromatic ring, may totally change the crystallization. Compared with other halogenated cyanocinnamic acid derivatives, only Cl-CCA has this beneficial property.
Materials and Methods
Materials and Reagents.
All chemicals were purchased from Sigma Aldrich and were analytical grade, except modified trypsin, which was proteomics grade, and when otherwise noted. The in-solution BSA digest (Starter Kit for MALDI-TOF MS, Part-No. 208241, Lot 2004–208241-001) and CHCA (Part-No. 201344, Lot 2007–201344-001) were purchased from Bruker Daltonics. The strong cation exchanger was from Merck (Ionenaustauscher I, pro analysis); vanillin (99%), 2-chloro-4-fluorobenzaldehyde (97%), and methanol (HPLC-grade) were from Acros. Acetonitrile (ACN) was Rotisolv HPLC-Grade and purchased from Roth, and 4-fluoro-3-methoxybenzaldehyde (98%) was acquired from ABCR. MilliQ water was prepared freshly in-house. The synthetic peptides ITITPNRITITPNK, ITITPNKITITPNV, and ITITPNRITITPNV were obtained from R. Pipkorn [Central Peptide Synthesis Unit, German Cancer Research Center (DKFZ)].
Synthesis of Substituted CCAs.
All CCA derivatives were synthesized by a standard Knoevenagel condensation using cyanoacetic acid or its derivatives and substituted benzaldehydes. Ammonium acetate was used as a catalyst. In a typical approach, 2 g of cyanoacetic acid (1 eq), 0.9 eq of the benzaldehyde, and 0.15 eq of ammonium acetate were refluxed with stirring in sufficient amounts of toluene (≈50 ml) for complete dissolving. After quantitative separation of the reaction water by a water separator (≈3 h), the reaction mixture was cooled to ≈50°C and filtered. The crude product was washed with sufficient amounts of distilled water and purified by repeated recrystallization from methanol/water (1:1). Cl-CCA was purified further by using cation-exchange columns followed by additional recrystallization from ACN/water. Overall yields were between 70 and 90%. 1H NMR (300 MHz, DMSO-d6) δ 7.66 (d, 2H, J = 9.4 Hz), 8.02 (d, 2H, J = 9.8 Hz), 8.33 (s, 1H). Accurate mass determination: (M+H)+ 208.01718 ± 0.00030 Da (error: 5.7 ± 1.5 ppm), Calc. 208.01598 Da.
One-Dimensional SDS-PAGE Electrophoresis.
One-dimensional gel electrophoresis was performed by using standard methods (20) on the Bio-Rad MiniProtean 3 Cell system (8 × 7.3 cm minigels). Ten-percent acrylamide gels of 0.75-mm thickness were used. BSA was dissolved in sample buffer to a final concentration of 25 fmol/15 μl, loaded into the wells, and subjected to electrophoresis using 50 mA per slab for 1–2 h. Gels were subsequently fixed and silver stained by using standard methods (21).
In-Gel Digestion.
Protein bands obtained after the application of 25 fmol of BSA to a 1D gel were faintly visible after silver staining. After in-gel digestion overnight at 37°C with trypsin in 20 μl of 10 mM aqueous ammonium hydrogencarbonate buffer, according to standard procedures (22), the combined extraction solutions were dried at 40°C and 35 mbar.
In-Solution Digestion.
Tryptic in-solution digestion of β-casein from bovine milk (≥90% β-casein) was performed by using standard conditions with 25 mM ammonium bicarbonate buffer solution.
Digestion of Synthetic Peptides ITITPNRITITPNK, ITITPNRITITPNV, and ITITPNKITITPNV.
A 10-μl sample of each peptide solution (1 mM) was treated with 9 μl of aqueous ammonium hydrogencarbonate (50 mM) and 1 μl of trypsin-solution (1 μg/μl) and digested overnight at room temperature. The resulting stock solution (500 pmol of digested peptides per μl) was diluted with 30% ACN/0.1% trifluoroacetic acid (TFA) to a final concentration of 1 μM and directly used for MALDI analysis.
Sample Preparation and Measurement.
A mixture of 0.5 μl of matrix solution (30 mM, 70% ACN) and 1 μl of BSA in-solution digest (diluted to 1 nM, 30% ACN, 0.01% TFA), 1 μl of the synthetic peptide digest, or 1 μl of the β-casein in-solution digest (diluted to 50 nM, 30% ACN, 0.1% TFA) were spotted without premixing onto a polished stainless steel target and air dried.
The 25 fmol/band BSA in-gel digest sample was redissolved in 4 μl of 30% ACN/0.01% TFA water. Then 2 μl of the analyte solution was spotted onto a polished steel target and mixed directly onto the plate, either with 0.5 μl of 30 mM CHCA or Cl-CCA in 70/30 ACN/1.5% TFA water. Neither CHCA nor Cl-CCA crystals were washed after air drying. NH4H2PO4 (aqueous, V = 1 μl, c = 10 mM) was added for suppression of matrix cluster ions (23).
Mass Spectrometry.
All MS measurements were carried out with an Applied Biosystems Voyager DE-STR TOF mass spectrometer with a 337-nm nitrogen laser at laser fluences optimized for the highest analyte S/N ratio. Mass spectra shown for BSA digests were accumulated from 500 single laser shots. Mass spectra shown for the β-casein digest were accumulated from five single mass spectra with 500 laser shots each. Data Explorer 4.5C was used for analysis of the spectra. Peak detection of phosphopeptides was performed by using a S/N threshold of 10 after internal calibration and advanced baseline correction with preset parameters.
Intensity ratios for cleaved tandem peptides are the average of six measurements with 500 laser shots per mass spectrum. MALDI-TOF parameters are polarity, positive; operation mode, reflector; accelerating voltage, 20 kV; grid voltage, 68.5%; delay time, 150 ns; shots per spectrum, 500; mass range, 500–3,500 Da; low mass gate, 450 Da; bin size, 0.5 ns; minimum S/N ratio for peptide detection of in-solution digested BSA, 4 [in the case of in-gel digestion samples S/N > 35 (800–1,000 Da), 15 (1,000–1,300 Da), 12 (1,300–1,500 Da), 8 (1,500–3,000 Da)]. Accurate mass determination of [Cl-CCA+H]+ was performed with a mixture of Cl-CCA and CHCA at a ratio of 1:100 (n/n) by using a Voyager DE-STR. The mass of [Cl-CCA+H]+ was calculated as the average value of eight measurements with 500 shots each. Ion peaks of CHCA*+ and [2CHCA+H]+ were used as internal calibrants.
PA Determination of Cl-CCA.
Determination of the PA value of Cl-CCA was carried out by postsource-decay fragmentation (24) of the proton-bound heterodimers (17, 19, 25) of CHCA and Cl-CCA by using different fluences, followed by a measurement of the peak area ratios of the resulting protonated matrix monomers (Fig. S8). The laser-fluence-dependent effective temperature, Teff, was detected by using the thermometer molecule N-p-methoxybenzylpyridinium (M+) chloride, and the MS/MS measurements were recorded at the same fluence (Fig. S9). Thermometer molecules yield only one intense fragment F+ and enable a correlation between the experimental survival yields SY = I(M+)/I(M+) + I(F+) and Teff by calculated dissociation rate constants from Rice-Ramsperger-Kassel-Marcus models (26, 27) (Fig. S10). To eliminate temperature influences caused by CHCA, the Cl-CCA:CHCA ratio was chosen at 10,000:1 (n/n), which was the highest possible ratio with the formation of intense heterodimer ions. By using CHCA as a reference base with a known PA, determination of the PA of Cl-CCA was possible by the simplified expression of the kinetic method:
 |
The chosen parameters for the MS/MS measurements were as follows: laser fluence, 2,200–2,800 units, step 100; polarity, positive; operation mode, reflector; accelerating voltage, 20 kV; grid voltage, 68.5%; delay time, 150 ns; shots per segment, 500; mirror ratios, 1, 0.6, and 0.45; spectrum processing, Gaussian smooth (25 points). The mirror ratios for the segment spectra were chosen in such a way that the protonated matrix monomers were recorded together in one segment.
The effective temperatures and the correlating matrix areas for the different laser fluences used are listed in Table S5.
Computational Methodology.
Visualization and initial building were done by using ChemDraw Pro 10.0 and Chem3D Ultra 10.0 running on a commercial PC system (C2D E6600, 2GB RAM). Initial geometries of the neutral and protonated compounds were constructed and optimized energetically by a slightly modified Allinger MM2 force field with a root-mean-square gradient of 0.1. For further optimization of the geometries, DFT calculations using the hybrid B3LYP method and 6–311G** basis set (28) were performed with the Gaussian03 implementation of DFT.
Supplementary Material
Footnotes
Conflict of interest statement: The invention of Cl-CCA as a MALDI matrix was filed as a German patent. The principle of tandem peptides was filed as a European patent.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803056105/DCSupplemental.
References
- 1.Karas M, Bachmann D, Hillenkamp F. The influence of the wavelength in high irradiance ultraviolet laser desorption mass spectrometry of organic molecules. Anal Chem. 1985;57:2935–2939. [Google Scholar]
- 2.Karas M, Bachmann D, Bahr U, Hillenkamp F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int J Mass Spectrom Ion Proc. 1987;78:53–56. [Google Scholar]
- 3.Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988;60:2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- 4.Vorm O, Roepstorff P, Mann M. Improved resolution and very high sensitivity in MALDI TOF of matrix surfaces made by fast evaporation. Anal Chem. 1994;66:3281–3287. [Google Scholar]
- 5.Sleno L, Volmer DA. Some fundamental and technical aspects of the quantitative analysis of pharmaceutical drugs by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1928–1936. doi: 10.1002/rcm.2006. [DOI] [PubMed] [Google Scholar]
- 6.Kovarik P, Grivet C, Bourgogne E, Hopfgartner G. Method development aspects for the quantitation of pharmaceutical compounds in human plasma with a matrix-assisted laser desorption/ionization source in the multiple reaction monitoring mode. Rapid Commun Mass Spectrom. 2007;21:911–919. doi: 10.1002/rcm.2912. [DOI] [PubMed] [Google Scholar]
- 7.Henzel WJ, Watanabe C, Stults JT. Protein identification: The origins of peptide mass fingerprinting. J Am Soc Mass Spectrom. 2003;14:931–942. doi: 10.1016/S1044-0305(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 8.Beavis RC, Chaudhary T, Chait BT. α-Cyano-4-hydroxycinnamic acid as a matrix for matrix-assisted laser desorption mass spectrometry. Org Mass Spectrom. 1992;27:156–158. [Google Scholar]
- 9.Tholey A, Heinzle E. Ionic (liquid) matrices for matrix-assisted laser desorption/ionization mass spectrometry—Applications and perspectives. Anal Bioanal Chem. 2006;386:24–37. doi: 10.1007/s00216-006-0600-5. [DOI] [PubMed] [Google Scholar]
- 10.Peterson DS. Matrix-free methods for laser desorption/ionization mass spectrometry. Mass Spectrometry Reviews. 2007;26:19–34. doi: 10.1002/mas.20104. [DOI] [PubMed] [Google Scholar]
- 11.Krause E, Wenschuh H, Jungblut PR. The dominance of arginine-containing peptides in MALDI-derived tryptic mass fingerprints of proteins. Anal Chem. 1999;71:4160–4165. doi: 10.1021/ac990298f. [DOI] [PubMed] [Google Scholar]
- 12.Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 13.Winkler C, Denkler K, Wortelkamp S, Sickmann A. Silver- and coomassie-staining protocols: Detection limits and compatibility with ESI-MS. Electrophoresis. 2007;28:2095–2099. doi: 10.1002/elps.200600670. [DOI] [PubMed] [Google Scholar]
- 14.Krüger R, Karas M. Ion formation in MALDI: The cluster ionization mechanism. Chem Rev. 2003;103:427–439. doi: 10.1021/cr010376a. [DOI] [PubMed] [Google Scholar]
- 15.Knochenmuss R, Zenobi R. MALDI ionization: The role of in-plume processes. Chem Rev. 2003;103:441–452. doi: 10.1021/cr0103773. [DOI] [PubMed] [Google Scholar]
- 16.Knochenmuss R. Ion formation mechanisms in UV-MALDI. Analyst. 2006;131:966–986. doi: 10.1039/b605646f. [DOI] [PubMed] [Google Scholar]
- 17.Mirza SP, Prasada RN, Vairamani M. Estimation of proton affinity values of fifteen matrix-assisted laser desorption/ionization matrices under electrospray conditions using the kinetic method. J Am Soc Mass Spectrom. 2004;15:431–453. doi: 10.1016/j.jasms.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Cooks RG, Kruger TL. Intrinsic basicity determination using metastable ions. J Am Chem Soc. 1977;99:1279–1281. [Google Scholar]
- 19.Cooks RG, Patrick JS, Kotiaho T, McLuckey SA. Thermochemical determinations by the kinetic method. Mass Spectrom Rev. 1994;13:287–339. [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 22.Shevchenko A, Tomas H, Havliš J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 23.Smirnov IP, et al. Suppression of α-cyano-4-hydroxycinnamic acid matrix clusters and reduction of chemical noise in MALDI-TOF mass spectrometry. Anal Chem. 2004;76:2958–2965. doi: 10.1021/ac035331j. [DOI] [PubMed] [Google Scholar]
- 24.Spengler B, Kirsch D, Kaufmann R, Jaeger E. Peptide sequencing by matrix assisted laser desorption mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:105–108. doi: 10.1002/rcm.1290060207. [DOI] [PubMed] [Google Scholar]
- 25.McLuckey SA, Cameron D, Cooks RG. Proton affinities from dissociations of proton-bound dimers. J Am Chem Soc. 1981;103:1313–1317. [Google Scholar]
- 26.Luo G, Marginean I, Vertes A. Internal energy of ions generated by matrix- assisted laser desorption/ionization. Anal Chem. 2002;74:6185–6190. doi: 10.1021/ac020339z. [DOI] [PubMed] [Google Scholar]
- 27.Gabelica V, Schulz E, Karas M. Internal energy build-up in matrix-assisted laser desorption/ionization. J Mass Spectrom. 2004;39:579–593. doi: 10.1002/jms.651. [DOI] [PubMed] [Google Scholar]
- 28.Wróblewski T, Ziemczonek L, Alhasan AM, Karwasz GP. Ab initio and density functional theory calculations of proton affinities for volatile organic compounds. Eur Phys J Special Topics. 2007;144:191–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.