Abstract
A major goal for developmental biologists is to define the behaviors and molecular contents of differentiating cells. We have devised a strategy for isolating cells from diverse embryonic regions and stages in the zebrafish, using computer-guided laser photoconversion of injected Kaede protein and flow cytometry. This strategy enabled us to perform a genome-wide transcriptome comparison of germ layer precursor cells. Mesendoderm and ectoderm precursors cells isolated by this method differentiated appropriately in transplantation assays. Microarray analysis of these cells reidentified known genes at least as efficiently as previously reported strategies that relied on artificial mesendoderm activation or inhibition. We also identified a large set of uncharacterized mesendoderm-enriched genes as well as ectoderm-enriched genes. Loss-of-function studies revealed that one of these genes, the MAP kinase inhibitor dusp4, is essential for early development. Embryos injected with antisense morpholino oligonucleotides that targeted Dusp4 displayed necrosis of head tissues. Marker analysis during late gastrulation revealed a specific loss of sox17, but not of other endoderm markers, and analysis at later stages revealed a loss of foregut and pancreatic endoderm. This specific loss of sox17 establishes a new class of endoderm specification defect.
Keywords: microarray, zgc:55423, gastrulation, mkp2, sox17
The establishment of germ layers and their coordinated rearrangement during gastrulation is a core developmental process (1). In organisms ranging from humans to flatworms, three primary germ layers arise: the mesoderm forms deep tissues such as blood and muscle, the endoderm produces viscera like the digestive tract and liver, and the ectoderm forms the epidermis and central nervous system. Cell lineage tracing studies have mapped the precursor cells that are fated to form these germ layers, and there are key commonalities to the fate maps of different vertebrates (2). Germ layer precursor cells are typically distributed in extended fields, making their quantitative isolation by manual microsurgery particularly challenging.
Fluorescent proteins have opened the door to new microdissection approaches in developmental biology. Placing different fluorescent proteins behind different promoters, distinct colors have been expressed in cell types comprising the nascent skin or late gastrula endoderm of developing mice (3, 4). In these studies, fluorescently labeled cells from either newborn or embryonic mice were dissociated, separated by FACS, and mRNA was harvested to identify tissue-specific genes by microarray analysis. More recently, photoconvertible fluorescent proteins such as Kaede (5) have become available, allowing researchers to interactively convert expressing cells from one color to another. Several promising uses of photoconvertible proteins in embryology have been described; however, they have not yet been used to facilitate tissue-specific microdissection.
A reproducible map of embryonic cell fates in zebrafish is established ≈5 h post-fertilization (hpf), at the late blastula stage (6). At this time, two to four embryonic cell layers are arranged in a dome that surrounds one hemisphere of a single large yolk cell (Fig. 1A). The endoderm precursor field lies within the lowest four tiers of this dome (Fig. 1 A and B) (7). The mesoderm precursor field overlaps the endoderm precursor field and extends to higher tiers (6). The cells that comprise these overlapping fields are collectively termed mesendoderm precursors. Above the sixth tier, there is a staggered transition from mesoderm fates to ectoderm fates, with ectoderm precursors continuing to the top of the embryo (6).
Fig. 1.
Use of Kaede protein to label embryonic cells and trace their lineages. (A) Schematic of a late blastula stage (40% epiboly) embryo. The embryonic layer (EL) is color coded according to germ layer precursor distribution, with dark red representing endoderm precursor-enriched, red representing mesoderm precursors, and green representing ectoderm precursors. Also indicated are the yolk cell (YC, tan), the enveloping layer (EVL, purple), and the yolk syncytial layer (YSL, light blue). For clarity, the yolk cell is represented as separated from the overlying tissues. (B) Fate map of a late-blastula stage embryo after Kimmel et al. (6) using the same color scheme as A and including precursor domains for the indicated outcomes in H. (C) Coomassie-blue stained polyacrylamide gel, showing a 34-kDa monomer of bacterially expressed Kaede after column purification. (D) Four-cell-stage (1 hpf) embryo shortly after injection with 3.5 ng of purified Kaede protein, lateral view. (E) 40% epiboly-stage (5 hpf) embryo just before photolabeling along the margin, animal pole view. (F) Same embryo as in E, just after photolabeling along the margin, animal pole view. (G) Close-up of a Kaede protein-injected embryo, directly after photolabeling of the margin, lateral view. Only optical sections from near the surface are shown. Brackets and numbers indicate the lowest cellular tiers. (H) Same embryo as in G, but now at pharyngula stage (24 hpf), lateral view, anterior to left. Representative tissues are indicated in H and their relative pixel values (% red of total red + green) match expected outcomes as follows: 1 (22%), 2 (25%), 3 (52%), 4 (52%), 5 (77%), and 6 (78%).
Traditional methods for isolating early mesendoderm and ectoderm cells are technically challenging (excising the tops of embryos with an eyebrow hair or explantation of individual cells with a micropipet) and only a few studies have been published that examined the fate changes they undergo when transplanted to host embryos (8, 9) or when explanted and cultured (10). Transgenic zebrafish lines with mesoderm-, endoderm-, or ectoderm-restricted fluorescent protein expression have been reported (11, 12), but at the late-blastula stage, none of these are bright enough for FACS-based purification (J.L.B. and B.F., unpublished observations).
To further our understanding of germ-layer differentiation, we have developed FACS-assisted microdissection of photolabeled (FAM-P) cells, a strategy for isolating zebrafish embryonic cells. FAM-P uses recombinant photoconvertible Kaede protein, which overcomes the spatiotemporal limitations of transgenically expressed fluorescent proteins or injected mRNA and allows the quantitative isolation of early embryonic zebrafish cells from user-defined regions of interest. We isolated endogenous mesendoderm and ectoderm precursors by FAM-P and transplanted them into host embryos, where they contributed to a similar range of tissues as mesendoderm and ectoderm precursors that were traditionally explanted by micropipet. DNA microarray analysis of FAM-P-isolated mesendoderm and ectoderm precursors revealed they continue to express molecular markers specific to their regions of origin. Microarray analysis also identified dozens of new mesendoderm precursor-enriched genes. Morpholino knockdown of one these genes, dusp4, produced a loss-of-function phenotype, characterized by the down-regulation of a single endoderm marker during gastrulation, sox17, although other endoderm markers were expressed normally, and a loss of foregut and pancreatic endoderm at later stages. Our studies illustrate just some of the ways that FAM-P can be applied to experimental embryology. Given the spatial and temporal flexibility that FAM-P offers, it should be useful for many other experiments in zebrafish and possibly in other model organisms.
Results
Differential Labeling of Germ Layer Precursors with the Kaede Protein.
The mesendoderm precursor field of zebrafish late blastula stage (40–50% epiboly, ≈5 hpf) embryos consists of a narrow band of cells that encircle a delicate yolk cell (Fig. 1 A and B). Because an efficient isolation of these cells by using traditional microsurgical tools is untenable, we set out to develop a method for fluorescently labeling cells of interest and isolating them by FACS. For this purpose, we selected the photoconvertible fluorescent reagent Kaede, a stony coral protein that fluoresces green (518 nm) in its full-length form but red (582–627 nm) after photocleavage by near-UV light (405 nm) (5). We discovered that nascent fluorescent protein maturation is slow relative to zebrafish development, such that late blastula-stage fluorescence from injected kaede mRNA [see supporting information (SI) Fig. S1] and zygotically expressed transgenic fluorescent proteins (J.L.B. and B.F., unpublished observations) were not bright enough for FACS. To circumvent this difficulty, we generated recombinant Kaede protein (Fig. 1C). Injection of purified Kaede protein was nontoxic and brightly labeled embryos from the time of injection (Fig. 1D) through the late blastula stage (Fig. 1 E–G) and persisted until 24 hpf (Fig. 1H), after which time the signal gradually faded (data not shown), presumably because of cellular catabolism.
To specifically label mesendoderm precursors, we microinjected Kaede protein into fertilized eggs and at the late blastula stage these embryos were mounted on a scanning confocal microscope, with the top of the embryo nearest to the lens (Fig. 1E). Orientation of multiple specimens was facilitated by mounting embryos, their transparent chorions intact, in arrayed conical molds (13, 14). A circular swath was defined comprising the outermost, and therefore lowest, tiers of cells, and these mesendoderm precursors were photoconverted from green to red by computer-controlled scanning with a near-UV laser (Fig. 1F). Using this method, we can label the mesendoderm precursors of 8–12 embryos in a single experiment.
Traditional fate mapping requires the lineage tracing of single cells in dozens of embryos. Labeling the entire margin visualized key features of the fate map (Fig. 1B) in a single embryo. Image analysis on day 2 shows tissues normally derived from lower tiers with more red pixels, tissues normally from higher tiers with more green pixels, and tissues normally derived from near the margin/nonmargin boundary with similar numbers of red and green pixels, presumably representing a mixture of red, green, and half-labeled yellow cells (Fig. 1H). This analysis demonstrates that we could correctly target the mesendoderm precursor field and indicates that Kaede protein labeling does not alter cellular destinies. We have similarly used Kaede protein to label and trace outcomes of other embryonic regions (Fig. S1). Such lineage tracing experiments are facilitated by the ability of cells labeled with Kaede protein to hold their color over time. When kaede mRNA is injected, photolabeled lineages become significantly yellowed because of newly synthesized green Kaede protein (see Fig. S1).
Isolation of Mesendoderm and Ectoderm Precursors.
To quantitatively purify mesendoderm and ectoderm precursors, we dissociated labeled embryos into single cell suspensions and sorted cells by FACS (Fig. S2). To minimize losses of labeled cells during processing (e.g., pipetting, centrifugation, and FACS steps), we mixed them with unlabeled cells from uninjected sibling embryos at the point of trypsinization. Forward and side scatter values for single embryonic cells were determined, and events within those gates were further sorted by their relative red and green fluorescence. Using these parameters, we could purify homogenous populations of green and red cells, with a recovery rate of input embryonic cells close to 75% and over 99% viability (data not shown).
A critical test of FAM-P's utility is to determine whether sorted cells retain their developmental potentials and biases. It has previously been shown that ectoderm precursors transplanted to the animal pole are reliably incorporated into host ectoderm (8, 9). We find the same is true for FAM-P-isolated ectoderm precursors, which contributed to ectoderm fates, such as retinal neurons and forebrain cells (Fig. S2 and Table S1). It was previously shown that early-stage mesendoderm precursors (4.7–5 hpf) are readily reprogrammed to ectoderm (8, 9), but shortly thereafter [beyond 50% epiboly (5.3 hpf)] become committed to mesendoderm fates (8). Our FAM-P purified mesendoderm cells come from an intermediate stage [40–50% epiboly (5.0–5.3 hpf)] and consistent with this, they contributed both to ectoderm- and mesendoderm-derived tissues, as did marginal cells of the same stage that we traditionally transplanted directly from one embryo to another (Fig. S2 and Table S1). We conclude that FAM-P-purified mesendoderm and ectoderm precursors maintain their endogenous commitment status and developmental potential.
Identification of Previously Characterized and Uncharacterized Germ Layer-Specific Genes.
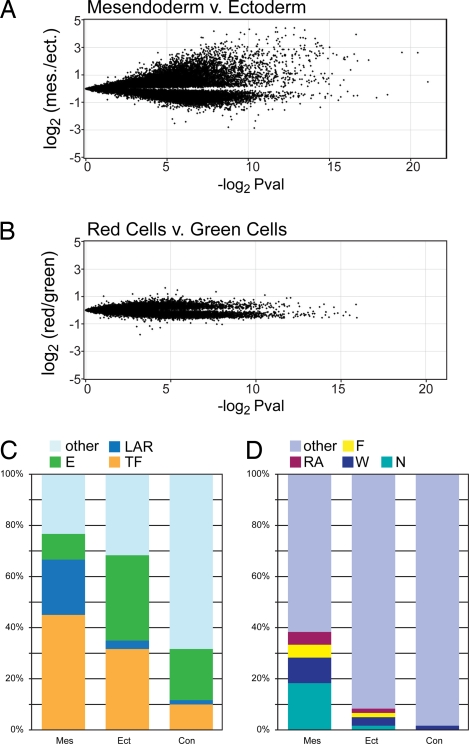
To study the transcriptomes of both the mesendoderm and ectoderm precursors, we harvested and amplified the RNA of purified precursor cells and cohybridized them onto oligo chips for microarray analysis (Fig. 2A). As a control against photolabeling effects, we also prepared and cohybridized amplified RNA probes from green and red control cells, yielding no substantially enriched genes (Fig. 2B). Thus, the expression profile of embryonic cells is not substantially altered by photolabeling per se. We identified 188 full-length cDNAs that were significantly enriched (P < 0.05) in the mesendoderm precursor pool and 106 similarly enriched cDNAs in the ectoderm precursor pool.
Fig. 2.
Transcriptome analysis of mesendoderm and neurectoderm precursor cells. (A and B) Volcano plots showing 31,356 (A) and 25,837 (B) oligos each. Each oligo is mapped along the y axis according to its relative enrichment in a direct comparison of the two source tissues (average normalized Log2 of the hybridization signal ratio) and along the x axis according to its significance (negative Log2 scale of uncorrected P value). (A) FAM-P isolated mesendoderm precursor cells (> 0 on Y axis) vs. cognate FAM-P isolated ectoderm precursor cells (< 0 on Y axis). (B) Kaede-injected and photoconverted embryo cells (> 0 on Y axis) vs. cells from Kaede-injected embryos that were not photoconverted (< 0 on Y axis). (C and D) Bar graphs showing the representation of selected molecular functions (C) and signaling pathways (D) among the 60 most-enriched genes (Table S2). Mes, mesendoderm precursor enriched; Ect, ectoderm precursor enriched; Con, red control cell enriched; E, Enzyme; LAR, ligand (agonist), antagonistic ligand or receptor; TF, transcription factor; N, Nodal pathway; W, Wnt pathway; F, FGF pathway; RA, retinoic acid pathway. Genes acting in multiple pathways were arbitrarily assigned to one pathway with the following priority: RA > F > W > N.
We compiled cohorts of the top 60 unique genes from each comparison for which a minimum of annotated information was available (Table S2) and examined them for over-represented gene ontology terms (Fig. 2C and Table S2). A striking percentage (45%) of the mesendoderm precursor genes encode transcription factors (Fig. 2C). The frequency of transcription factors on the entire microarray, by contrast, is below 10%, and a similar frequency is seen in the control cohort (Fig. 2C). We also noted an excess of ligands, agonists, and receptors and a paucity of enzymes among the mesendoderm precursors compared with the control cohort (Fig. 2C). An elevated number of transcription factors (32%) was also seen among ectoderm precursors (Fig. 2C), but no other molecular class seems to be over or underrepresented.
We also scored the mesendoderm, ectoderm, and control cohorts for membership in four signaling pathways—Nodal, WNT, FGF, and retinoic acid—which are known to be active among mesendoderm precursors (15) and for known ectoderm-specific genes. We found that a full 38.3% of the mesendoderm precursor cohort genes fall into at least one of these four pathways, whereas ectoderm and control cohort incidences were 8.3% and 1.7%, respectively (Fig. 2D). Although we identified far fewer highly enriched ectoderm genes, a slightly increased false discovery filter of 5.5% reveals a substantial enrichment of several well characterized neurectoderm genes, such as sox3 (16), sox 19a (17), lhx5 (18) and otx2 (19), as well as the nonneural ectoderm gene foxi1 (20) (Fig. 2C and Table S2).
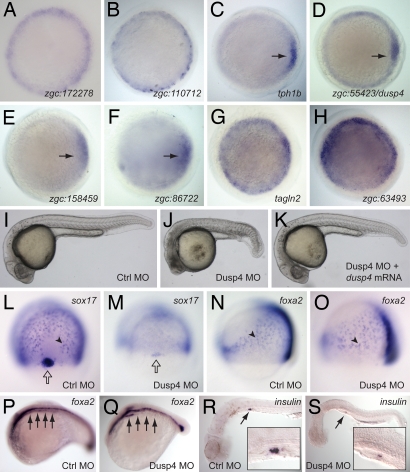
A number of the mesendoderm-enriched genes we identified have no reported expression in the late blastula stage. We characterized 21 such genes from the mesendoderm precursor pool by cloning and performing whole mount in situ hybridizations on late blastula-stage embryos. Ten of these genes showed margin-specific staining in late blastula embryos, validating their enrichment among mesendoderm precursors (Fig. 3 A–H and data not shown). We also validated the ectoderm-specific expression of several previously uncharacterized ectoderm precursor genes (data not shown).
Fig. 3.
Expression and function of new mesendoderm genes. (A–H) Whole mount in situ hybridizations (WISH) on late-blastula-stage (5 hpf) embryos, validating the mesendoderm-specific expression of eight mesendoderm precursor-enriched genes, as indicated. Animal pole views are shown, and C–F have their dorsal sides to the right (black arrows), as confirmed by double stains with the chordin gene (data not shown). (I–K) Embryos injected with a control morpholino (Ctrl MO), Dusp4 morpholino (Dusp4 MO), or a rescuing combination of Dusp4MO and dusp4 mRNA, shown at the pharyngula stage (28 hpf). (L–O) WISH on late-gastrula-stage (80% epiboly) control and Dusp4 MO-injected embryos, visualizing expression of the endoderm markers sox17 (L and M, dorsal views) and foxa2 (N and O, lateral views). Black arrowheads indicate sample endoderm cells, and open arrows point to stained dorsal forerunner cells. (P–S) WISH on 18 somite-stage control (P), pharyngula-stage (26 hpf) Dusp4 MO-injected (Q), and pharyngula-stage (30 hpf) control and morphant (R and S) embryos, visualizing foxa2 (P and Q) and insulin (R and S). Embryos in P and Q were stage matched rather than time matched to account for developmental delays of morphants. Black arrows in P and Q point to posterior endoderm, including foregut region. Black arrows and magnifications in R and S highlight pancreatic stains.
We used antisense morpholino oligonucleotides (MOs) to block the translation of several validated mesendoderm precursor genes and found one to associate with a distinct and reproducible phenotype: zgc:55423, which has 86% homology (Clustal alignment) to human dual-specificity phosphatase 4 (DUSP4, alias: MKP2) and which we have accordingly termed dusp4. Dusps attenuate mitogen-activated protein kinase (MAPK) signaling via de-phosphorylation of two key residues, with human DUSP4 reported to target the MAPKs ERK and JNK (21). At the pharyngula stage, Dusp4-depleted embryos displayed developmental delays and small and necrotic heads, suggesting an essential role for Dusp4 in anterior development (Fig. 3J). Given dusp4's expression in the margin, we investigated the expression of mesoderm and endoderm markers in Dusp4 morphants. We observed a drastic reduction in the expression of sox17 in gastrula-stage endoderm cells as well as in a specialized group of cells termed the dorsal forerunners (Fig. 3 L and M) (22). Intriguingly, at this stage, other endoderm markers such as foxa2 (23) (Fig. 3 N and O) were expressed normally in Dusp4 morphants. These phenotypes are specific as we were able to phenocopy the morphological and molecular defects with a second, nonoverlapping MO against Dusp4 (data not shown) and we could rescue them by coinjection of dusp4 mRNA (Fig. 3K). Analysis of more differentiated endoderm with probes for foxa2 (24) and insulin (24) transcripts revealed a loss of foregut (Fig. 3 P and Q) and pancreatic (Fig. 3 R and S) endoderm.
Discussion
This paper introduces a method for viably isolating embryonic cells from user-defined regions and stages of interest. Injected Kaede protein can brightly label cells much earlier than exogenous mRNA, and there is no problem with de novo synthesis over the course of lineage tracing experiments. Caged fluorophores might be suitable alternatives to Kaede protein; however, these reagents have been commercially unavailable for several years. Another possible alternative is recombinant photoconvertible EosFP protein, which has been used to achieve early cell labeling in frog embryos (25).
For the studies described here, we microdissected embryos within 1 h of labeling; however, labeled cells can be allowed to further differentiate before isolation. For instance, we easily isolated early-labeled mesendoderm precursors at mid- and late-gastrula stages (data not shown). Thus, a diverse range of embryonic cell types can be labeled and purified by FAM-P. One topological limitation should be noted. The cells we labeled were conveniently arranged in a shallow half dome. Differential labeling of deep and superficial cells is not feasible with the single-photon apparatus we used but might be achievable with multiphoton systems, as has been reported for photoconversion of KikGR (26).
To our knowledge, our microarray analysis is the first such study on endogenous germ layer precursors in zebrafish; however, three papers have reported on the transcriptomes of zebrafish embryos forced to over- or underproduce mesendoderm via alterations in Nodal signaling (27–29). Each of these studies yielded important and complementary data, but it is useful to perform a detailed comparison to assess the endogenous approach. Our method was best for identifying non-Nodal mesendoderm pathway members (i.e., FGF, WNT, and retinoic acid), with 12 of our top 60 genes being in these categories, compared with 9 of 98 (28) and 6 of 75 (27) in the other studies that presented their nonvalidated hits (Table S2). Our approach was also successful for identifying Nodal pathway genes. The highest number of validated Nodal-regulated genes emerging from the four studies was 34 of a curated list of 72 (29). Our 188 mesendoderm genes included 26 genes from this list and we have dozens more, such as those in Fig. 3, pending validation (Table S3). Thus, screening endogenous cells yielded an overall greater diversity of pathway-specific genes. Our proposed explanation for this difference is that hyperactivation of Nodal signaling extinguishes the expression of Nodal and non-Nodal pathway genes that are uniquely expressed at lower endogenous Nodal signaling levels.
Many of the mesendoderm precursor-enriched genes we identified were not previously shown to be expressed by mesendoderm precursors, and half of those we tested showed margin-enriched expression in situ, including dusp4. The loss of expression of sox17, but not foxa2, that we observed in Dusp4 morphants during gastrulation is unusual and may define a new class of endoderm defect; although reduction of sox17 has been seen in zebrafish sqt, cyc, oep, bon, fau, and cas mutants, foxa2 has been seen to be coordinately down-regulated where examined (30). We also report a loss of foregut and pancreatic endoderm in Dusp4 morphants. This outcome is consistent with sox17 decreases, as a mouse knockout of Sox17 has been reported to lack gut endoderm (31).
In summary, the ability to microdissect a wide range of early cells in zebrafish opens up numerous experimental possibilities for developmental biologists. Future studies might include, for instance, comparisons of dorsal vs. ventral or younger vs. older embryonic cell populations. We have shown that FAM-P-purified zebrafish cells can be used in transplantation assays and microarray studies, but many other applications can be envisioned, such as direct transcriptome sequencing, proteome analysis, or the establishment of new embryonic cell lines.
Materials and Methods
Microinjection and Kaede Photoconversion.
Embryos were obtained from WT adult zebrafish (AB, WIK, or EK) via natural mating. pKaede-S1 vector (MBL International) was subcloned into pRSET B (Invitrogen), expressed in E. coli, and purified using Ni-NTA agarose affinity, sizing, and hydroxyapatite columns (expression and purification by ProteinOne Inc.). The protein was stored in 5 mM Hepes (pH 7) and 0.2 M KCl buffer at −80°C. For kaede and dusp4 mRNA, NotI-linearized Kaede-pCS2+ (32) or dusp4-pCS2 was used as a template for mMessage mMachine synthesis using SP6 RNA polymerase (Ambion). Kaede protein injections of 3.5 ng/embryo and mRNA injections of 300 pg/embryo were carried out at the 1–2 cell stage and embryos were subsequently incubated at 28°C. Embryos at the stage of interest were mounted in their chorions in an agarose array of conical imprints (13, 14). By using a laser scanning microscope (Carl Zeiss LSM 510 META), Kaede was photoconverted in regions of interest, such as the margin, with a 405-nm laser and imaged via 488-nm and 543-nm excitation for green and red Kaede, respectively. Emitted light was respectively filtered through 505–530-nm BP and 560-nm LP filters. For loss-of-function studies, 1–3 ng of an antisense splicing MO (Gene Tools), targeting zygotic dusp4 translation (5′-TAGCCCCCTGTAAAAGTGGAAAAGG-3′), was injected into one cell-stage embryos. A similar, nonoverlapping MO (5′-TGCTGCTTGTGTATTTACCTGGTCG-3′) yielded indistinguishable results. For control embryos, 5 ng of a standard control MO (5′-TAGTTAAGCCTAGCTCTCATAAACT-3′) was injected. For rescue, 50-pg full-length dusp4 mRNA was coinjected.
Embryonic Cell Sorting and Transplantation.
Embryos were dechorionated with Pronase (P8811, Sigma) and transferred to siliconized glass vials. Uninjected embryos were added to samples with <20 labeled embryos to bring the total number to 20–30. For dissociation, embryos were incubated for 6 min in 300 μl of trypsin solution (27) and triturated once with a transfer pipette midincubation. After dissociation with trypsin, 30 μl of heat-inactivated FBS (HI-FBS) (Gibco) was added and the vial was then flooded with L-15 Medium (Sigma) containing 0.1% BSA, 0.8 mM CaCl2, 2 mM L-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The cells were centrifuged at 100 × g for 4 min at 4°C and all but ≈700 μl of media was removed. The cells were transferred to a Titertube Micro Tube (Bio-Rad) inside of a 5-ml polystyrene tube, and 70 μl of HI-FBS was added. The cells were transported on ice to a flow cytometer (FACSDiVa Vantage, BD Biosciences) where they were sorted based on their forward scatter vs. side scatter and green vs. red fluorescence by using a 100-μm tip at 8–10 psi into either TRIzol (Invitrogen) for RNA isolation or cell media for cell viability assays or transplants. Methods for transplantation experiments can be found in the legend of Fig. S2.
RNA Isolation, Amplification, and Cy Labeling.
Cells were sorted into TRIzol, vortexed, frozen on dry ice, stored at −80°C, and extracted according to the manufacturer's protocol with slight modifications. Approximately 300 ng to 1 μg RNA was linearly amplified by using the Amino Allyl MessageAmp II aRNA Amplification kit (Ambion) with yields ranging from 12 to 30 μg of aRNA. aRNA samples were split and labeled, half with Cy3 mono NHS ester and half with Cy5 mono NHS ester (CyDyes from GE Healthcare; post-labeling reagents from the MessageAmp II kit). Labeled aRNAs were fragmented by incubation at 94°C for 15 min in fragmentation buffer (Affymetrix). Paired probes were prepared for four independent experiments microdissecting mesendoderm and ectoderm precursors and three independent experiments using control red and control green cells prepared as follows. A number of embryos were injected with Kaede and photoconverted to red in toto, and an equivalent number of Kaede-injected embryos were left in their green-fluorescent state. These red-labeled and green-labeled embryos were then pooled together with unlabeled stage-matched embryos, disaggregated, and the entire cell population was sorted to isolate red and green control cells.
Microarray Hybridization, Transcriptomes Analysis, and Validation.
In-house microarray chips were used (13). Four biological replicates of mesendoderm and ectoderm precursor probes were cohybridized, each with one dye swap, for a total of eight hybridizations. Similarly, three biological replicates of red-cell and green-cell probes with one dye swap were cohybridized for a total of six additional hybridizations. Hybridizations were performed overnight at 45°C in Maui Mixer FL hybridization chambers (BioMicro Systems). Post-hybridization processing and scanning were as previously described (13). Data points with quality values of <0.85 were eliminated, and the dataset was normalized by median shift following a log2 transformation by using the Avadis 4.0 software program. P values and average log ratios were determined for each oligo of the array as described in ref. 13 and predicted genes, ESTs, and duplicates were disregarded. Representation of molecular categories among the most enriched genes in mesendoderm precursor vs. ectoderm precursor comparisons as well as red vs. green control-cell comparisons was preliminarily assessed with GeneSifter software (VizX Labs), looking at approximately the top 100 genes with available RefSeq IDs. This assessment was followed by a hand-curated analysis of the 60 most-enriched genes from each category (Table S2).
For validation studies, primers were designed to amplify the coding sequence as well as the 5′ and 3′ UTRs of genes selected for study and cloned by PCR into TOPO TA vectors (Invitrogen). DIG-labeled RNA probes were prepared by using PCR-generated DNA templates and a DIG RNA labeling kit with either SP6 or T7 polymerase (Roche), and in situ hybridizations were carried out essentially as described in ref. 33, with post-hybridization washes performed by an automated liquid exchanger (Biolane HTI, Hölle & Hüttner).
All zebrafish work was approved by the National Human Genome Research Institute's Animal Care and Use Committee.
Supplementary Material
Acknowledgments.
We thank Darryl Leja for artwork and expert assistance with graphics; Alexandra Joyner, Paul Liu, David Bodine, Igor Dawid, and Mary LaMarca for helpful comments on the manuscript; Erich Roessler and Stacie Anderson for valuable discussions; and Atsushi Miyawaki for sharing the Kaede-pCS2+ vector. This research was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE8654).
This article contains supporting information online at www.pnas.org/cgi/content/full/0805589105/DCSupplemental.
References
- 1.Haeckel E. Die gastraea-theorie: Die phylogenetische classification des thierreichs und die homologie der keimblätter. Jenaische Zeitschrift für Naturwissenschaften. 1874;8:1–55. [Google Scholar]
- 2.Hatada Y, Stern CD. A fate map of the epiblast of the early chick embryo. Development. 1994;120:2879–2889. doi: 10.1242/dev.120.10.2879. [DOI] [PubMed] [Google Scholar]
- 3.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwood RI, et al. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304:541–555. doi: 10.1016/j.ydbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- 7.Warga RM, Nusslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- 8.David NB, Rosa FM. Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signaling. Development. 2001;128:3937–3947. doi: 10.1242/dev.128.20.3937. [DOI] [PubMed] [Google Scholar]
- 9.Ho RK, Kimmel CB. Commitment of cell fate in the early zebrafish embryo. Science. 1993;261:109–111. doi: 10.1126/science.8316841. [DOI] [PubMed] [Google Scholar]
- 10.Sagerstrom CG, Gammill LS, Veale R, Sive H. Specification of the enveloping layer and lack of autoneuralization in zebrafish embryonic explants. Dev Dyn. 2005;232:85–97. doi: 10.1002/dvdy.20198. [DOI] [PubMed] [Google Scholar]
- 11.Ellingsen S, et al. Large-scale enhancer detection in the zebrafish genome. Development. 2005;132:3799–3811. doi: 10.1242/dev.01951. [DOI] [PubMed] [Google Scholar]
- 12.Fan X, et al. Nodal signals mediate interactions between the extra-embryonic and embryonic tissues in zebrafish. Dev Biol. 2007;310:363–378. doi: 10.1016/j.ydbio.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei W, et al. An early requirement for maternal FoxH1 during zebrafish gastrulation. Dev Biol. 2007;310:10–22. doi: 10.1016/j.ydbio.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroter C, et al. Dynamics of zebrafish somitogenesis. Dev Dyn. 2008;237:545–553. doi: 10.1002/dvdy.21458. [DOI] [PubMed] [Google Scholar]
- 15.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Ann Rev Gen. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 16.Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong SH, Emelyanov A, Teh C, Korzh V. Wnt signalling mediated by Tbx2b regulates cell migration during formation of the neural plate. Development. 2005;132:3587–3596. doi: 10.1242/dev.01933. [DOI] [PubMed] [Google Scholar]
- 18.Toyama R, et al. The LIM class homeobox gene lim5: Implied role in CNS patterning in Xenopus and zebrafish. Dev Biol. 1995;170:583–593. doi: 10.1006/dbio.1995.1238. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen VH, et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- 20.Nissen RM, Yan J, Amsterdam A, Hopkins N, Burgess SM. Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development. 2003;130:2543–2554. doi: 10.1242/dev.00455. [DOI] [PubMed] [Google Scholar]
- 21.Teng CH, Huang WN, Meng TC. Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress. J Biol Chem. 2007;282:28395–28407. doi: 10.1074/jbc.M705142200. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi Y, et al. Notch signaling can regulate endoderm formation in zebrafish. Dev Dyn. 2004;229:756–762. doi: 10.1002/dvdy.10483. [DOI] [PubMed] [Google Scholar]
- 23.Strahle U, Blader P, Henrique D, Ingham PW. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993;7:1436–1446. doi: 10.1101/gad.7.7b.1436. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, et al. Genetic analysis of early endocrine pancreas formation in zebrafish Molecular endocrinology. Mol Endocrinol. 2006;20:194–203. doi: 10.1210/me.2005-0189. [DOI] [PubMed] [Google Scholar]
- 25.Wacker SA, Oswald F, Wiedenmann J, Knochel W. A green to red photoconvertible protein as an analyzing tool for early vertebrate development. Dev Dyn. 2007;236:473–480. doi: 10.1002/dvdy.20955. [DOI] [PubMed] [Google Scholar]
- 26.Hatta K, Tsujii H, Omura T. Cell tracking using a photoconvertible fluorescent protein. Nature protocols. 2006;1:960–967. doi: 10.1038/nprot.2006.96. [DOI] [PubMed] [Google Scholar]
- 27.Dickmeis T, et al. Identification of nodal signaling targets by array analysis of induced complex probes. Dev Dyn. 2001;222:571–580. doi: 10.1002/dvdy.1220. [DOI] [PubMed] [Google Scholar]
- 28.Link V, et al. Identification of regulators of germ layer morphogenesis using proteomics in zebrafish. J Cell Sci. 2006;119:2073–2083. doi: 10.1242/jcs.02928. [DOI] [PubMed] [Google Scholar]
- 29.Bennett JT, et al. Nodal signaling activates differentiation genes during zebrafish gastrulation. Dev Biol. 2007;304:525–540. doi: 10.1016/j.ydbio.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda K, Kikuchi Y. Endoderm development in vertebrates: Fate mapping, induction and regional specification. Dev Growth Differ. 2005;47:343–355. doi: 10.1111/j.1440-169X.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 31.Kanai-Azuma M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 32.Mutoh T, Miyata T, Kashiwagi S, Miyawaki A, Ogawa M. Dynamic behavior of individual cells in developing organotypic brain slices revealed by the photoconvertable protein Kaede. Exp Neurol. 2006;200:430–437. doi: 10.1016/j.expneurol.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Thisse C, Thisse B. High resolution whole-mount in situ hybridization. Zebrafish Sci Monitor. 1998:5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.