Abstract
With the goal of identifying changes in gene expression in CD4+ T cells during the development of diabetes in the nonobese diabetic (NOD) mouse, we used DNA microarrays to analyze gene expression in CD4+ T cells from the pancreatic draining lymph nodes of NOD/BDC 2.5 T cell receptor transgenic and WT NOD mice at different ages. At 4 and 6 weeks of age, we found up-regulation of a number of genes that are known to be induced by IFN-α. IFN-α levels and IFN-α–producing plasmacytoid dendritic cells were increased in the PLNs of 3- to 4-week-old NOD mice. Moreover, blockade of IFN-α receptor 1 in NOD mice by a neutralizing antibody at 2–3 weeks of age significantly delayed the onset and decreased the incidence of type 1 diabetes, increased the relative number of immature dendritic cells in the PLNs, and enhanced the ability of spleen CD4+ T cells to produce IL-4 and IL-10. These findings demonstrate that IFN-α in the PLNs is an essential initiator in the pathogenesis of type 1 diabetes in NOD mice.
Type 1 diabetes (T1D) is a T cell-mediated autoimmune disease in which the insulin-producing β cells of the pancreatic islets are progressively destroyed (1). Nonobese diabetic (NOD) mice, which spontaneously develop diabetes, serve as a model of human T1D. In NOD mice, infiltration of lymphocytes and macrophages into the pancreatic islets (insulitis) begins at 4–5 weeks of age followed by overt diabetes beginning at approximately 12 weeks of age. In NOD/BDC 2.5 mice, more than 90% of T cells express a Vα1Vβ4 CD4+ T cell receptor that reacts with an islet antigen (2). The explosive insulitis that occurs in these mice at 15–18 days of age is recognized as the “3-week checkpoint.” Physiological programmed cell death of pancreatic β cells, which peaks at 14–17 days of age, is responsible for the abrupt onset of insulitis in NOD/BDC2.5 mice (2, 3). Thus, the first 3 weeks of life is a critical period for the initiation of T cell autoimmunity to β cell antigens in the pancreatic draining lymph nodes (PLNs) of NOD and NOD/BDC 2.5 mice (4–6).
IFN-α is a group of pleiotropic cytokines in the type I family of IFNs. Plasmacytoid dendritic cells (pDCs), which can be activated through toll-like receptors (TLRs)-7 and -9 by single-stranded RNA and double-stranded DNA, respectively, to produce large amounts of IFN-α (7). IFN-α exerts broad but distinct effects on innate and adoptive immune responses by signaling through a heterodimeric receptor composed of IFN-α receptor 1 (IFNAR1) and IFNAR2 (8). Many studies suggest that IFN-α is involved in the development of T1D. For example, higher levels of IFN-α mRNA and protein were detected in the pancreata of T1D patients than in pancreata of nondiabetic patients (9, 10). IFN-α treatment of patients with tumors or viral hepatitis is associated with an increased incidence of T1D (11, 12). Over-expression of IFN-α on β cells induced T1D in non-autoimmune-prone C57BL/6 mice (13). Additionally, IFN regulatory factor 1-deficient NOD mice failed to develop insulitis and diabetes (14). However, some studies have shown that oral treatment of prediabetic NOD mice with IFN-α suppressed insulitis and diabetes (15, 16). Thus, the role that IFN-α plays in the pathogenesis of T1D in NOD mice is controversial.
We have a longstanding interest in the molecular mechanisms that control the early development of autoimmunity to pancreatic β cell autoantigens and the pathogenesis of T1D in NOD mice (2, 17, 18). In the present study, DNA microarrays were used to identified significant up-regulation of several IFN-α-inducible gene transcripts in CD4+ T cells from PLNs of 6-week-old NOD/BDC 2.5 and WT NOD mice, as compared with 2-week-old mice. Blockade of IFN-α signaling by anti-IFNAR1 mAb in 2- to 3-week-old NOD mice markedly delayed the onset and decreased the incidence of diabetes. Suppression of T1D by anti-IFNAR1 mAb treatment was associated with significantly increased numbers of immature dendritic cells (DCs) in PLNs and enhanced immunoregulatory cytokine (IL-4 and IL-10) production by spleen CD4+ T cells. These results indicate that IFN-α produced by pDCs in the PLN is critical for initiating the development of T1D in NOD mice.
Results and Discussion
Distinct IFN-α-induced Gene Expression Profile in NOD/BDC2.5 and WT NOD Mice.
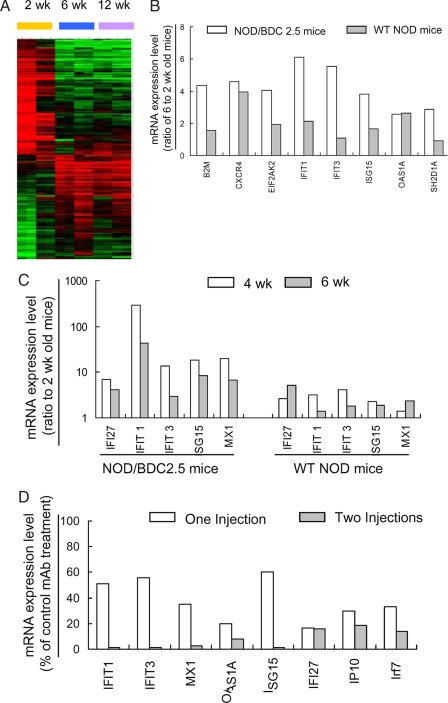
To identify the diabetes related genes that changed expression during the development of T1D, we analyzed gene expression profiles in CD4+ T cells purified from the PLNs of NOD/BDC 2.5 and WT NOD mice at 2, 6, and 12 weeks of age, using cDNA microarray analysis. The gene expression levels from 2-week-old WT NOD mice were used as the baseline for the other samples. A total of 251 genes were identified with a twofold or greater change in expression between 2- and 6-week-old NOD/BDC 2.5 (Fig. 1A). In contrast, only six gene transcripts were differentially expressed when comparing 6- and 12-week-old NOD/BDC 2.5 mice (data not shown). Among these 251 genes, 119 genes were up-regulated and 132 genes were down-regulated. These genes fell into multiple functional categories, including apoptosis, transcription regulation, cell cycle/proliferation, DNA/RNA binding/editing, and signal transduction. In particular, we identified 31 down-regulated and 32 up-regulated immune-related genes: 8 of the 32 up-regulated genes were IFN-α-inducible (Fig. 1B). For example, IFN-stimulated gene 15 (ISG15), a well-known type I IFN signature gene in viral infections, showed 3.8-fold and 1.6-fold increases in 6-week-old NOD/BDC 2.5 and NOD mice, respectively. Moreover, an additional 14 of the 32 up-regulated genes were commonly inducible by both IFN-α and IFN-γ (data not shown). Next, we used quantitative real-time PCR to analyze expression of IFN-α signature genes by PLN CD4+ T cells from 2-, 4-, and 6-week-old NOD/BDC 2.5 mice. Notably, the expression levels of these genes in 4-week-old NOD/BDC 2.5 mice were frequently much higher than those in 6-week-old mice (Fig. 1C). Thus, these results demonstrate that the increase in IFN-α gene signature expression is spontaneous, and develops between 2 and 4 weeks of age, i.e., at the same time as the 3-week checkpoint in the development of T1D in NOD/BDC 2.5 mice (2).
Fig. 1.
A distinct IFN-α-induced gene expression profile in NOD/BDC2.5 and NOD mice. (A) Overview of the gene expression profile in CD4+ T cells from the PLNs of NOD/BDC 2.5 mice at 2, 6, and 12 weeks of age. All genes shown were selected by Significance analysis of microarray (SAM) analysis for a fold change value >2 between the 2- and 6-week samples. Individual values are expressed as the ratio of the expression value to the control (2-week-old NOD mice) value. Data from individual genes are depicted as a single row. Different replicates and different time points are shown as columns. Red and green indicate genes expressed at higher or lower levels than control. The intensity of the color reflects the magnitude of the change from baseline. Duplicated arrays at each time point and the results of two separately repeated experiments are included. (B) IFN-α-inducible genes with more than a twofold change in expression level between 2- and 6-week-old NOD/BDC 2.5 mice (open bars). The fold changes in WT NOD counterpart are shown in parallel (gray bars). These genes include B2m (β-2 microglobulin), Cxcr4 (Chemokine [C-X-C motif] receptor 4), Eif2ak2 (eukaryotic translation initiation factor 2-α kinase 2), Ifit1 (IFN-induced protein with tetratricopeptide repeats 1), Ifit3 (IFN-induced protein with tetratricopeptide repeats 3), Isg15 (IFN-stimulated gene 15), Oas1a (2′-5′ oligoadenylate synthetase 1A), and Sh2d1a (SH2 domain protein 1A). (C) The mRNA expression ratio of IFN-α-inducible genes, compared at 4 (open bar) or 6 (gray bar) weeks with the 2-week control of NOD/BDC 2.5 and WT NOD mice by using real-time quantitative PCR. (D) Repressed mRNA expression of IFN-α-inducible genes by treatment with anti-IFNAR1 mAb. NOD/BDC 2.5 mice were treated i.p. with anti-IFNAR1 or control mAb on day 15 (one injection, open bar), or days 15 and 20 (two injections, gray bar) after birth. CD4+ T cells were purified from the PLNs at 25 days of age for analysis of the mRNA expression of IFN-α-inducible genes by using real-time quantitative PCR.
To determine if the up-regulation of IFN-α-induced genes was mediated by IFN-α receptor signaling, we treated NOD/BDC2.5 mice i.p. with 1 mg of neutralizing anti-IFNAR1 mAb on day 15, or on days 15 and 20, after birth (19). The expression of IFN-α signature genes in PLN CD4+ T cells was analyzed by real-time PCR on day 25. Whereas one injection of anti-IFNAR1 mAb partially suppressed the expression of IFN-α signature genes, two injections almost completely blocked the expression of these genes (Fig. 1D). Therefore, the increase of IFN-α-inducible gene expression is mediated through IFN-α receptor signaling.
pDCs in the PLNs Produce IFN-α.
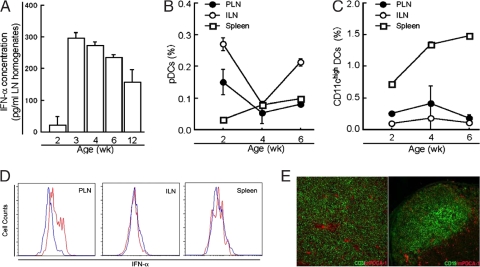
Increased IFN-α-inducible gene expression in the CD4+ T cells of the PLNs encouraged us to investigate the presence of IFN-α protein and IFN-α-producing pDCs in the PLNs. We measured IFN-α levels in PLN homogenates of NOD mice at different ages by ELISAs. Little or no IFN-α was detected at 2 weeks of age. However, IFN-α levels increased sharply at 3–4 weeks and decreased at 6 weeks (Fig. 2A). We then stained single cell suspensions from PLNs, inguinal lymph nodes (ILNs), and spleens of NOD mice using a mAb against mouse pDC antigen 1 (mPDCA-1), which is specifically expressed on pDCs, and an anti-CD11c mAb, and these were quantified by flow cytometry. The percentage of pDCs in the PLNs and ILNs sharply decreased from 2 to 4 weeks of age and increased at 6 weeks (Fig. 2B). In contrast, the percentage of myeloid CD11chigh DCs in the PLNs and ILNs increased slightly but statistically significantly from 2 to 4 weeks of age and decreased at 6 weeks (Fig. 2C). The percentage of both pDCs and CD11chigh DCs in the spleen increased with age. Consistent with increased IFN-α protein in the PLNs of 3-week-old NOD mice, intracellular staining revealed that a greater number of pDCs in the PLNs of 3-week-old mice spontaneously produced IFN-α than did those from 10-day-old mice (Fig. 2D). In frozen sections of the PLNs, most mPDCA-1+ pDCs were found in the CD3+ T cell areas, whereas rare pDCs were found in the CD19+ B cell areas (Fig. 2E). These results demonstrate that pDCs are present in the PLNs of NOD mice at various ages and contribute to the increased production of IFN-α. The sudden increase in production of IFN-α in PLNs at 3 weeks of age closely follows a wave of programmed cell death in pancreatic β cells that peaks at postnatal days 14–17 (3). It has been reported that TLR-2 is involved in the initiation of autoimmune responses and the development of T1D by sensing apoptotic cells in pancreas (20). The development of T1D is significantly decreased in TLR-2-deficient NOD mice. However, this study does not rule out the possible involvement of other TLRs such as TLR-3, TLR-7, or TLR-9, which have been implicated in the pathogenesis of other autoimmune diseases, in the development of T1D.
Fig. 2.
pDCs in the PLNs produce IFN-α. (A) Homogenates were prepared from the PLNs of 2-, 3-, 4-, 6-, and 12-week-old NOD mice and used for IFN-α ELISA. Data were obtained from three independent experiments, each pooled from 5 mice. (B and C) Single cell suspensions were prepared from the PLNs, ILNs, and spleen of 2-, 4-, and 6-week-old NOD mice (n = 3 per group), stained with mAbs against CD11c and mPDCA1, and examined by FACS. Data for the frequency of pDCs (B; CD11cintmPDCA-1+) and CD11chigh mDCs (C; CD11chighmPDCA-1−) were obtained from 3–4 mice at each time point. (D) Single cell suspensions were prepared from the PLNs of 10-day-old (blue) and 3-week-old (red) NOD mice and stained with mAbs against mPDCA-1, CD11c, and IFN-α. The overlay histogram shows the intracellular IFN-α level on gated CD11intmPDCA1+ pDCs. (E) Frozen sections of 4-week-old NOD mouse PLNs were stained with mAbs against mPDCA1 and CD3 (left) or mAbs against mPDCA-1 and CD19 (right) and visualized on a fluorescent microscope. Most pDCs (red) were localized in the CD3+ T cell area (green), whereas very few pDCs (red) were found in the CD19+ B cell area (green). The isotype control mAb for anti-CD3, CD19, or mPDCA1 mAb did not stain any cells (data not shown). These staining results are representative of those from 4 mice.
Our results support the hypothesis that the debris of apoptotic β cells, particularly ssRNA and dsDNA, activates pDCs through TLR-7 and -9, which leads to IFN-α secretion from PLN pDCs. This is followed by increased expression of IFN-α-inducible genes in PLN CD4+ T cells. Earlier studies have shown that β cell-derived IFN-α can induce insulitis and diabetes in transgenic mice, autoimmune-prone BioBreeding rats, and chemically induced models of T1D (13, 21, 22). Our findings indicate that increased IFN-α in the PLNs is an early molecular event in the spontaneous triggering of T cell-mediated autoimmunity.
Blockade of IFNAR1 Suppresses T1D in NOD Mice.
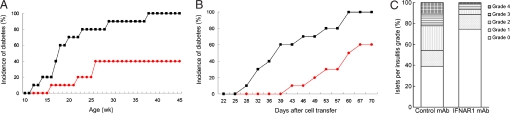
To examine the role of IFN-α at the initial stage of T1D development, we injected NOD mice with anti-IFNAR1 mAb or a control mAb on days 15 and 20 after birth and monitored the development of T1D. In the control mAb-treated group the first diabetic mouse was found at 12 weeks of age. In contrast, there was a 4-week delay in diabetes onset in anti-IFNAR1 mAb-treated mice. By 45 weeks of age, 100% of control mAb-treated mice and 40% of anti-IFNAR1 mAb-treated mice were diabetic (Fig. 3A, P < 0.01, χ2 test, 10 mice per group).
Fig. 3.
Blockade of IFNAR1 decreases the onset of T1D in WT NOD mice. (A) NOD mice were treated i.p. with 1 mg of anti-IFNAR1 (red) or control mAb (black) on days 15 and 20 after birth, and monitored for the onset of diabetes weekly (n = 10 per group). (B) Lymphocytes (1 × 107) were prepared from anti-IFNAR1-treated (red) or control mAb-treated (black) 12-week-old NOD mice, transferred i.v. into 6- to 8-week-old female NOD/SCID mice (n = 10 per group). The onset of diabetes in the recipient mice was monitored twice a week. (C) Insulitis was scored in 10- to 12-week-old, anti-IFNAR1-treated (n = 7) or control mAb-treated (n = 6) mice.
To determine if the suppression of T1D by anti-IFNAR1 mAb treatment results from alteration in the diabetic potential of lymphocytes, we transferred spleen lymphocytes from 12-week-old NOD mice that have been treated with anti-IFNAR1 or control mAb at 15 and 20 days of age, into NOD/SCID mice. Treatment of donors with anti-IFNAR1 mAb delayed the onset (43 vs 26 d after transfer) and decreased the incidence (60% vs 100% at d 70) of diabetes in the recipient mice, compared with treatment of donors with control mAb (Fig. 3B, P < 0.01, χ2 test, n = 10 mice/group). Histological sections of pancreata showed no inflammation in ≈40% of islets in control mAb-treated mice; peri-insulitis and insulitis were found in approximately 40% and 20% of islets, respectively (Fig. 3C). In contrast, in anti-IFNAR1 mAb-treated mice, more than 70% of islets were inflammation-free, whereas the percentages of islets with peri-insulitis and insulitis were less than 20% and 7%, respectively. Although the mean inflammation score in the islets of control mAb-treated group was higher than that in anti-IFNAR1 mAb-treated group, there was no significant statistical difference (1.2 ± 1.1 vs 0.5 ± 0.7, P > 0.05, n = 6–7 per group).
Our data indicate that IFN-α signaling is important in the development of T1D in NOD mice. Blockade of this signaling effectively prevents or delays the onset of the disease. This is consistent with the role of IFN-α in other models of T1D, as noted earlier. In non-autoimmune-prone mice, transgenic expression of IFN-α in islets, treatment of mice transgenically expressing CD80 on the islets with the IFN-α inducer (poly I:C), or treatment of mice with streptozotocin promotes insulitis and diabetes (13, 21, 22). More importantly, NOD mice lacking IFN regulatory factor-1 did not develop insulitis and diabetes, although T cells from these NOD mice proliferated more profoundly to Con A or the glutamic acid decarboxylase isoform GAD65 peptide than did those from WT NOD mice (14).
Previous studies have shown that oral IFN-α treatment of NOD mice 5 week or older significantly suppressed the development of insulitis and diabetes (15, 16). By 5 weeks of age, however, the principal autoimmune responses have already been established in the PLNs (1). Thus, the results obtained in these latter studies may reflect the effect of IFN-α on the progress of established autoimmunity, rather than on initiation of autoimmunity in the PLNs. Interestingly, in addition to the increase in IFN-α production in the PLNs at 3 weeks of age, the 3-week checkpoint also marks an abrupt reversal of TNF-α effects on the diabetic process (4). Therefore, these data show that IFN-α is important for initiating the early development of autoimmunity and the diabetogenic process in NOD mice.
Blockade of IFNAR1 Increases Immature DCs in the PLNs.
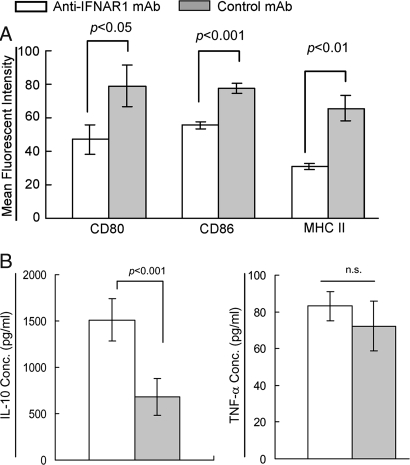
Given that DCs are critical for the initiation of the autoimmune response against β cell autoantigens in the PLNs in NOD mice, it is reasonable to investigate whether the suppression of T1D by anti-IFNAR1 mAb treatment is attributable to changes in the phenotype and maturation of DCs in the PLNs. The percentage of CD11c+ DCs was slightly increased in the PLNs, but not in ILNs or spleens, of anti-IFNAR1 mAb-treated 25-d-old mice compared with control mAb-treated mice (2.3 ± 0.2% vs. 1.8 ± 0.1% of gated mononuclear cells). Anti-IFNAR1 mAb treatment did not affect the relative percent of pDCs in the PLNs, ILNs, or spleens, although it slightly down-regulated expression of CD80, CD86, and MHC II, on the pDCs in the spleen (data not shown). CD11c+ DCs in the PLNs of anti-IFNAR1 mAb-treated mice displayed an immature phenotype with lower expression levels of CD80, CD86, and MHC II compared with those of control mAb-treated mice (Fig. 4A). In parallel with the immature phenotype, splenic CD11c+ DCs in anti-IFNAR1 mAb-treated mice acquired the ability to produce greater amounts of the immunoregulatory cytokine IL-10 in response to LPS compared with control mAb-treated NOD mice (Fig. 4B). However, anti-IFNAR1 mAb treatment had no effect on TNF-α production (Fig. 4B) by splenic DCs, whereas IL-12 and IFN-α were undetectable in both groups (data not shown).
Fig. 4.
Blockade of IFNAR1 increases immature DCs in the PLNs of NOD mice. NOD mice were treated i.p. with two injections of anti-IFNAR1 or control mAb at days 15 and 20 after birth (n = 4 per group). On day 25, single cell suspensions were prepared from the PLNs, ILNs, and spleens, and stained with mAbs against CD11c, mPDCA-1, CD80, CD86, and MHC II and analyzed by FACS. (A) Data represent the mean fluorescent intensity of CD80, CD86, or MHC II staining on gated CD11c+ DCs. (B) Splenic CD11c+ cells were magnetically enriched and stimulated with LPS (1 μg/ml) for 48 h in vitro. IL-10 or TNF-α levels in the supernatant were measured using an ELISA.
The function of DCs is largely dependent on their phenotype and activation status. Whereas immunogenic mature DCs are strong inducers of effector T cells, immature DCs are considered to be tolerogenic (23). Immature DCs that express lower levels of costimulatory molecules induce and maintain peripheral tolerance by induction of regulatory T cells and T cells secreting immunoregulatory cytokines (23). It has been shown that IFN-α promoted and sustained the maturation and migration of mouse and human DCs by inducing expression of CD80, CD86, CD40, and MHC (24, 25). DCs derived from IFNAR1-deficient mice failed to mature in response to IFN-α or IFN-α-inducing reagents, although these DCs migrated in vivo as well as did WT DCs (26, 27). Therefore, in this study, the increase of tolerogenic immature DCs and production of IL-10 in the PLNs may be causal in inhibiting inflammatory pathologic processes. Additionally, we do not preclude the possibility that anti-IFNAR1 mAb treatment may affect maturation of DCs during their migration into the PLNs from pancreatic islets through afferent lymphatics.
Blockade of IFNAR1 Enhances IL-4 and IL-10 Production by CD4+ T Cells.
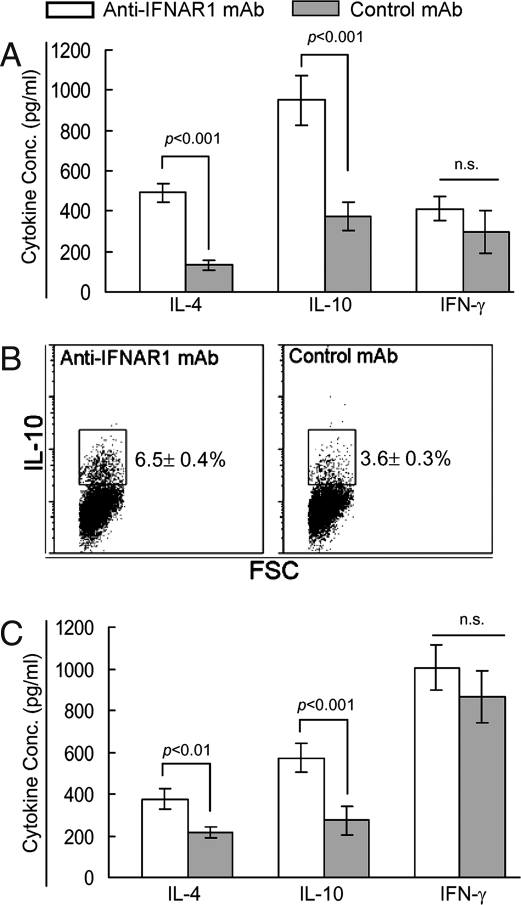
Because T1D is a T cell-mediated disease, we further examined whether blockade of IFN-α signaling altered T lymphocyte phenotype and functions in NOD mice, thus potentially influencing the development of diabetes. There were no significant differences between the treatment groups in the relative numbers of CD4+ and CD8+ T cells and their activation status as determined by the expression levels of CD44, CD62L, and CD69 molecules in the PLNs, ILNs, or spleens at 25 days or 12 weeks of age (data not shown). We then used cytokine ELISAs to examine splenic CD4+ T cells, which contained autoreactive memory/effector CD4+ T cells generated in the PLNs, for their ability to secret Th1 cytokines (i.e., IFN-γ) and Th2 cytokines (i.e., IL-4 and IL-10) in response to anti-CD3 and anti-CD28 mAbs in vitro. In accord with the relative increase of immature DCs in the PLNs, CD4+ T cells from anti-IFNAR1 mAb-treated, 25-day-old mice produced greater amounts of IL-4 and IL-10 than did those from control mAb-treated mice (Fig. 5A). Intracellular cytokine staining revealed that anti-CD3/CD28 mAbs-stimulated CD4+ T cells from the spleens of anti-IFNAR1 mAb-treated mice contained greater numbers of IL-10-prodcuing cells than control mAb-treated mice (Fig. 5B, 6.5 ± 0.4% vs. 3.6 ± 0.3%, P < 0.001). Similar effects on IL-4 and IL-10 production by splenic CD4+ T cells were also seen in the anti-IFNAR1 mAb-treated 12-week-old mice (Fig. 5C). However, anti-IFNAR1 mAb treatment did not affect the frequency of IFN-γ-producing CD4+ T cells or IFN-γ levels in the culture supernatants. Many studies have demonstrated that immature DCs suppress insulitis and T1D by selectively increasing IL-4- and/or IL-10-producing CD4+ T cells in the PLNs and/or pancreas (28, 29). Conversely, IFN-α has been shown to directly increase IFN-γ production by CD4+ T cells in vitro (30). Our data indicate that blockade of IFN-α signaling enhances the ability of CD4+ T cells to produce IL-4 and IL-10 but not IFN-γ, thereby creating an immunoregulatory cytokine-dominant environment in NOD mice. This immunoregulatory cytokine (IL-4 and IL-10) dominance, which led to the suppression of T1D, is driven by immature DCs generated after anti-IFNAR1 mAb treatment at 2–3 weeks of age.
Fig. 5.
Blockade of IFNAR1 enhances IL-4 and IL-10 production by CD4+ T cells. NOD mice were treated i.p. with anti-IFNAR1 or control mAb on days 15 and 20 after birth, and killed at 25 days or 12 weeks of age (n = 4 per group). CD4+ T cells were purified from spleen and stimulated with anti-CD3 and CD28 mAbs for 48 h in vitro. (A) IL-4, IL-10, and IFN-γ secretion in culture supernatants by stimulated CD4+ T cells of 25-day-old mice was measured by ELISA. (B) Representative FACS plots show intracellular IL-10 staining in CD4+ T cells from anti-IFNAR1- or control mAb-treated 25-day-old NOD mice after stimulation with anti-CD3 and CD28 mAbs, followed by PMA and ionomycin. (C) IL-4, IL-10, and IFN-γ secretion in culture supernatants by stimulated CD4+ T cells from 12-week-old mice was measured by ELISA.
In summary, we have demonstrated increased expression of several IFN-α-inducible genes in CD4+ T cells and increased production of IFN-α in the PLNs of 3- to 4-week-old NOD mice. Blockade of IFN-α signaling by an anti-IFNAR1 mAb during this period significantly delayed the onset and decreased the incidence of T1D in NOD mice. These results reveal that IFN-α is a primary initiator of the type 1 diabetic process, which can occur spontaneously in germ-free animals, without the intervention or action of an external agent. Because there are many genes in the IFN-α family (14 in mouse and 13 in human), further efforts should be made to identify which IFN-α(s) are involved in the initiation of the diabetic process, and subsequently to develop strategies to block these IFN-α molecules and prevent the initiation of T1D in the NOD mouse. Our results also have implications for the many other autoimmune diseases such as systemic lupus and rheumatoid arthritis, in which endogenous IFN-α may play a greater or lesser role in the initiation and continuation of the autoimmune disease.
Materials and Methods
Mice.
NOD/LtJ and NOD/SCID mice were purchased from The Jackson Laboratory. NOD/BDC 2.5 T cell receptor transgenic mice were kindly provided by Dr. Diane Mathis (Joslin Diabetes Center, Harvard University, Boston, MA). All animals were housed and bred under barrier conditions in Stanford University animal facilities. All animal studies have been approved by Stanford University's Administrative Panel for Laboratory Animal Care.
Magnetic Sorting of Cells.
Single mononuclear cells were prepared from the PLNs and spleens of NOD or NOD/BDC 2.5 mice by a routine mechanical dissection method. CD4+ T cells and CD11c+ DCs were purified using a CD4+ T Cell Isolation Kit and anti-CD11c microbeads (Miltenyi Biotec), respectively, following the manufacturer's instructions.
Microarray Analysis.
CD4+ T cells from the PLNs of 2-, 6-, and 12-week-old female NOD and NOD/BDC 2.5 mice were purified as mentioned earlier. mRNA was extracted and linearly amplified using an Amino Allyl MessageAmp II amplified RNA kit (Ambion). An amplified RNA sample from 2-week-old NOD mice was used as a standard reference control and labeled with Cy3 fluorophore, whereas an aRNA sample from the other groups was labeled with a Cy5 fluorophore. We used Mouse Exonic Evidence Based Oligonucleotide (MEEBO) arrays containing 38,784 70mer probes provided by Stanford Functional Genomics Facility. Significance Analysis of Microarrays was used to identify differentially expressed genes in the time-course study.
Quantitative Real-Time PCR.
Total RNA was extracted from purified CD4+ T cells with an RNeasy Kit (Qiagen). Reverse transcription was done using a QuantiTect Reverse Transcription Kit (Qiagen). Real-time PCR was carried out with a pathway-focused RT2 Profiler PCR Array (SuperArray Bioscience).
mAbs for Flow Cytometric Analysis.
Fluorescent-labeled mAbs specific for CD4 (L3T4), CD8 (H35–17.2), CD25 (PC61.5), Foxp3 (FJK-16s), MHC II (I-A/I-E) (M5/114.15.2), CD69 (H1.2F3), CD44 (IM7), CD62L (MEL-14), CD11c (N418), CD11b (M1/70), IL-4 (BVD6–24G2), IL-10 (JES5–16E3), and IFN-γ (XMG1.2) were purchased from eBioscience; mPDCA-1 (JF05–1C2.4.1) was from Miltenyi; CD80 (16–10A1) and CD86 (GL-1) were from BioLegend; and mouse IFN-α (RMMA) was from PBL Biomedical Laboratories.
DC Analysis.
Single cell suspensions from the PLNs, ILNs, and spleens were stained with mAbs against CD11c, mPDCA-1, MHC II, CD80, and CD86, and analyzed by FACS. For cytokine analysis, CD11c+ DCs were purified from spleens and cultured in 96-well plates (1 × 105 cells/well) with stimulation by LPS (1 μg/ml) for 48 h. The supernatant was collected to measure the secretion of IL-10, IL-12, TNF-α, and IFN-α by ELISA.
For immunostaining of pDCs, frozen sections were prepared from the PLNs of 4-week-old NOD mice and fixed with cold acetone. The sections were incubated with rat anti-mouse CD3 (17A2, eBiosciences), rat anti-mouse CD19 (1D3, eBiosciences), or control mAb at room temperature for 1 h followed by 30 min incubation with Alexa Fluor 488 anti-rat IgG antibody (Vector). The sections were then sequentially incubated with 20% normal rat serum, biotin rat anti-mouse mPDCA1 (or control mAb), and Alexa Fluor 546 streptavidin (Vector). The slides were mounted with mounting medium and visualized and imaged on a fluorescent microscope using Image-Pro software (Media Cybernetic).
In Vivo Treatment.
For IFNAR1 blocking experiments, WT NOD mice were treated i.p. with 1 mg of anti-mouse IFNAR-1 antibody (MAR1–5A3) or an irrelevant isotype-matched control mAb (GIR-208) at days 15 and 20 after birth. Animals were killed at 25 days or 12 weeks of age.
Adoptive Transfer Studies and Assessment of T1D and Insulitis.
Ten million unfractionated spleen cells from anti-IFNAR1- or control mAb-treated, 12-week-old NOD mice were injected i.v. into 5–8-week-old female NOD.SCID mice. The final volume of all injections was 250 μl per mouse in PBS. Urine glucose levels were monitored using Chemstrips (Roche Diagnostics). Mice were considered diabetic on the first of three consecutive high glucose readings and were killed after the third high reading.
Pancreata were harvested from 10–12-week-old NOD mice that were treated i.p. with anti-IFNAR1 or control mAb on days 15 and 20 after birth, and embedded in optimum cutting temperature medium. For each pancreas, 10 sections were collected every 100 μm, fixed with acetone, and stained with hematoxylin and eosin. Inflammation in each islet was graded on a scale from 0 to 4 as follows: grade 0 (no inflammation), grade 1 (peri-insulitis involving <50% of the islet circumference), grade 2 (peri-insulitis involving >50% of the islet circumference), grade 3 (insulitis involving <50% of the islet area) and grade 4 (insulitis involving >50% of the islet area). The inflammation score for each mouse was calculated based on inflammation grading in all islets.
Cytokine Assays.
Ninety-six-well plates were precoated with 5 μg/ml of anti-CD3 mAb. Purified spleen CD4+ T cells (2 × 105) were loaded in each well with 1 μg/ml of anti-CD28 mAb and cultured in a humidified 37°C incubator for 48 h. Supernatants were collected for ELISA analysis of IL-4, IL-10, and IFN-γ production (all of the antibody pairs for ELISA were purchased from eBiosciences). Cells were then re-stimulated with PMA (50 ng/ml) and ionomycin (2 μg/ml) for 6 h. Brefeldin A was added during the final 2 h of the stimulation. The cells were fixed, permeabilized and stained with FITC-IL-4, PE-IL-10, and PE/Cy7-IFN-γ, and analyzed by FACS.
To measure IFN-α production, pancreatic LNs from 2-, 3-, 4-, 6-, and 12-week-old NOD mice (n = 5 per group) were dissected, pooled, and lysed with radioimmunoprecipitation assay buffer (Sigma-Aldrich) in the presence of protease inhibitor (Roche Diagnostics). Lysates were centrifuged and the supernatants were removed and titrated for the level of IFN-α by ELISA. Mouse IFN-α ELISA kits were obtained from PBL Biomedical Laboratories.
Statistical Analysis.
Statistical significance between groups was determined by a two-tailed Student t test or χ2 test. All P values less than 0.05 were considered to represent statistically significant differences. Results are shown as the mean ± SEM, with SEM represented by the error bars.
Acknowledgments.
This work was supported by Juvenile Diabetes Research Foundation grants 2004–794 and 2007–745 (to H.O.M.); American Diabetes Association Mentor-Based Postdoctoral Fellowship (to H.O.M.); and National Institute of Health grant R01 DK67592 (to S.A.M.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 3.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 4.Yang XD, et al. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 8.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44:658–664. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- 10.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;2:1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- 11.Guerci AP, et al. Onset of insulin-dependent diabetes mellitus after interferon-alfa therapy for hairy cell leukaemia. Lancet. 1994;343:1167–1168. doi: 10.1016/s0140-6736(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 12.Fabris P, et al. Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Aliment Pharmacol Ther. 2003;18:549–558. doi: 10.1046/j.1365-2036.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 13.Stewart TA, et al. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa T, et al. Complete suppression of insulitis and diabetes in NOD mice lacking interferon regulatory factor-1. J Autoimmun. 2001;17:119–125. doi: 10.1006/jaut.2001.0531. [DOI] [PubMed] [Google Scholar]
- 15.Brod SA, Malone M, Darcan S, Papolla M, Nelson L. Ingested interferon alpha suppresses type I diabetes in non-obese diabetic mice. Diabetologia. 1998;41:1227–1232. doi: 10.1007/s001250051056. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka-Kataoka M, et al. Oral use of interferon-alpha delays the onset of insulin-dependent diabetes mellitus in nonobese diabetes mice. J Interferon Cytokine Res. 1999;19:877–879. doi: 10.1089/107999099313398. [DOI] [PubMed] [Google Scholar]
- 17.Yang XD, Karin N, Tisch R, Steinman L, McDevitt HO. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proc Natl Acad Sci USA. 1993;90:10494–10498. doi: 10.1073/pnas.90.22.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee LF, et al. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: analysis of dendritic cell maturation. Proc Natl Acad Sci USA. 2005;102:15995–16000. doi: 10.1073/pnas.0508122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehan KC, et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Devendra D, et al. Interferon-alpha as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes. 2005;54:2549–2556. doi: 10.2337/diabetes.54.9.2549. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Hultgren B, Dybdal N, Stewart TA. Islet expression of interferon-alpha precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity. 1994;1:469–478. doi: 10.1016/1074-7613(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 24.Luft T, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 25.Ito T, et al. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 26.Dalod M, et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda K, et al. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hancock WW, Polanski M, Zhang J, Blogg N, Weiner HL. Suppression of insulitis in non-obese diabetic (NOD) mice by oral insulin administration is associated with selective expression of interleukin-4 and -10, transforming growth factor-beta, and prostaglandin-E. Am J Pathol. 1995;147:1193–1199. [PMC free article] [PubMed] [Google Scholar]
- 29.Ploix C, et al. Protection against autoimmune diabetes with oral insulin is associated with the presence of IL-4 type 2 T-cells in the pancreas and pancreatic lymph nodes. Diabetes. 1998;47:39–44. doi: 10.2337/diab.47.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Cho HJ, et al. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J Immunol. 2002;168:4907–4913. doi: 10.4049/jimmunol.168.10.4907. [DOI] [PubMed] [Google Scholar]