Abstract
Amplification of the receptor tyrosine kinase ErbB2 is frequently observed in breast cancer. Amplification of erbB2 is also associated with multiple genomic gains and losses; however, the importance of these associated changes is largely unknown. We demonstrate that Brk, a cytoplasmic tyrosine kinase, is coamplified and coexpressed with ErbB2 in human breast cancers. ErbB2 interacts with Brk and increases its intrinsic kinase activity. Expression of Brk enhances the ErbB2-induced activation of Ras/MAPK signaling and cyclin E/cdk2 activity to induce cell proliferation of mammary 3-dimensional acini in culture. In a murine model of breast cancer, expression of Brk was found to shorten the latency of ErbB2-induced tumors by promoting cell proliferation, with no effect on protection from apoptosis. Furthermore, overexpression of Brk conferred resistance to the ability of Lapatinib, an ErbB2 kinase inhibitor, to inhibit ErbB2-induced proliferation. Thus, we identified Brk as a drug target for ErbB2-positive cancers.
Keywords: amplification, lapatinib, tumorigenesis
ErbB2 belongs to the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (1). Amplification of erbB2 occurs in approximately 25% of breast cancers and is correlated with poor clinical outcomes (2). Although ErbB2 is not activated by direct binding of a soluble ligand, it is activated by ligand-induced formation of heterodimers with other EGFR family members, namely, EGFR/ErbB1, ErbB3, and ErbB4. Overexpression of ErbB2 can also result in ligand-independent receptor homodimerization and activation (1, 3). Overexpression of neu (the murine homologue of human erbB2) under the control of mouse mammary tumor virus (MMTV) long terminal repeat (LTR) results in mammary tumorigenesis (4), suggesting that erbB2 can initiate tumorigenesis in vivo.
ErbB2 is a drug target for human breast cancer. A humanized monoclonal antibody against the extracellular domain of ErbB2, Herceptin, in combination with chemotherapy, is used to treat patients expressing high levels of ErbB2 (5). In patients who respond to the treatment, Herceptin can delay mortality anywhere between 9 months and 3 years (5). However, almost 50% of patients with tumors that contain erbB2 amplification do not respond to Herceptin (6), and those who do respond develop resistance to the drug, highlighting the need for better options to treat patients with ErbB2-positive cancers. A better understanding of the ErbB2 signaling pathway can identify novel targets for combination therapy that will significantly aid our ability to treat patients with ErbB2-amplified breast cancers.
Members of the ErbB2 signaling pathway can also be coamplified with ErbB2 in breast cancer (7–10). For example, Grb7, a Src homology domain (SH2) containing an adaptor molecule that associates with ErbB2 and amplifies signaling by ErbB2, is a component of the ErbB2 amplicon (10). In addition to the erbB2 amplicon, erbB2-amplified tumors also are characterized by several gains and losses located elsewhere in the genome (7, 8). Whether these genomic alterations contain novel members of the ErbB2 signaling pathway that are involved in initiation, progression, and maintenance of erbB2-amplified breast tumor is poorly understood.
The nonreceptor tyrosine kinase Brk (breast tumor kinase, also known as PTK6), located on Chr 20q13.3, was identified from a metastatic human breast carcinoma (11, 12). Brk is overexpressed in approximately two-thirds of breast cancers and is also overexpressed in cancers such as melanomas and colon and prostate tumors. Brk expression is limited or undetectable in normal breast epithelial cells (11), suggesting a tumor cell–specific function for this kinase. Overexpression of Brk sensitizes human mammary epithelial cell to epidermal growth factor (EGF) and heregulin stimulation and increases anchorage-independent growth. Down-regulation of Brk interferes with EGF- and Heregulin-induced cell proliferation, suggesting that Brk contributes to signaling by members of the EGF receptor family (12–14). Brk and ErbB2 are simultaneously overexpressed in tissue from invasive ductal breast carcinomas (15). Immunohistochemistry analyses demonstrated that expression of Brk and ErbB2 and ErbB4 correlated with metastasis-free survival (16). The coexpression of these 2 oncogenic kinases suggested a clinically relevant link between them, but the significance of this coexpression is not known.
Here, we demonstrate that brk is frequently coamplified with erbB2. Brk associates with ErbB2, induces prolonged activation of the Ras/MAPK pathway, and promotes cell proliferation. Overexpression of Brk cooperated with ErbB2 during murine mammary tumorigenesis. In addition, coexpression of Brk conferred resistance to the ability of ErbB2 kinase inhibitors to inhibit ErbB2-induced cell proliferation. Thus, we identify Brk as coamplification partner for ErbB2, and demonstrate that Brk cooperates with ErbB2 to increase the proliferative potential of ErbB2 positive tumors in vivo and to confer resistance to anti-ErbB2 therapies.
Results
ErbB2 and Brk Are Coamplified and Coexpressed in Human Breast Cancers.
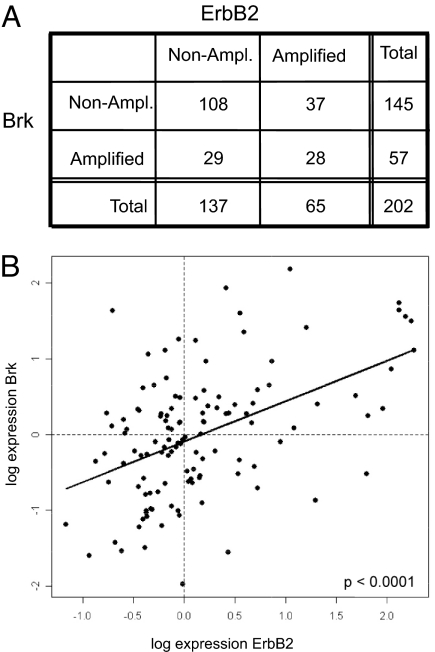
Brk localizes to Chr 20q13.3, a region of the genome that is frequently amplified in breast cancer (7, 8). To determine whether brk is coamplified with ErbB2 in breast cancer, we analyzed genomic changes in breast cancer, using data generated by representational oligonucleotide microarray analyses (ROMA), an array-based approach that uses representation to reduce genomic complexity (17). A detailed analysis of genomic changes observed by ROMA analysis of 202 breast cancer tumor samples of Swedish and Norwegian origin was published elsewhere (8). To determine whether amplification of erbB2 correlates with amplification of genomic locus containing brk, a 2 × 2 contingency table was generated for 2 categorical variables, erbB2 and brk, in 2 observation states, amplified and nonamplified (Fig. 1A). Each entry represents the number of observations for a given gene (variable) in 1 observation state. Amplification of erbB2 was strongly associated with amplification of brk, as indicated by Fisher's exact test (P = 0.0024; odds ratio 2.8), suggesting a nonrandom association between erbB2 and brk.
Fig. 1.
Coamplification and cooverexpression of ErbB2 and Brk in human breast cancer. (A) A 2 × 2 contingency table was generated for 2 categorical variables, ErbB2 and PTK6, in 2 observation states, amplified and not amplified; Each entry represents the number of observations for a given gene (variable) in 1 observation state. Fisher's exact demonstrated that there is a nonrandom association between ErbB2 and PTK6 with a P value of 2 × 10e-3 (odds ratio, 2.8; 95% confidence interval, 1.410561–5.601979). (B) Scatter plot of gene expression levels of Brk versus ErbB2 expression for 113 Norwegian tumors. Vertical and horizontal dotted lines represent median values for ErbB2 and Brk, respectively. Linear least-squares fit to the data are shown as a solid straight line; dashed coordinate axis lines are drawn through the origin. Slope of fit is 0.53 ± 0.09 (slope is different from 0 with a P value of 3.4 × 10−8).
To determine whether coamplification correlated with cooverexpression, we reanalyzed changes in expression of erbB2 and brk, using a recently published cDNA microarray analysis dataset of 113 Norwegian breast tumors (18). The values are median-polished log ratios of expression in tumor cells to that in a reference cell mixture. A scatter plot of brk versus erbB2 expression for 113 Norwegian tumors was generated, and tumors with below-median and above-median erbB2 and brk expression are separated by vertical and horizontal dotted lines, respectively (Fig. 2B). A linear least-squares fit to the data are shown as a solid straight line. The slope of the fit is 0.53 ± 0.09 (the slope is different from 0 with a P value of 3.4 × 10e-8), suggesting that there is a significant correlation between expression of erbB2 and brk.
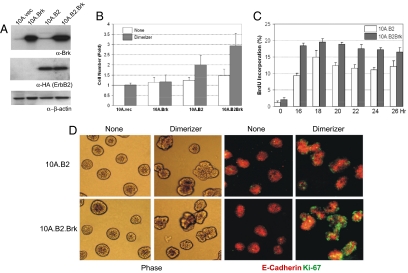
Fig. 2.
Brk enhances ErbB2-induced proliferation of MCF-10A cells. (A) MCF-10A cell populations transfected with empty vector pLPCX (10A.vec), or vectors expressing Brk (10A.Brk), chimeric ErbB2 (10A.cB2), or both Brk and chimeric ErbB2 (10A.cB2.Brk) were generated, and expression of Brk and chimeric ErbB2 was determined by immunoblotting. Blot was reprobed with β-actin to serve as loading control. Cells were incubated in the absence of serum and EGF overnight, and were stimulated with carrier (None) or 1 μmol/L AP1510 (Dimerizer) for 24 h. Changes in cell numbers (B) or in the ability to incorporate BrdU (C) were determined. Data presented in (B) and (C) represent an average of three independent experiments. (D) 10A.cB2 and 10A.cB2Brk cells were cultured on Matrigel for 14 days. The acini structures were treated with either carrier (none) or 1 μM AP1510 (dimerizer) for 24 h. Phase-contrast or immunoflorescence images for Ki-67 (green) and E-cadherin (red) are shown.
Brk Overexpression Promotes ErbB2-Induced Cell Cycle Progression.
To investigate the effect of coexpressing Brk and ErbB2, we expressed Brk in MCF-10A cells together with an inducible chimeric form of ErbB2 (herein referred to as chimeric-ErbB2 or cB2) (19). Unlike the wild-type ErbB2 receptors, the chimeric ErbB2 cannot be activated by ligand-induced heterodimerization because the extracellular domain is replaced with p75 nerve growth factor receptor (20). The chimera has an FK506 binding protein (FKBP) fused to the C terminus and can be homodimerized by using a cell-permeable, bivalent FKBP ligand AP1510 (dimerizer) (see Materials and Methods for details). An immortalized EGF-dependent, nontumorigenic, human mammary epithelial cell line, MCF-10A, was used in these studies. MCF-10A cells expressing Brk alone (10A.Brk), ErbB2 alone (10A.cB2), or Brk with ErbB2 (10A.cB2.Brk) were generated by retroviral infections. All of the cells used in this study were generated as pooled populations of drug-resistant cells to avoid any clonal variations. 10A.cB2 and 10A.cB2.Brk cells expressed similar levels of the ErbB2 chimera, as detected by anti-HA immunoblotting. 10A.Brk and 10A.cB2.Brk cells showed comparable expression of Brk (Fig. 2A), which was higher than the endogenous levels detected in 10A.Vec and 10A.cB2.
We have shown that dimerization of ErbB2 induces EGF-independent proliferation of MCF-10A cells (19). We investigated whether coexpression of Brk affected cell proliferation. Although expression of Brk alone was not sufficient to promote EGF-independent proliferation of MCF-10A cells, it cooperated with ErbB2 to enhance the ability of ErbB2 to induce an increase in cell number (Fig. 2B). In addition, the cells were analyzed by flow cytometry to monitor changes in cell cycle. 10A.cB2.Brk cells maintained a higher percentage of cells in the S phase at all time points analyzed. In addition, there was a 2-fold greater percentage of cells in the S phase in 10A.cB2.Brk cells than that observed in 10A.cB2 cells (18% vs. 9%) within the first 16 h after activation [Fig. 2C and supporting information (SI) Fig. S1], suggesting that the cells entered the S phase faster and maintained high proliferation rates in the presence of Brk. Thus, Brk increases ErbB2-induced cell proliferation by promoting rapid S phase entry.
Brk Enhances ErbB2-Induced Reinitiation of Proliferation in 3D Epithelial Acini.
We have demonstrated that although both ErbB1 and ErbB2 can induce EGF-independent proliferation of MCF-10A cells on plastic dishes, only ErbB2 reinitiates proliferation of proliferation arrested, 3-dimensional (3D) acini structures grown in a bed of extracellular matrix, Matrigel (19). We investigated whether Brk cooperates with ErbB2 during reinitiation of proliferation in proliferation-arrested MCF-10A 3D acini. Both parental ErbB2-expressing cells and the cells coexpressing ErbB2 and Brk had low proliferation rates, as observed using Ki-67 as a proliferation cell marker, in the absence of ErbB2 activation. Stimulation of ErbB2 induced 2- to 3-fold higher proliferation rates in 10A.cB2 Brk acini than that observed in 10A.cB2 (Fig. 2D). These results demonstrate that overexpression of Brk enhanced the ability of ErbB2 to reinitiate cell proliferation in 3D acini structures.
Overexpression of Brk Promotes ErbB2-Induced Activation of CyclinE/cdk2 Complex.
To determine how coexpression of Brk enhanced ErbB2-induced proliferation, we analyzed changes in the expression of cyclins and cell-cycle inhibitors that regulate the G1 phase of the cell cycle. Activation of ErbB2 in serum-starved 10A.cB2.Brk cells did not affect the ErbB2-induced expression of cyclin D1 (Fig. S2A). By contrast, expression of Brk significantly increased the ErbB2-induced increase in Cyclin E and decrease of the cell cycle inhibitor p27kip1 within 3 to 6 h, which was faster than that observed for 10A.cB2 cells (6–9 h) (Fig. S2B). These observations suggest that coexpression of Brk enhances ErbB2-induced expression of cyclin E and decreased levels of p27kip1 to promote progression through G1 phase of the cell cycle.
The change in the levels of cyclin E and p27 suggested that Brk may promote ErbB2-induced activation of cyclin E/Cdk2 activity. Activation of ErbB2-induced maximal activation of Cdk2 kinase activity within 6 h of activation of ErbB2 in cells expressing Brk, whereas a similar increase in Cdk2 activity was not observed until 9–12 h in the absence of Brk (Fig. S2C).
Brk Interacts with ErbB2 and Induces Prolonged Activation of MAPK Pathway.
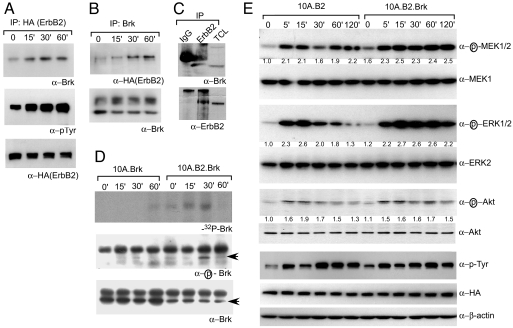
To gain insight into how ErbB2 and Brk cooperate to promote cell cycle progression, we investigated whether Brk is a novel component of the ErbB2 signal transduction pathway. Activation of ErbB2 resulted in an association between ErbB2 and Brk as determined by coimmunoprecipitation analysis (Fig. 3 A and B). In addition, ErbB2 and Brk interact with each other in an ErbB2 overexpressing breast cancer-derived cell line, T47D (Fig. 3C). To determine whether ErbB2 increased the intrinsic kinase activity of Brk, anti-Brk immunoprecipitates were subjected to in vitro autophosphorylation assays. Although Brk immunoprecipitated from cell lysates that do not express ErbB2 did not show any significant change in response to stimulation with dimerizer, activation of ErbB2-induced the intrinsic kinase activity of Brk (Fig. 3D Top). The modest increase in the kinase activity in 10.Brk cells at 60 min is due to higher level of total Brk present in the immunoprecipitate (Fig. 3D Bottom). Interestingly the highest change in ErbB2-induced increase in Brk activity was observed at 30 min and returned to basal levels by 60 min, suggesting that there is temporal regulation of Brk activity by ErbB2. We have demonstrated that activation of Brk is associated with phosphorylation of Tyr-342 within its kinase domain (21). We generated an antibody that can recognize phosphorylated Tyr-342 to serve as an independent assay to monitor changes in Brk activation. Dimerization of ErbB2 induced a significant increase in phosphorylation of Tyr-342 that was not observed in Brk immunoprecipitates from lysates derived from cells that do not express ErbB2 (Fig. 3D). As observed in the in vitro kinase assay, the highest levels of phospho-Brk were observed 30 min after ErbB2 activation and retuned to basal levels by 60 min. Taken together, these analyses demonstrate that ErbB2 directly recruits Brk and transiently activates the intrinsic kinase activity of Brk.
Fig. 3.
Brk associates with activated ErbB2 and enhances downstream signaling by ErbB2. 10A.cB2Brk cells were starved and stimulated with 1.0 μM dimerizer for indicated time. (A) ErbB2 was immunoprecipitated and was immunoblotted with anti-Brk or anti-pTyr. (B) Conversely, anti-Brk immunoprecipitates were immunoblotted with anti-ErbB2 antibodies. (C) ErbB2 or a nonspecific control (IgG) was immunoprecipiated from T47D cell lysates and immunoprecipitates were immunoblotted with anti-Brk antibodies. (D) Anti-Brk immunoprecipitates were subjected to autophosphorylation assays (Top). Blots were reprobed by using a phospho-specific (Middle) or total-Brk (Lower) antibody. (E) 10.B2 or 10A.cB2.Brk cells were stimulated with dimerizer, and lysates were immunoblotted by using phospho-specific and total antibodies, as indicated. β-Actin immunoblot serves as the loading control. Fold changes were calculated by normalizing the values to levels of unphosphorylated proteins.
Two key events downstream of ErbB2 are activation of the Ras/MAPK and PI3K/Akt pathways (1). We monitored changes in phosphorylation of Mek, Erk, and Akt to determine whether coexpression of Brk affects ErbB2-induced downstream signaling. ErbB2 induced a maximal increase in phosphorylation of Mek and Erk within 15 min of dimerization both in parental and Brk overexpressing cells (Fig. 3E). In the absence of Brk coexpression, phosphorylation of Erk began to decrease by 30 min and reached basal levels by 60–120 min. In contrast, in cells coexpressing Brk, ErbB2-induced phosphorylation of Erk was sustained throughout the 120 min (Fig. 3E). Coexpression of Brk did not have an effect on ErbB2-induced changes in phosphorylation of Akt (Fig. 3E), suggesting that among the signaling pathways downstream of ErbB2, Brk selectively enhances the Ras/MAPK pathways but not the PI3K/Akt pathway. The prolonged activation was not to due an increase in autophosphorylation of ErbB2, because we did not observe any difference in the levels of tyrosine phosphorylated ErbB2 in cells expressing Brk (Fig. 3E). Thus, we demonstrate that Brk is a novel component of the ErbB2 signaling pathway that prolongs activation of Ras/MAPK.
Brk Kinase Activity Is Required for ErbB2-Induced Proliferation and Decreases Lapatinib Efficacy.
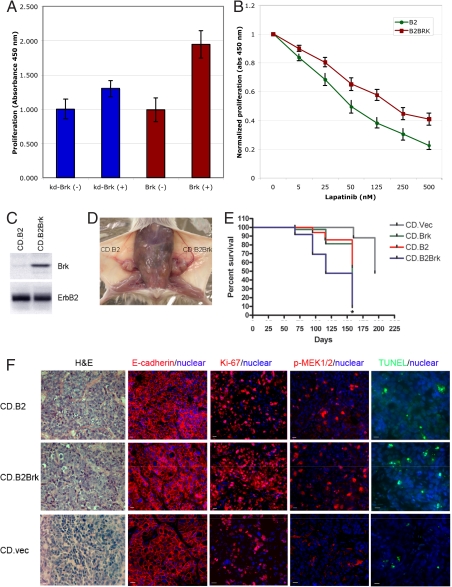
To determine whether Brk is required for ErbB2-induced cell proliferation, we expressed a Brk mutant (K219M) that lacks intrinsic kinase activity and that will function as a dominant negative to interfere with the ErbB2-Brk signaling axis. Expression of the kinase-dead version of Brk (kd.Brk) significantly inhibited ErbB2-induced BrdU incorporation (Fig. 4A), demonstrating that the Brk kinase plays a critical role during ErbB2-induced cell proliferation in mammary epithelial cells.
Fig. 4.
Brk enhances ErbB2-induced mammary tumorigenesis in mice. (A) 10A.cB2.Brk (Brk) or 10A.cB2.kd-Brk (kd-Brk) cells were starved for 36 h and stimulated with AP1510 (±). BrdU was added for the last 4.0 hours of stimulation. The amount of BrdU incorporation was quantitated as outlined in Materials and Methods. (B) 10A.cB2 or 10A.cB2.Brk cells were starved and stimulated with AP1510 in the presence of increasing concentrations of Lapatinib. The amount of BrdU incorporation was determined as outlined above. (C) Comma-1D cells expressing full-length ErbB2 (CD.B2) or full-length ErbB2 and Brk (CD.B2.Brk) were generated. (D) CD.B2 or CD.B2Brk cells were transplanted into epithelium-free mammary fat pads of syngenic BALB/c mice, and mammary tumorigenesis was monitored. Image shows representative animal transplanted with CD.B2 and CD.B2.Brk in contralateral sides. (E) Tumor growth was monitored over time starting from 8 weeks post-transplantation. Kaplan–Meier curve shows incidence of mammary tumor in mice transplanted with different cell types. Statistical significance for the difference between CD.Brk and CD.B2.Brk was determined by the log-rank test (*, P < 0.05) (F) Tumors from CD.B2 or CD.B2.Brk transplantation were fixed and sectioned to perform H&E staining; expression of ki-67 and E-cadherin, and the presence of phosphorylated Mek (p-Mek) was monitored by immunohistochemistry. The presence of apoptotic nuclei was monitored by TUNEL.
Molecules that cooperate with ErbB2 to promote proliferation are likely to have an impact on anti-ErbB2 therapeutics. To determine whether coexpression of Brk affects the ability of an ErbB2 kinase inhibitor to inhibit proliferation, we investigated the ability of ErbB2 to induce proliferation in the presence of increasing concentrations of Lapatinib, an ATP-competitive small- molecule inhibitor of ErbB2 and EGFR. Coexpression of Brk significantly increased the amount of Lapatinib required to inhibit ErbB-induced proliferation (Fig. 4B) demonstrating that Brk decreases the efficacy of Lapatinib action in ErbB2-responsive mammary epithelial cells.
Brk Synergizes with ErbB2 in Promoting Murine Mammary Tumorigenesis.
The in vivo significance of the ErbB2-Brk pathway was determined by using an orthotopic transplantation-based mammary tumorigenesis model with an immortalized pluripotent mammary epithelial cell line, Comma-1Dβgeo (CD). When transplanted into epithelium-free mammary fat pads of syngenic BALB/c mice, parental CD cells form hyperplastic mammary ductal outgrowths (22) and spontaneously form tumors by 4 months. To determine the effect of coexpressing ErbB2 and Brk, Comma-1D cells stably overexpressing ErbB2 alone or both Brk and ErbB2 (referred as CD.B2 and CD.B2.Brk, respectively) were generated (Fig. 4C). The engineered cells were injected into the cleared fat pads of 3-week-old female BALB/c mice, and tumor occurrence was monitored over time. Both CD.B2- and CD.B2.Brk-transplanted mice developed mammary tumors (Fig. 4D). CD.B2.Brk-transplanted mice started to develop mammary tumors an average of 73 days after transplantation, compared with the average latency of 95 days in CD.B2-transplanted mice (Fig. 4E) (P < 0.01). Comma-1D cells expressing Brk alone or empty vector could also induce tumors in cleared fat pads, with latencies of 98 and 125 days on average, respectively.
Both CD.B2 and CD.B2.Brk cells gave rise to tumors with epithelial properties, as monitored by expression of E-cadherin (Fig. 4F). To determine whether the shorter latency was due to changes in rates of cell proliferation or to a decrease in cell apoptosis or both, tumor tissues were analyzed by using a proliferation marker Ki-67, or for the presence of terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL)-positive cells. Consistent with the in vitro cell cycle data, CD.B2.Brk tumors showed increased proliferation, as reflected by a higher percentage of Ki-67-positive cells compared with CD.B2 tumors (78% cells in CD.B2.Brk tumors and 45% cells in CD.B2 tumors) (Fig. 4F). Furthermore, coexpression of Brk increased the level of phosphorylated-MEK in CD.B2.Brk tumors (32% positive cells) compared with CD.B2 tumors (17% positive cells) (Fig. 4F), suggesting that the ErbB2-Brk coexpression enhances signaling to the Ras/MAPK pathway both in culture and in vivo. There was no significant difference in the rates of cell apoptosis (6% for CD.B2 and 8% for CD.B2.Brk) as monitored by TUNEL labeling (Fig. 4F), suggesting that the ErbB2-Brk pathway promotes mammary tumorigenesis by regulating proliferation and not by protecting cells from apoptosis.
Discussion
We have identified Brk as a novel component of the ErbB2 signaling pathway that is coamplified and cooverexpressed with ErbB2 in human breast cancer.
We demonstrate that coexpression of Brk and ErbB2 promotes proliferation by inducing prolonged activation of the Ras/MAPK pathway. The relationship between prolonged activation of the Ras/MAPK pathway and cell cycle is consistent with several reports, using fibroblasts in which extended activation of Ras/MAPK is required for stimulation of cell cycle progression (23, 24). Our results are consistent with previous reports in which cooperation between growth factor and integrin signaling is required for induction of CyclinE/Cdk2 but not cyclin D1 in epithelial cells (25). Overexpression of cyclin E is observed in ER-negative tumors and is associated with cancer relapse and patient death (26–28). The mechanisms by which cyclin E levels increase in breast tumors are poorly understood. Our observation provides an insight into this process. It identifies activation of Brk as an inducer of cyclinE/Cdk2 activity.
Brk contains a modular arrangement of domains that resembles the arrangement in Src-family kinases (SH3-SH2-catalytic). We have showed that, like Src-family kinases, Brk is also regulated by intramolecular interactions involving the SH3 and SH2 domains (21). Mutation of the C-terminal tyrosine 447, which is analogous to the regulatory Y527 of Src, increased the activity of Brk toward cellular substrates and synthetic peptides (21, 29). Disruption of the SH2- and SH3-mediated interactions promotes autophosphorylation at Ty342 (in the kinase activation loop) and enhances kinase activity toward exogenous substrates. Activation of ErbB2 induced an increase phosphorylation of Tyr-342 and activation of the intrinsic kinase activity of Brk. Consistent with our findings, Brk is potentially the target of other receptor tyrosine kinases implicated in breast cancer. For example, Brk interacts with EGFR and promotes activation of PI 3′K/Akt pathway (30). In addition, stimulation with insulin-like growth factor-1 (IGF-1) activates Brk (31). Both EGFR and IGF-1R are activated in human breast cancer. Thus, in addition to the ErbB2 pathway, activation of Brk may play a critical role in multiple signaling pathways implicated in breast cancer.
Brk expression is low or undetectable in normal mammary tissues or in benign lesions. However, approximately two-thirds of the breast tumors that were examined expressed significant levels of Brk (11). Current evidence suggests that Brk has a direct role in promoting carcinoma cell proliferation (12–14). Our results demonstrate the Brk is required for ErbB2-induced cell proliferation in breast epithelial cells. Furthermore, we show that Brk decreases the efficacy of Lapatinib, an ErbB2 kinase inhibitor. Together these results suggest that expression of Brk contributes to the development of ErbB2-postive breast cancer and the acquisition of resistance to anti-ErbB2 therapeutic strategies. Brk is potentially an attractive therapeutic target because Brk does not promote proliferation in normal adult cells (its expression in normal tissues is restricted to nonproliferative cells in the gastrointestinal tract and skin) (12, 29). This relatively restricted role in normal physiology suggests that targeting Brk might produce fewer unwanted side effects than other oncogenic kinases (12). Along these lines, targeted disruption of Sik, the murine homolog of Brk, resulted in mice that were viable and fertile (36). Thus, Brk inhibitors could potentially be used in combination therapy with ErbB2 inhibitors to treat patients with advanced ErbB2-positive breast cancer, and in patients who develop resistance to ErbB2 inhibitors.
Materials and Methods
Materials.
MCF-10A cells were obtained from ATCC, MCF-10A cells expressing ErbB2 chimera (10A.cB2) were described in ref. 19. Culturing of MCF-10A-based cell lines and 3D culture assays were performed as outlined in ref. 32. Comma-1Dβgeo cells were a kind gift from Daniel Medina and were cultured in DMEM/F12 as described in ref. 22. The dimerizing compound, AP1510, was generously provided by ARIAD Pharmaceuticals (www.ariad.com). Antibodies against p27, E-cadherin, ERK2 and MEK1, and phosphotyrosine were obtained from BD Transduction Laboratories. Anti-phospho-ERK1/2 was from Biosource. Anti-phospho-MEK1/2 and anti-phospho-Akt were from Cell Signaling. Antibodies against cyclin D1, cyclin E, Brk, and cdk2 were from Santa Cruz Biotechnology. Anti-phospho-Brk (Y342) was from Upstate Biotech. Anti-HA was from Covance. Anti-ErbB2 and anti-phospho-ErbB2 (Y1248) were from NeoMarkers. Anti-β-actin was from Sigma. Anti-BrdU was from Roche. Anti-Ki-67 was from Oncogene. Anti-mouse or anti-rabbit antibody conjugated with Alexa Fluor dyes were from Molecular Probes. Goat F(ab′)2 anti-mouse IgG (H+L) was from Caltag Laboratories. Anti-fade Prolong reagent was from Molecular Probes. BrdU was from Sigma. Histone H1 were purchased from Roche. [γ-32P] ATP was from Amersham Biosciences. Trilogy solution was from Cell Marque. TUNEL assay kit was from Molecular Probes.
BrdU Incorporation.
Serum- and growth factor-starved cells were treated with AP1510 and BrdU was added to medium at final concentration of 110 mM at 4 h before fixation. Cells were fixed with methanol at room temperature for 5 min. Cells were then washed with PBS:Glycine (1 M glycine in PBS) and digested with EcoR1 and Exonuclease III at 37°C for 30 min sequentially. Cells were blocked for 60 min in 10% goat serum in washing buffer and stained with anti-BrdU antibody for 60 min followed by incubation of Alexa Fluor-conjugated secondary antibody. DNA was stained with DAPI.
Proliferation assays were also done by using the BrdU Cell Proliferation kit from Chemicon. All assays were performed in triplicate. MCF10A cells expressing WT Brk or K219M Brk, or for other experiments, cells expressing ErbB2 chimera alone or in combination with WT BRK, were plated at a density of 2 × 104 cells per well of a 96-well culture dish. The cells were starved for 36 h and treated with 1 mM AP1510 or ethanol as control. For detecting resistance to Lapatinib, 1 mM AP1510 with varying concentrations of Lapatinib (0–500 nM) was added. BrdU was added 12 h after AP1510 stimulation for 4 h. The cells were then fixed and incubated with anti-BrdU antibody, and washed as per the manufacturer's instructions. The absorbance in the wells was read at 450 nm wavelength. The absorbance values were normalized against the control wells (DMSO). Averaged data from multiple experiments were plotted along with the SEM.
Cdk2 Kinase Activity Assay.
The kinase assay was performed as outlined in ref. 33. The PVDF membrane was exposed to film and then incubated with anti-Cdk2 antibodies by after Western blot analysis to determine the total amount of Cdk2 in the immunoprecipitates.
Assays for BRK Activation.
Immediately following AP1510 treatment, the cells were washed once with ice-cold PBS containing 1 mM sodium orthovanadate and scraped into 500 μl of lysis buffer (25 mM Tris-Cl pH 8.5, 1 mM EDTA, 100 mM NaCl, 1% Nonidet-P40, 0.1 mM sodium orthovanadate, 0.05 μg/m each aprotinin and leupeptin, and 1 mM PMSF). One-half of the anti-BRK (Santa Cruz Biotechnology) immunoprecipitate was incubated in a kinase assay mixture containing 100 mM Tris-Cl pH 7.5, 10 mM MgCl2, 1 mg/ml BSA, 0.1 mM sodium orthovanadate, 500 μM substrate peptide, and 500 μM unlabeled ATP with 0.13 μM (10 μCi) radiolabeled ATP. The other half of the immunoprecipitate was used for immunoblotting with anti-phospho BRK antibody to detect phosphorylation of the BRK activation loop tyrosine (Y342).
3D Culture and Immunofluorescence.
The 3D culture of MCF-10A cells on basement membrane and immunofluorescence analysis were carried out as described (19, 32, 34).
Fat Pad Transplantation.
Fat pad transplantation in BALB/c mouse was performed as described in ref. 22. Briefly, Comma-1D cells stably expressing vector, Brk, ErbB2, or ErbB2 and Brk were trypsinized and collected in serum-containing medium. Cells were spin down and resuspended in serum-free DMEM/F12 medium. A 10-μl quantity of cell resuspension containing 100,000 cells was injected into the epithelium-cleared mammary fat pad of 3-week-old, female BALB/c mice (Jackson Laboratories). Tumor growth was monitored every week after 8 weeks of transplantation. All animal protocols were approved by the Cold Spring Harbor Laboratory IACUC.
Hematoxylin and Eosin Stain and Immunohistochemistry.
The mammary tumors isolated from mice that had undergone transplantation were fixed in 4% formalin, embedded in paraffin, and sectioned. Hematoxylin and eosin staining for histological evaluation was performed in the Animal Shared Resource at Cold Spring Harbor Laboratory. For immunohistochemical analysis, the tissue section was deparaffinized in Xylene, antigen retrieval in Trilogy solution, using a pressure cooker, blocking in 10% goat serum in PBS-T (0.01% Triton X-100 in PBS), primary antibodies incubation, and Alexa Fluor-conjugated secondary antibodies incubation. The nuclei were stained with DAPI. The slides were mounted with antifade prolong reagent.
Analysis of Human Breast Cancer Samples.
A complete description of the patient samples, ROMA, and statistical analysis have been described elsewhere (8). To determine whether overexpression of ErbB2 correlated with overexpression of Brk, we analyzed changes in gene expression of 113 Norwegian tumors, using a published dataset generated by cDNA microarray analysis (18); a subset of those tumors were also analyzed for DNA copy number changes, using ROMA. The values are median-polished log ratios of expression in tumor cells to that in a reference cell mixture.
Supplementary Material
Acknowledgments.
We thank Anne-Lise Borresen-Dale and Therese Sørlie for generously sharing the gene expression data on primary breast tumors before publication, ARIAD pharmaceuticals for AP1510, GSK Pharmaceuticals for Lapatinib, and members of the Muthuswamy and Miller laboratories for helpful discussions. This work was supported by U.S. Army Postdoctoral Fellowship DAMD17–03-1-0403 (to B.X.); National Institutes of Health Grants CA098830 and CA105388, Find a Cure Today, The V Foundation, Long Islanders Against Breast Cancer, and a Rita Allen Scholar award (to S.K.M.); and National Institutes of Health Grant CA58530 and Susan G. Komen Foundation Grant BCTR0600409 (to T.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805009105/DCSupplemental.
References
- 1.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 4.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Romond EH, Perez EA. Advances in adjuvant therapy for breast cancer. Clin Adv Hematol Oncol. 2006;4(suppl 1):4–9. discussion suppl 10; quiz 2 p following suppl 10. [PubMed] [Google Scholar]
- 7.Chin K, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Hicks J, et al. Novel patterns of genome rearrangement and their association with survival in breast cancer. Genome Res. 2006;16:1465–1479. doi: 10.1101/gr.5460106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauraniemi P, Barlund M, Monni O, Kallioniemi A. New amplified and highly expressed genes discovered in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res. 2001;61:8235–8240. [PubMed] [Google Scholar]
- 10.Stein D, et al. The SH2 domain protein GRB-7 is co-amplified, overexpressed and in a tight complex with HER2 in breast cancer. EMBO J. 1994;13:1331–1340. doi: 10.1002/j.1460-2075.1994.tb06386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 12.Harvey AJ, Crompton MR. The Brk protein tyrosine kinase as a therapeutic target in cancer: Opportunities and challenges. Anticancer Drugs. 2004;15:107–111. doi: 10.1097/00001813-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kamalati T, et al. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J Biol Chem. 1996;271:30956–30963. doi: 10.1074/jbc.271.48.30956. [DOI] [PubMed] [Google Scholar]
- 14.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–4209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 15.Born M, et al. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J Pathol. 2005;205:592–596. doi: 10.1002/path.1720. [DOI] [PubMed] [Google Scholar]
- 16.Aubele M, et al. PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br J Cancer. 2007;96:801–807. doi: 10.1038/sj.bjc.6603613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucito R, et al. Representational oligonucleotide microarray analysis: A high-resolution method to detect genome copy number variation. Genome Res. 2003;13:2291–2305. doi: 10.1101/gr.1349003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naume B, et al. Presence of bone marrow micrometastasis is associated with different recurrence risk within molecular subtypes of breast cancer. Mol Oncol. 2007;1:160–171. doi: 10.1016/j.molonc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19:6845–6857. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu H, Miller WT. Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J Biol Chem. 2002;277:34634–34641. doi: 10.1074/jbc.M203877200. [DOI] [PubMed] [Google Scholar]
- 22.Danielson KG, Oborn CJ, Durban EM, Butel JS, Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci USA. 1984;81:3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharrocks AD. Cell cycle: Sustained ERK signalling represses the inhibitors. Curr Biol. 2006;16:R540–R542. doi: 10.1016/j.cub.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 24.Murphy LO, Blenis J. MAPK signal specificity: The right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Bill HM, et al. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol. 2004;24:8586–8599. doi: 10.1128/MCB.24.19.8586-8599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keyomarsi K, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 27.Keyomarsi K, Tucker SL, Bedrosian I. Cyclin E is a more powerful predictor of breast cancer outcome than proliferation. Nat Med. 2003;9:152. doi: 10.1038/nm0203-152. [DOI] [PubMed] [Google Scholar]
- 28.Hunt KK, Keyomarsi K. Cyclin E as a prognostic and predictive marker in breast cancer. Semin Cancer Biol. 2005;15:319–326. doi: 10.1016/j.semcancer.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–419. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- 30.Kamalati T, Jolin HE, Fry MJ, Crompton MR. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene. 2000;19:5471–5476. doi: 10.1038/sj.onc.1203931. [DOI] [PubMed] [Google Scholar]
- 31.Qiu H, Zappacosta F, Su W, Annan RS, Miller WT. Interaction between Brk kinase and insulin receptor substrate-4. Oncogene. 2005;24:5656–5664. doi: 10.1038/sj.onc.1208721. [DOI] [PubMed] [Google Scholar]
- 32.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 33.Neve RM, et al. Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene. 2000;19:1647–1656. doi: 10.1038/sj.onc.1203470. [DOI] [PubMed] [Google Scholar]
- 34.Xiang B, Muthuswamy SK. Using three-dimensional acinar structures for molecular and cell biological assays. Methods Enzymol. 2006;406:692–701. doi: 10.1016/S0076-6879(06)06054-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.