Abstract
Approximately 90% of alcoholics relapse within 4 years, in part because of an enhanced motivation to seek alcohol (EtOH). A novel G protein modulator (Gpsm1/AGS3) was up-regulated in the rat nucleus accumbens core (NAcore) but not in other limbic nuclei during abstinence from operant EtOH self-administration. Furthermore, NAcore AGS3 knockdown reduced EtOH seeking to pre-abstinence levels in a novel rat model of compulsive, human EtOH seeking. AGS3 can both inhibit G protein Giα-mediated signaling and stimulate Gβγ-mediated signaling. Accordingly, sequestration of Gβγ, but not Giα knockdown, significantly reduced EtOH seeking to pre-abstinence levels. Thus, AGS3 and Gβγ are hypothesized to gate the uncontrolled motivation to seek EtOH during abstinence. AGS3 up-regulation during abstinence may be a key determinant of the transition from social consumption to compulsion-like seeking during relapse.
Keywords: self-administration, reinstatement, alcohol deprivation effect, Giα, G protein
One of the most insidious aspects of addiction is the high propensity for relapse (1), which in alcoholics is marked by a heightened motivation to seek alcohol (EtOH) despite mounting adverse consequences (2). Accordingly, there is considerable interest in neuroadaptations that develop during periods of abstinence that may promote drug seeking and relapse (3). Enhanced EtOH seeking during abstinence is observed in heavy social drinkers (4), non-human primates (5), and rodents (2, 6–9), where this EtOH deprivation effect is hypothesized to model many aspects of compulsive EtOH seeking and consumption (refs. 2, 6, 8, but see ref. 9).
Since the first characterization of cellular responses to EtOH (10) and other commonly abused drugs (11), molecules involved in G protein signaling have received much attention in the addiction literature (cf. refs. 3 and 12). The G protein signaling modulator one/activator of G protein signaling 3 (AGS3) has recently surfaced as a common gatekeeper of cocaine- and heroin-primed relapse (13, 14).
AGS3 binds Giα subunits, which inhibits Giα and facilitates Gβγ signaling (15–17). Manipulating AGS3 expression modulates several addiction-linked phenomena, including sensitivity of Giα-coupled D2-like dopamine receptors, cocaine-induced glutamate release, and reinstatement of cocaine seeking by a drug prime (13). Very little is known about the role of AGS3 in regulating motivation during other relapse-precipitating events commonly encountered by human alcoholics, such as conditioned, drug-predictive stimuli that may prime compulsive seeking (1, 2). Although it also remains unknown whether common molecular gatekeepers of relapse exist, a common mechanism is suggested by comparable relapse rates across drug classes (18, 19) and a shared modulation of dopamine-mediated signaling (20). Thus, we examined the role of AGS3 in the enhanced, aberrant motivation to seek EtOH that is apparent after EtOH self-administration and protracted abstinence.
Results
NAcore AGS3 Expression Increased During EtOH Abstinence.
The NAcore is critical for relapse to cocaine and heroin seeking (21), and AGS3 protein expression increased in both the prefrontal cortex (PFC) and NAcore during abstinence from repeated cocaine treatment (13). Accordingly, we hypothesized that increased AGS3 protein expression might represent a common molecular determinant of the enhanced drug- and EtOH-seeking behavior typifying relapse.
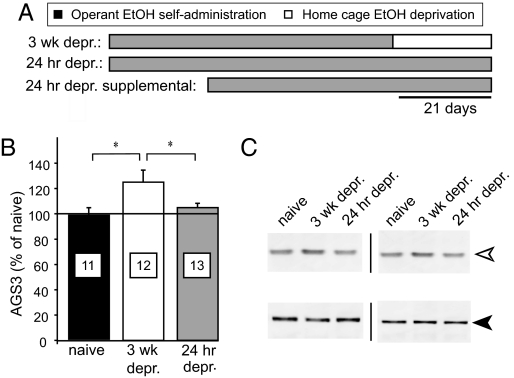
NAcore AGS3 protein expression increased relative to EtOH-naïve rats after 3 wk of EtOH abstinence (“3 wk depr”), but not after 24 h of abstinence (“24 h depr”) [Fig. 1; F(2, 33) = 3.69, P < 0.05], and returned to baseline after 3 wk of additional abstinence [supporting information (SI) Table S1]. EtOH abstinence did not alter AGS3 expression in several other forebrain nuclei related to motivated, goal-directed behavior (Table S1), indicating relatively specific regulation of AGS3 expression in the NAcore during EtOH abstinence. Like the EtOH deprivation effect (2, 6–9), the increase in NAcore AGS3 expression was apparent after several weeks but not after 24 h of abstinence. Thus, increased NAcore AGS3 expression may represent a neural substrate of the enhanced motivation to seek EtOH during protracted abstinence.
Fig. 1.
AGS3 protein expression increased in the NAcore during EtOH abstinence. (A) Schematic demonstrating the self-administration and abstinence paradigm, wherein rats self-administered EtOH for 45–50 contiguous days, followed by 3 wk of EtOH abstinence (“3 wk depr.”) or followed by a further 3 wk of self-administration (“24 h depr.”), or were started 3 wk later (“24 h depr.”; data in SI Results). (B and C) AGS3 expression in the NAcore, normalized to expression in naïve rats (n = 11), was elevated in 3 wk depr (n = 12) but not 24 h depr (n = 13) rats. Representative blots of AGS3 and calnexin, to control for loading, transfer, and blotting conditions across the gel, are shown in C. The open arrowhead indicates AGS3 at ≈72 kDa; the filled arrowhead at ≈90 kDa indicates calnexin. Optical density data were normalized to percent of naïve values within each blot compared with intralane calnexin immunoreactivity. Data represent mean ± SEM. *, P < 0.05—comparing 3 wk depr to EtOH-naïve and 24 h depr rats by using a one-way ANOVA with Scheffé post hoc comparisons.
Motivation to Seek EtOH Is Enhanced During EtOH Abstinence.
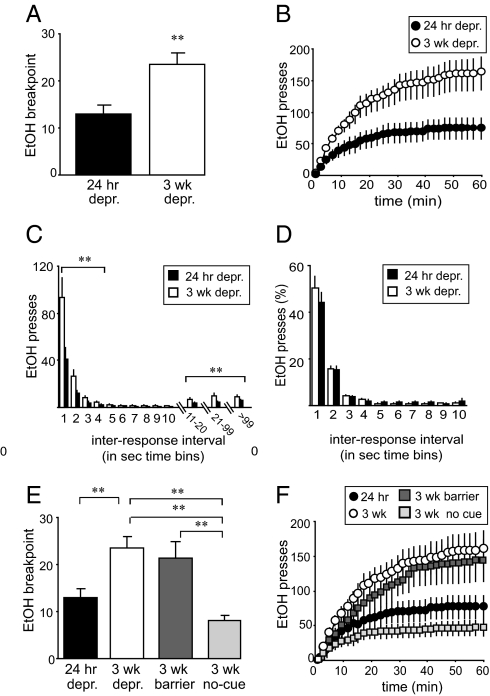
There is considerable interest in identifying neuroadaptations that develop during drug abstinence that may drive the enhanced drug seeking that typifies relapse (3, 12). Thus, we assessed motivation to seek EtOH during abstinence using a progressive ratio schedule (PR) in rats previously trained to respond for EtOH under a fixed ratio to test the hypothesis that AGS3 is a neural substrate of this motivation. Under the PR, the response requirement increased with each subsequent drink obtained. Responding on the PR is thus influenced by previous and anticipated reinforcing efficacy in relation to a mounting work requirement (22). The point at which the rat stops responding, the breakpoint, is therefore considered a quantitative indicator of motivation (22), in this case, to pursue the next drink. Breakpoint was nearly doubled after 3 wk of EtOH abstinence compared with rats that had undergone only 24 h of abstinence [Fig. 2A; F(1, 34) = 11.77, P < 0.01]. Moreover, no differences in breakpoint were observed after 24 h depr following 7 or 10 wk self-administration or in self-administration before an abstinence period of 3 wk or 24 h (SI Results). Taken together, these data indicate that protracted (3 wk) abstinence increased the motivation to seek EtOH.
Fig. 2.
Abstinence-induced enhancement of EtOH seeking. (A) Three weeks of EtOH abstinence significantly enhanced the breakpoint under a PR (3 wk depr: n = 18; 24 h depr: n = 18). (B) Cumulative response timeline showing that enhanced EtOH seeking during abstinence was apparent throughout the session. (C and D) Increased EtOH seeking manifested as an increased number of both fast and slow interresponse intervals (C), but there was no difference in the response pattern when the number of interresponse intervals in each time bin was expressed as a percentage of total responses (D). (E) EtOH-seeking behavior was not impeded by a physical barricade that prevented access to EtOH (“3 wk barrier,” dark gray squares, n = 9) but was significantly blunted by removal of EtOH and all EtOH-associated cues (“3 wk no-cue,” light gray squares, n = 10). (F) Cumulative response data from experiments in E overlain with data from B. Data represent mean ± SEM. *, P < 0.05; **, P < 0.01—using ANOVA with Scheffé post hoc comparisons.
Enhanced EtOH seeking during abstinence was apparent throughout the PR session [Fig. 2B; depr: F(1, 34) = 7.98, P < 0.01; depr × time: F(59, 2,006) = 6.50, P < 0.01]. Because increased responding exhibited by abstinent rats could represent differences in motoric capacity, responding was analyzed in terms of interresponse interval distribution, which quantitatively describes detailed response structure (23). A greater number of both fast (<5 sec) and slow (>10 sec) responses were emitted by 3-wk-EtOH-abstinent rats during the PR session [Fig. 2C; <5 sec, depr: F(1, 34) = 7.73, P < 0.01; depr × time: F(3, 102) = 7.17, P < 0.01; >10 sec, depr: F(1, 34) = 6.83, P < 0.01; depr × time: F(89, 3,026) = 2.56, P < 0.01]. However, there was no difference when interresponse intervals were expressed as a percentage of total responses (Fig. 2D and Table S2). Hence, protracted abstinence resulted in an increase in the overall number of EtOH-seeking responses without changing the temporal structure of lever-pressing behavior.
Enhanced EtOH-Seeking During Abstinence May Be Compulsive.
The enhanced PR responding during abstinence described above was reinforced by EtOH delivery; thus, acute EtOH effects, rather than the cuing effects of stimuli previously associated with EtOH, may have driven EtOH seeking. Because conditioned cues can increase craving and prime relapse in alcoholics (1, 24–28), we examined whether EtOH-associated cues could maintain responding under the PR when a clear Plexiglas barrier blocked access to the EtOH-containing dipper cup (“3 wk barrier”). Bedding, treated as above, provided an EtOH odor cue. Interestingly, breakpoint attained by 3 wk depr rats was unaltered when EtOH access was prevented [Fig. 2E; 3 wk depr vs. 3 wk barrier: F(1, 25) = 0.264, P = 0.61; 24 h depr vs. 3 wk barrier: F(1, 25) = 5.21, P < 0.05]. Moreover, breakpoint expressed by 3 wk depr rats was reduced to 24 h depr level when both EtOH and the conditioned, cuing stimuli previously associated with EtOH were omitted (“3 wk no-cue”) [Fig. 2E; 3-wk no-cue vs. 24 h depr: F(1, 26) = 3.24, P = 0.083; 3 wk no-cue vs. 3 wk depr barrier: F(1, 16) = 13.09, P < 0.01]. Changes in breakpoint were mirrored by concomitant differences in cumulative responding (Fig. 2F and Table S2). These data suggest that enhanced EtOH seeking during abstinence may be driven by cues predicting EtOH delivery. Importantly, increased breakpoint and continued drug seeking in the absence of reinforcer delivery, but in the presence of drug-related cues, are considered to be hallmarks of uncontrolled, compulsive EtOH seeking (refs. 2, 6, and 8, but see ref. 9). Thus, these data suggest that enhanced motivation to seek EtOH during abstinence may represent some aspects of human alcoholic, compulsive behavior (1, 2, 29). In addition, the enhanced willingness to work for EtOH was dose-dependently reduced by naltrexone (Fig. S2), which, because of clinical efficacy of naltrexone in alcoholics, suggests that the motivation modeled here may possess predictive validity for and thus relevance to the development of clinical interventions for alcoholism (30).
AGS3 Knockdown Normalized EtOH-Seeking to Pre-Abstinence Levels.
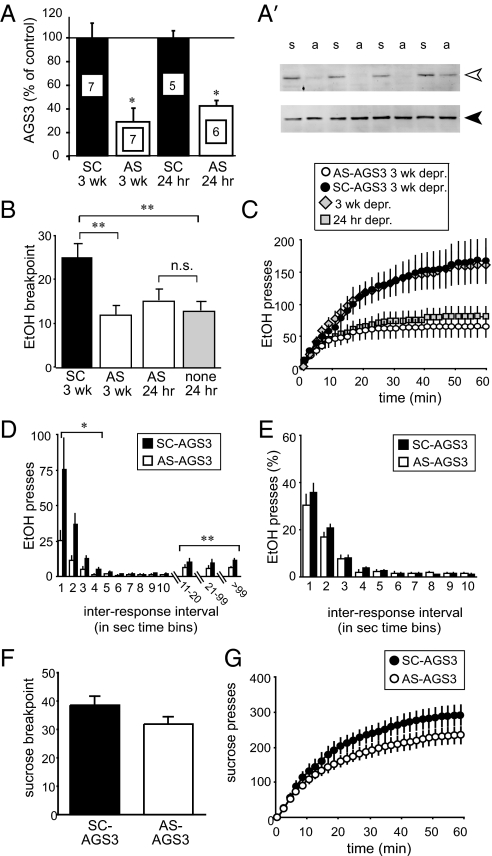
Because AGS3 expression was up-regulated in the NAcore after 3 wk of abstinence (Fig. 1B), a time point when EtOH seeking was robust (Fig. 2A) (see ref. 7), but not after 24 h of abstinence (Fig. 1B), elevated AGS3 expression was hypothesized to drive the enhanced motivation to seek EtOH during abstinence. Thus, motivation was examined after decreasing AGS3 expression in the NAcore with antisense (AS-AGS3).
To examine AS-AGS3 efficacy, one NAcore hemisphere of 3-wk-EtOH-abstinent rats was transfected with AS-AGS3 while the other was transfected with a control, scrambled construct (SC-AGS3) not predicted to bind any known gene. AGS3 protein expression was knocked down by AS-AGS3 compared with SC-AGS3 [Fig. 3A; 3 wk depr: F(1, 12) = 13.58, P < 0.01; 24 h depr: F(1, 9) = 63.18, P < 0.01], confirming that AS-AGS3 was capable of reducing NAcore AGS3 expression in EtOH-deprived rats. Expression was not altered after SC-AGS3 infusion [F(1, 18) = 0.17, P = 0.68]. This AS-AGS3 sequence was previously shown to reduce AGS3 expression and to enhance signaling through Giα-coupled receptors in the PFC of cocaine-deprived rats without affecting expression of Gβ, the Giα1/3 isoforms, or two receptors that signal through Giα (13).
Fig. 3.
AGS3 knockdown reduced the enhanced EtOH seeking during abstinence. (A) Virus expression of antisense was used to knock down AGS3 in the NAcore of 3-wk-EtOH-deprived rats (SC-AGS3: n = 7; AS-AGS3: n = 7) and in 24 h depr rats (SC-AGS3: n = 6; AS-AGS3: n = 6). Representative AGS3 and calnexin control blots at 3 wk depr are shown in (A′). (B) AGS3 knockdown (AS-AGS3) reduced EtOH seeking in 3 wk depr rats to 24 h depr levels (n = 12), with no effect in 24 h depr rats (n = 12), whereas a scrambled control construct (SC-AGS3) had no effect on breakpoint at 3 wk depr (n = 15). (C and D) Reduced motivation to seek EtOH in 3 wk depr rats was also evident throughout the PR testing period in terms of reduced cumulative responding (C) and in decreased number of fast and slow interresponse intervals (D). (E) No difference in response pattern. (F and G) AGS3 knockdown failed to reduce breakpoint or responding for 5% sucrose, a reinforcing substance not commonly thought to be addictive (AS-AGS3, sucrose: n = 17; SC-AGS3, sucrose: n = 17). The open arrowhead in A′ AGS3 at ≈72 kDa; the filled arrowhead in A′ at ≈90 kDa indicates calnexin. Data represent mean ± SEM. s, scrambled; a, antisense. *, P < 0.05; **, P < 0.01—using ANOVA with Scheffé post hoc comparisons.
Importantly, bilateral NAcore AGS3 knockdown during 3 wk of abstinence reduced the EtOH-seeking breakpoint relative to SC-AGS3-treated, 3-wk-EtOH-deprived [Fig. 3B; F(1, 26) = 8.48, P < 0.01] or intact, EtOH-deprived rats not undergoing transfection [compare Fig. 3 B and C with Fig. 2 A and B; F(1, 29) = 12.03, P < 0.01]. The breakpoint attained after AGS3 knockdown was similar to that observed in 24 h depr, intact rats (Fig. 3B and Table S2), suggesting that AGS3 knockdown normalized the enhanced motivation to seek EtOH during abstinence to control, 24 h depr levels. In addition, breakpoint observed in SC-AGS3-treated, 3-wk-EtOH-deprived rats was similar to that in intact, 3-wk-EtOH-deprived rats (Table S2). Cumulative responding mirrored these data [Fig. 3C; AS-AGS3 vs. SC-AGS3, knockdown: F(1, 26) = 6.84, P < 0.01; knockdown × time: F(59, 1,534) = 7.36, P < 0.01; see Table S2]. Thus, NAcore AGS3 knockdown during abstinence dramatically reduced the motivation to seek EtOH.
AGS3 knockdown also reduced the number of fast and slow interresponse intervals [Fig. 2D; <5 sec, transgene: F(1, 26) = 6.22, P < 0.05; transgene × time: F(3, 78) = 3.44, P < 0.05; >10 sec, transgene F(1, 26) = 6.32, P < 0.05, transgene × time: F(89, 2,314) = 6.08, P < 0.01]. However, the pattern of interresponse intervals when expressed as a percentage of total responding [Fig. 3E; transgene: F(1, 26) = 3.68, P = 0.07; transgene × time: F(9, 234) = 0.71, P = 0.70] was not altered, suggesting that knockdown did not yield nonspecific motoric impairments.
Knockdown of NAcore AGS3 did not significantly alter sucrose seeking after 3 wk of deprivation from operant sucrose self-administration [Fig. 3F; breakpoint: F(1, 32) = 2.56, P = 0.12], suggesting that decreased EtOH seeking during AGS3 knockdown was not due to nonspecific motoric or motivational effects (see also Fig. S5). Although there was a significant treatment × time interaction [Fig. 3G; F(59, 1,888) = 2.37, P < 0.01], there was no significant main effect of treatment [Fig. 3G; F(1, 32) = 1.71, P = 0.20] or significant treatment effects at any individual time point (Fig. 3G; all P > 0.15), perhaps indicating a very weak effect of AGS3 knockdown on responding for sucrose.
Importantly, AGS3 knockdown in 24 h EtOH depr rats did not alter breakpoint (Fig. 3B and Table S2) despite a significant protein reduction [Fig. 3A; F(1, 9) = 63.18, P < 0.01], further suggesting a lack of nonspecific impairments from cannulation and AS-AGS3 transfection. Taken together, these data suggest that AS-AGS3 reduction of EtOH seeking in deprived rats was not due to nonspecific motoric or motivational impairments. In addition, AGS3 knockdown of EtOH, but not sucrose seeking, suggests that elevated NAcore AGS3 may selectively modulate maladaptive drug seeking after prolonged drug exposure without effecting the motivation to seek non-drug reinforcers (see also ref. 13).
Gβγ Sequestration Reduced EtOH-Seeking.
Signaling through the Gα subunit and the Gβγ complex of heterotrimeric G proteins is regulated predominately by the activation state of Gα; when Gα is inactive, Gβγ is inactive, because the inactive state of Giα is stabilized by the Gβγ complex (31). However, AGS3 can also stably maintain inactive Giα (17, 32–34) and in this way can augment signaling through Gβγ (15). Accordingly, the role of Giα and Gβγ in enhanced EtOH seeking was examined in the NAcore during EtOH abstinence.
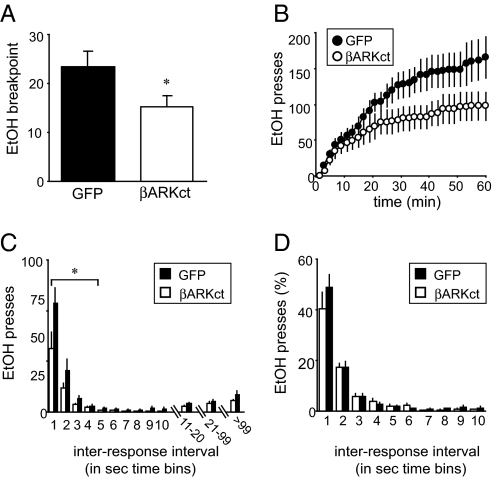
Because AGS3 binding to Giα can increase signaling through Gβγ (15, 16), the role of Gβγ in EtOH seeking was assessed by overexpression of the β-adrenergic receptor kinase C-terminal fragment (βARKct) in the NAcore of 3-wk-EtOH-abstinent rats. βARKct exhibits high affinity for Gβγ dimers, via a pleckstrin homology domain, preventing Gβγ participation in signaling (35). Importantly, sequestration of Gβγ with βARKct reduced breakpoint in 3-wk-EtOH-deprived rats relative to 3 wk depr controls in which green fluorescent protein (GFP) was overexpressed [Fig. 4A; F(1, 12) = 4.98, P < 0.05]. This reduction was also apparent across cumulative responses [Fig. 4B; treatment: F(1, 12) = 3.23, P = 0.09; treatment × time: F(59, 708) = 3.29, P < 0.01]. Breakpoint after βARKct overexpression was also reduced relative to that expressed by intact, 3 wk depr rats [F(1, 24) = 4.23, P < 0.05]. Furthermore, βARKct overexpression reduced breakpoint to levels observed in the 24 h depr rats and in deprived rats with AGS3 knocked down (Table S2). Interresponse interval analysis revealed that βARKct overexpression reduced the number of fast (<5 sec) responses exhibited [Fig. 4C; <5 sec, transgene: F(1, 12) = 3.99, P = 0.06; transgene × time: F(3, 36) = 3.18, P < 0.05], but did not alter the pattern of interresponse intervals when expressed as a percentage of total responding (Fig. 4D and Table S2), suggesting that nonspecific motor impairments did not occur as a result of Gβγ sequestration. Because inhibition of either AGS3 or Gβγ signaling produced a similar reduction in EtOH seeking, elevated NAcore AGS3 expression may facilitate EtOH seeking via enhanced signaling through Gβγ.
Fig. 4.
Gβγ inhibition reduced EtOH seeking during abstinence. (A and B) Gβγ sequestration in the NAcore by overexpression of the βARKct minigene significantly reduced the breakpoint (A) and cumulative responding (B) in 3-wk-EtOH-deprived rats compared with controls overexpressing GFP (βARKct: n = 8; GFP: n = 6). (C and D) βARKct overexpression reduced the number of fast (<5 sec) responses exhibited (C), but did not reduce motoric capacity (D). Data represent mean ± SEM. *, P < 0.05; **, P < 0.01—using ANOVA with Scheffé post hoc comparisons.
Although both AGS3 knockdown and βARKct overexpression may affect many signaling pathways that could result in nonspecific reductions in motoric output, our data suggest that there were no differences in motoric and/or motivational capacity with AS-AGS3 or βARKct (see Fig. 3 F and G, and Fig. S3), including no change in relative interresponse interval distribution (Figs. 3E and 4D) and no effect of AGS3 knockdown upon responding for sucrose (Fig. 3 F and G) or food (13). These results suggest that nonspecific effects did not contribute to either the βARKct- or AS-AGS3 reduction of EtOH seeking.
Finally, because AGS3 binds to and inhibits signaling through multiple Giα subunits (17, 36), the motivation to seek EtOH during knockdown of Giα1 or Giα3 protein expression in the NAcore of 3-wk-EtOH-deprived rats was examined. One week of antisense oligonucleotide infusion into the NAcore of 3-wk-EtOH-deprived rats knocked down Giα1/3 expression, but did not affect EtOH seeking (Fig. S4). Thus, both βARKct-mediated Gβγ sequestration and AGS3 knockdown, but not Giα1/3 knockdown, normalized the enhanced motivation to seek EtOH during abstinence.
Discussion
AGS3 was up-regulated in the NAcore after 3 wk of abstinence from operant EtOH self-administration but not in several other forebrain regions. Knockdown of AGS3 in the NAcore normalized the compulsion-like motivation to work for EtOH that was observed during abstinence. Reduced signaling through Gβγ, which is positively regulated by AGS3, also normalized EtOH seeking during abstinence. Thus, the enhanced motivation to seek EtOH during abstinence from operant EtOH self-administration was regulated by the G protein modulator AGS3 and the G protein Gβγ complex.
An important aspect of the present study was the use of a PR to assess motivational changes, because many reports have examined EtOH consumption, which does not delineate motivation per se (2, 29). Thus, these results, in combination with continued cue-driven seeking in the absence of reinforcer attainment (Fig. 2 E and F), suggest that the enhanced motivation to seek EtOH during abstinence could represent some aspects of compulsive EtOH-seeking behavior observed in human alcoholics (1, 2).
Human alcoholic drinking patterns are often marked by periods of abstinence and intake (cf. ref. 8). Interestingly, several studies noted that repeated periods of drinking and abstinence in rodents can be necessary for, or at least increase the magnitude and persistence of, enhanced EtOH intake after deprivation (8, 29, 37). Our model using an outbred rat strain yielded a significant increase in EtOH-seeking behavior after a single deprivation (Fig. 2 A–C), suggesting that significant stimulus-driven EtOH seeking can develop during a single abstinence period. Additionally, naltrexone inhibited the enhanced EtOH seeking (Fig. S2), suggesting some predictive validity of the increased EtOH seeking described here as a preclinical model potentially relevant to relapsing alcoholics (30). Naltrexone, however, can reduce both concurrent EtOH and sucrose self-administration (38, 39) in addition to reducing relapse propensity (39) (see Fig. S2). However, AGS3 knockdown selectively modulated excessive motivation to pursue EtOH during abstinence but did not suppress the motivation to seek sucrose, a highly reinforcing substance. AGS3 knockdown also did not affect the motivation to obtain EtOH in the absence of protracted abstinence (Fig. 3B), suggesting a lack of nonselective motoric or motivational effects of AGS3 knockdown.
The NAcore guides behavior in response to salient stimuli (40, 41) and is critical for many types of cocaine and heroin relapse (21), as well as enhanced EtOH seeking during abstinence (results here). Moreover, EtOH-associated cues that activate the nucleus accumbens/striatum (1, 24–28) can significantly facilitate EtOH seeking that accompanies relapse in human alcoholics (1, 24–26, 42, 43). Thus, it is intriguing that AGS3 up-regulation, which may drive EtOH seeking during abstinence, is restricted to the NAcore (Fig. 1B and Table S1), and that cues previously associated with EtOH initiate and/or maintain a heightened seeking in EtOH-abstinent rats (Fig. 2 E and F). Furthermore, increased breakpoint (Fig. 2A) and continued EtOH seeking in the absence of EtOH delivery but in the presence of EtOH-associated cues (Fig. 2 E and F) suggest that the increased EtOH-seeking motivation modeled here may represent uncontrolled, compulsive responding (2, 29).
Because NAcore AGS3 expression increased during abstinence from repeated EtOH (Fig. 1B) or cocaine (13) intake and AGS3 knockdown normalized EtOH (Fig. 3 A–D), cocaine (13) or heroin (14) seeking, elevated AGS3 may represent a common neuroadaptation increasing relapse propensity. Importantly, AGS3 knockdown did not effect responding for sucrose (Fig. 3 F and G), food (13), or novel environment exploration (13), suggesting that AGS3 may selectively regulate drug seeking without altering the motivational impact of natural reinforcers.
Although we hypothesize that altered AGS3 expression may represent an important, common neuroadaptation facilitating the seeking of several abused substances, the role of AGS3 is varied among substances. For example, AGS3 expression was not altered during EtOH abstinence in the prelimbic/infralimbic regions of the PFC, where increased expression is critical for cocaine relapse (13). Additionally, AGS3 remained elevated in the NAcore 8 wk after the last cocaine exposure (13), but returned to baseline after a 6-wk EtOH abstinence. This may stem, in part, from the lower relative reinforcing efficacy of EtOH compared with cocaine as well as drug-specific pharmacokinetics/dynamics. Because elevated NAcore AGS3 expression was transient, we hypothesize that AGS3 may also facilitate the development of more persistent neuroadaptations that support enhanced drug seeking during more prolonged periods of abstinence.
Our study found that NAcore Giα1/3 knockdown in 3-wk-EtOH-abstinent rats did not impact EtOH seeking (Fig. S4), suggesting that Giα may not participate in cue-primed EtOH seeking. However, Giα1/3 isoforms likely contribute to EtOH-related behaviors under other conditions because a number of Giα-linked receptors regulate EtOH self-administration (44–47). Interestingly, CB1 receptor antagonism reduced both EtOH and sucrose self-administration (47, 48), which contrasts with the relatively specific effects of AGS3 knockdown that reduced EtOH and cocaine seeking but left food and sucrose seeking intact (results here, and see ref. 13). Thus, AGS3 may bind a subclass of Giα subunits or act predominately in distinct cellular microdomains.
These results extend and further substantiate a growing literature suggesting that reduced signaling through Giα-mediated pathways, as may be affected by high AGS3 expression, represents an important neuroadaptation in addiction (cf. ref. 13). Results from human alcoholics have, however, produced mixed but suggestive evidence of lasting perturbations in G protein signaling (49, 50) that, in some studies, were not apparent without an abstinence period (51, 52). In addition, decreased availability and function of D2 dopamine receptors in the ventral striatum after repeated alcohol ingestion and subsequent abstinence (53) has been correlated with transynaptic network activation that may support craving (26); high AGS3 expression can impair signaling through D2 dopamine receptors (13, 16).
Inhibition of Gβγ signaling with the Gβγ scavenger βARKct reduced EtOH seeking to levels similar to that observed under AGS3 knockdown (Fig. 4 A and B) akin to that observed under a continuous-EtOH-access paradigm (54). Although the precise mechanisms whereby AGS3 regulates free Gβγ remain unclear (16, 36), these data suggest that increased AGS3 expression may enhance the willingness to work for EtOH during abstinence by augmenting signaling through Gβγ.
Thus, NAcore AGS3 up-regulation during abstinence is a novel neuroadaptation that selectively contributed to the development and/or maintenance of enhanced motivation to seek EtOH. Enhanced motivation was due to increased control of behavior by EtOH-predictive cues because responding persisted even when the EtOH reinforcer was not delivered (Fig. 2 E and F) and may therefore reflect uncontrolled or compulsive behavior (2, 29). Importantly, the enhanced EtOH seeking during abstinence was dramatically normalized by AGS3 knockdown (Fig. 3 B–D) or by sequestration of the downstream effector Gβγ (Fig. 4). Thus, increased AGS3 expression and the resulting augmentation of Gβγ signaling could together facilitate compulsive, pathological EtOH-seeking behavior observed in human alcoholics (2, 55–57).
Materials and Methods
Subjects.
Experiments conformed to the 1996 National Institutes of Health Guide for the Care and Use of Laboratory Animals and were conducted with local institutional animal care and use committee approval. Male Wistar rats (250–275 g; Harlan) were acclimated with ad libitum access to food and water in the home cage, unless otherwise stated, for 1 wk before experimentation. All experiments were conducted during the light phase at approximately the same time of day for each rat.
Immunoblotting was conducted as described in ref. 13, with the modifications noted in SI Methods.
Self-Administration Training and Maintenance.
After shaping and sucrose fade, rats were allowed 30 min/day on a fixed ratio 3 schedule of 10% EtOH reinforcement. After 45–50 contiguous days, rats were either placed into abstinence for 3 wk (“deprived”: 3 wk depr) or given additional, daily EtOH self-administration sessions for 3 wk and tested 24 h after the final self-administration session (24 h depr). No differences in breakpoint in 24 h depr rats were observed after 7 wk vs. 10 wk of self-administration or in self-administration levels before abstinence in 3 wk vs. 24 h depr regardless of 7 or 10 wk of self-administration (SI Results). For some AGS3 antisense experiments, rats underwent a similar schedule of training for 5% sucrose and abstinence. See SI Methods for details.
Breakpoint Determination.
Three weeks or 24 h after the final self-administration session, EtOH-seeking motivation was tested on a modified progressive ratio schedule (22). Rats were presented with an EtOH odor cue (58) generated by sprinkling ≈15 ml of ≈87% EtOH beneath the previously EtOH-paired lever. Rats were exposed to the EtOH odor for 2 min before lever extension and presentation of a compound cue, consisting of a tone, stimulus light above the active lever, and illumination of the EtOH-filled dipper cup that were previously paired with reinforcer delivery. For experiments with sucrose-trained rats, as well as with ethanol-trained rats tested under “no-cue” conditions, animals were not exposed to EtOH vapor, but the bedding was similarly sprinkled with water and rats were placed in the operant chamber 2 min before delivery of the compound cue described above.
The progressive ratio schedule of reinforcement was determined by
for 10% EtOH or 5% sucrose. Breakpoint was defined as the maximal number of responses emitted to complete the final ratio (22). Presses that did not complete the final ratio were not included in breakpoint determination. Sessions ended after 15 min of inactivity or after 62 min, whichever came first; sessions generally ended before the 62-min time limit. For some experiments, a clear Plexiglas barricade was placed between the dipper cup and faceplate to prevent dipper cup access. Bedding was sprinkled with EtOH, and rats were placed in the chamber, as above. In these experiments, the EtOH-filled dipper cup was raised but remained inaccessible. For additional details, see SI Methods and Fig. S1.
Antisense and viral-mediated delivery were performed as described in SI Methods. See Fig. S6 for histology.
Statistical Analysis.
Unless otherwise indicated, data were analyzed with a one- or two-way ANOVA followed by Scheffé post hoc comparison if significant main effects were found with a 95% confidence interval.
Supplementary Material
Acknowledgments.
We thank Peidong Fan for adenovirus production, Ling Wang for histological preparation, and Saleem Nicola for fruitful discussions. This work was supported by National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism Grants R37 AA010030 (to I.D.) and F32 AA015464 (to M.S.B.); by the State of California for medical research on alcohol and substance abuse through the University of California at San Francisco (A.B.); and by U.S. Army Medical Research Acquisition Activity Grants 21702-5014, 17-01-1-0803, and 17-03-1-0061 (to I.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706999105/DCSupplemental.
References
- 1.Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt's cognitive-behavioral model. Alcohol Res Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 4.Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42:1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- 5.Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: Effects of response requirement and duration of alcohol abstinence. Alcoholism Clin Exp Res. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- 7.Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcoholism Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- 8.Rodd ZA, et al. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- 9.Wolffgramm J, Galli G, Thimm F, Heyne A. Animal models of addiction: Models for therapeutic strategies? J Neural Transm. 2000;107:649–668. doi: 10.1007/s007020070067. [DOI] [PubMed] [Google Scholar]
- 10.Charness ME, Gordon AS, Diamond I. Ethanol modulation of opiate receptors in cultured neural cells. Science. 1983;222:1246–1248. doi: 10.1126/science.6316506. [DOI] [PubMed] [Google Scholar]
- 11.Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci USA. 1977;74:3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci STKE. 2005;2005:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- 13.Bowers MS, et al. Activator of G protein signaling 3: A gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao L, et al. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci USA. 2005;102:8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takesono A, et al. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 16.Webb CK, et al. D2 dopamine receptor activation of potassium channels is selectively decoupled by Gα-specific GoLoco motif peptides. J Neurochem. 2005;92:1408–1418. doi: 10.1111/j.1471-4159.2004.02997.x. [DOI] [PubMed] [Google Scholar]
- 17.De Vries L, et al. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Gαi subunits. Proc Natl Acad Sci USA. 2000;97:14364–14369. doi: 10.1073/pnas.97.26.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Marlatt GA, Gordon JR, editors. Determinants of Relapse: Implications of the Maintenance of Behavior Change. New York: Brunner/Mazel; 1980. [Google Scholar]
- 20.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 21.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 22.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 23.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig AM, Wikler A. “Craving” and relapse to drink. Q J Stud Alcohol. 1974;35:108–130. [PubMed] [Google Scholar]
- 25.Grusser SM, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 26.Heinz A, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 27.Kareken DA, et al. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: Preliminary findings. Alcoholism Clin Exp Res. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- 28.Myrick H, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 29.Turyabahika-Thyen K, Wolffgramm J. Loss of flexibility in alcohol-taking rats: Promoting factors. Eur Addict Res. 2006;12:210–221. doi: 10.1159/000094423. [DOI] [PubMed] [Google Scholar]
- 30.Tambour S, Quertemont E. Preclinical and clinical pharmacology of alcohol dependence. Fund Clin Pharmacol. 2007;21:9–28. doi: 10.1111/j.1472-8206.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 31.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 32.Natochin M, et al. AGS3 inhibits GDP dissociation from Gα subunits of the Gi family and rhodopsin-dependent activation of transducin. J Biol Chem. 2000;275:40981–40985. doi: 10.1074/jbc.M006478200. [DOI] [PubMed] [Google Scholar]
- 33.Peterson YK, et al. Stabilization of the GDP-bound conformation of Giα by a peptide derived from the G-protein regulatory motif of AGS3. J Biol Chem. 2000;275:33193–33196. doi: 10.1074/jbc.C000509200. [DOI] [PubMed] [Google Scholar]
- 34.Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP. Structural determinants for GoLoco-induced inhibition of nucleotide release by Gα subunits. Nature. 2002;416:878–881. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 35.Pitcher JA, et al. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 36.Bernard ML, Peterson YK, Chung P, Jourdan J, Lanier SM. Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J Biol Chem. 2001;276:1585–1593. doi: 10.1074/jbc.M005291200. [DOI] [PubMed] [Google Scholar]
- 37.Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: An animal model of alcoholism? Alcohol Alcoholism. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- 38.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour in the rat: Effects of naltrexone. Eur J Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- 40.Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci. 2003;23:9833–9841. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 42.Field M, Duka T. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology. 2002;159:325–334. doi: 10.1007/s00213-001-0923-z. [DOI] [PubMed] [Google Scholar]
- 43.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 44.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: Further examination of the role of dopamine receptors in the nucleus accumbens. Alcoholism Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 46.Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 47.Cippitelli A, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- 48.Economidou D, et al. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 49.Jope RS, et al. Selective increases in phosphoinositide signaling activity and G protein levels in postmortem brain from subjects with schizophrenia or alcohol dependence. J Neurochem. 1998;70:763–771. doi: 10.1046/j.1471-4159.1998.70020763.x. [DOI] [PubMed] [Google Scholar]
- 50.Ozawa H, et al. Reduced sensitivity to ethanol of Gsα and Gi/oα in the cerebral cortex of alcoholic patients. Alcohol Alcoholism. 1994;29:93–97. [PubMed] [Google Scholar]
- 51.Ortiz J, et al. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- 52.Nestby P, et al. Unrestricted free-choice ethanol self-administration in rats causes long-term neuroadaptations in the nucleus accumbens and caudate putamen. Psychopharmacology. 1999;141:307–314. doi: 10.1007/s002130050838. [DOI] [PubMed] [Google Scholar]
- 53.Rommelspacher H, Raeder C, Kaulen P, Bruning G. Adaptive changes of dopamine-D2 receptors in rat brain following ethanol withdrawal: A quantitative autoradiographic investigation. Alcohol. 1992;9:355–362. doi: 10.1016/0741-8329(92)90032-6. [DOI] [PubMed] [Google Scholar]
- 54.Yao L, et al. βγ Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- 55.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 56.Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: Recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koob GF. Alcoholism: Allostasis and beyond. Alcoholism Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 58.Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.