Abstract
Dopa-responsive dystonia (DRD) is a hereditary dystonia characterized by a childhood onset of fixed dystonic posture with a dramatic and sustained response to relatively low doses of levodopa. DRD is thought to result from striatal dopamine deficiency due to a reduced synthesis and activity of tyrosine hydroxylase (TH), the synthetic enzyme for dopamine. The mechanisms underlying the genesis of dystonia in DRD present a challenge to models of basal ganglia movement control, given that striatal dopamine deficiency is the hallmark of Parkinson's disease. We report here behavioral and anatomical observations on a transgenic mouse model for DRD in which the gene for 6-pyruvoyl-tetrahydropterin synthase is targeted to render selective dysfunction of TH synthesis in the striatum. Mutant mice exhibited motor deficits phenotypically resembling symptoms of human DRD and manifested a major depletion of TH labeling in the striatum, with a marked posterior-to-anterior gradient resulting in near total loss caudally. Strikingly, within the regions of remaining TH staining in the striatum, there was a greater loss of TH labeling in striosomes than in the surrounding matrix. The predominant loss of TH expression in striosomes occurred during the early postnatal period, when motor symptoms first appeared. We suggest that the differential striosome-matrix pattern of dopamine loss could be a key to identifying the mechanisms underlying the genesis of dystonia in DRD.
Keywords: basal ganglia, movement disorders, Parkinson's disease, striatum
Parkinson's disease (PD), Huntington's disease, and the dystonias represent the three main classes of basal ganglia disorders producing severe motor disturbances. Dystonias, the most common of these, are characterized by the occurrence of sustained muscle contractions and cocontractions leading to repetitive patterned movements (hyperkinetic dystonias) or fixed abnormal postures (hypokinetic dystonias). Many forms of dystonia now are recognized as genetic disorders, classified as DYT-1 to DYT-16 (1–4).
Dopa-responsive dystonia (DRD), a hypokinetic dystonia, was the first dystonic syndrome for which a causative gene was identified (GCH1) (5). DRD is remarkable among dystonias, because it is highly responsive to treatment with levodopa, the signature treatment for PD. Autosomal dominant DRD manifested by fixed dystonic postures can result from all of the inherited metabolic disorders involving synthesis of BH4 (L-erythro-5,6,7,8-tetrahydrobiopterin), because this enzyme is the essential cofactor for tyrosine hydroxylase (TH: EC1.14.16.3), itself the critical enzyme for synthesizing dopamine and other monoamines [supporting information (SI) Fig. S1] (5–8). Loss of dopamine in the striatum, together with reduced striatal TH activity and TH protein, are the key features that have been observed in the rare autopsied cases of autosomal dominant DRD (9). Some parkinsonian symptoms are present in DRD, especially with increasing age, but dystonia is predominant. DRD thus presents critical questions about how striatal dopamine affects motor behavior: why a striatal dopamine deficiency leads mainly to dystonia rather than to parkinsonism in DRD, and why levodopa treatment so effectively treats the dystonia (6, 7, 10, 11).
To address these issues, Sumi-Ichinose et al. (12) generated a mouse model of autosomal dominant DRD (the Pts−/− mouse) by disrupting the synthesis of BH4 through disabling of the PTS gene, necessary for BH4 synthesis (12). Because Pts−/− mice died shortly after birth, a rescue DRD model was generated by transgenic introduction of human PTS cDNA under the control of the 5′-promoter region of the human dopamine β-hydroxylase (DBH: EC1.14.17.1) gene (13). In these DPS-rescued Pts−/− mice (DPS mice), BH4 and TH concentrations are severely reduced in the striatum (to ≈20% and 15%, respectively, of wild-type Pts+/+ levels), but noradrenaline and adrenaline are largely spared (13).
In the experiments reported here, we show that DPS mice develop abnormal postures and movements, and that the development of such symptoms is correlated temporally with highly patterned loss of TH in the striatum involving both a loss of TH in striosomes and a failure of increase of TH in both striosomes and matrix. We suggest that differential loss of dopamine in striosomes, together with a more global dopaminergic deficit in the striatum, may be critical in producing dystonia in DRD.
Results
Motor Dysfunction in DPS Mice.
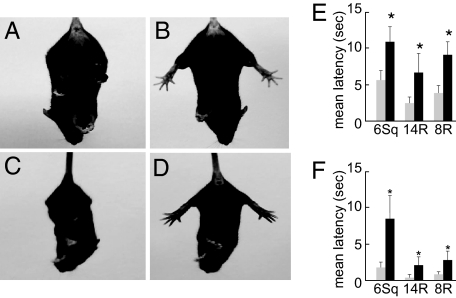
To determine whether the DPS mice had motor impairments, we evaluated mutants along with sibling controls in a series of behavior tests. At rest, adult DPS mice demonstrated no overt signs of movement disorder. However, when hung by their tails, the DPS mice exhibited dystonic posture of the hindlimbs with self-clasping (Fig. 1, also see Movie S1). The fixed clasping posture was first detected in young DPS mice at 2 weeks of age (Fig. 1C). We tested mature DPS mice and sibling controls with beam-walking protocols including three beams of different shapes and diameters. The DPS mice were impaired relative to wild-type controls on all tests. DPS mice were slower (Fig. 1E, latency to complete crossing; P = 0.001, ANOVA) and made more foot slips (Fig. 1F; P = 0.001, ANOVA) (see Movie S2). These results suggest that DPS mice manifest a phenotype of hypokinetic hindlimb dystonia that resembles the hindlimb dystonia observed in DRD, including in a patient with a PTS deficiency (8), specifically modeled genetically in the DPS mice.
Fig. 1.
Behavioral phenotype of DPS mice. (A–D) Hindlimb clasping dystonic phenotype in DPS mice (A and C) compared with behavior in wild-type sibling controls (B and D), at adulthood (A and B), and at 2 wk of age (C and D). Note normal splaying of hindlimbs in wild type. (E and F) Hypokinetic motor deficits in adult DPS mice. On the beam-walking tests with a 6-mm-wide square beam (6Sq) and with 14 mm (14R)- or 8 mm (8R)-wide round beams, DPS mice (black bars) showed an increase in the latency to complete crossing (E) and in the number of foot slips (F), relative to controls. *, P = 0.001.
Patterns of Loss of TH Immunostaining in the Striatum of Adult DPS Mice.
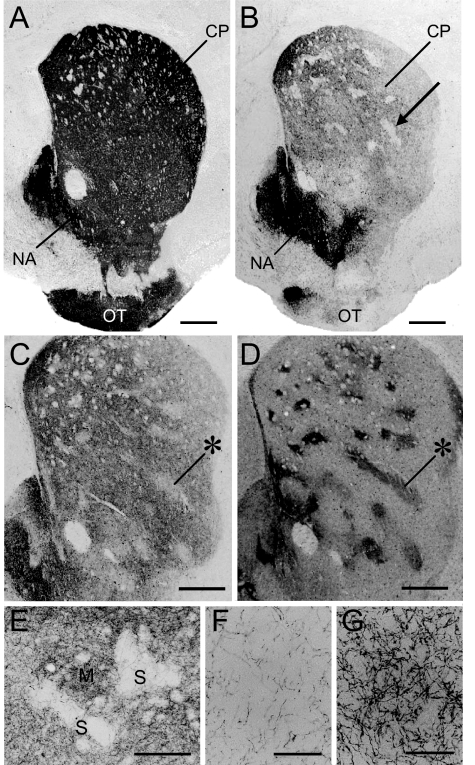
Immunohistochemistry for TH demonstrated striking abnormalities in the striatum of the adult DPS mice (Fig. 2). In contrast to the intense, nearly homogeneous TH staining in the striatum of wild-type controls, striatal TH immunostaining in the DPS mice was markedly reduced in the caudoputamen and in the lateral part of the olfactory tubercle, but not in the nucleus accumbens (Fig. 2B). Within the caudoputamen of the DPS mice, there were focal zones of particularly severe loss of TH staining (Fig. 2B). These zones of lowered TH labeling corresponded to striosomes identified in adjacent sections stained for a marker of striosomes, the μ-opiate receptor (MOR) (14) (Fig. 2 C and D). Fig. 2 E–G illustrate this marked depletion of TH-positive fibers in the striosomes relative to the nearby matrix.
Fig. 2.
Differential loss of TH labeling in striosomes in adult DPS mice. (A and B) TH-stained cross-sections through the striatum from a wild-type mouse (A) and a DPS mouse (B). An example of the focal zones of lowered TH-staining visible in the DPS mouse is indicated by the arrow in B. (C and D) Serially adjacent striatal sections immunostained for TH (C) and for MOR (D) from a DPS mouse. * indicates examples of MOR-positive striosomes that correspond to the TH-poor zones. (E) Photomicrograph illustrating magnified view of TH-immunostaining in caudoputamen of a DPS mouse. Striosome and matrix compartments are indicated by S and M, respectively. (F and G) Higher-power photomicrographs showing a severe loss of TH-immunostained afferent fibers in a striosome (F), but their relative sparing in the nearby matrix (G) in the caudoputamen of a DPS mouse. (Scale bars: A–D, 500 μm; E, 200 μm; and F and G, 50 μm.) CP, caudoputamen; NA, nucleus accumbens; OT, olfactory tubercle.
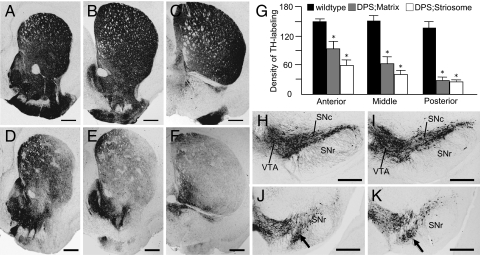
There was also a strong posterior-to-anterior gradient in the loss of TH staining in the caudoputamen (Fig. 3). Caudally, TH was severely depleted in all but the most medial part of the striatum, so that the difference in labeling between striosomes and matrix was mostly or fully obscured (Fig. 3F). Densitometric measurements confirmed this visual impression (Fig. 3G). Thus, DPS mice exhibit both differential loss of TH in striosomes rostrally and differential loss of TH in both compartments at caudal levels of the caudoputamen, with sparing of TH in the nucleus accumbens and medial olfactory tubercle.
Fig. 3.
Patterns of TH immunostaining in the striatum and midbrain of adult DPS and wild-type mice. (A–F) TH-immunostained cross-sections at anterior (A and D), middle (B and E), and posterior (C and F) levels of the caudoputamen, taken from wild-type (A–C) and DPS (D–F) mice. Note the strong posterior-to-anterior gradient of reduced TH labeling in the caudoputamen in the DPS mouse. (G) Optical density measurements of TH labeling in the striosome and matrix compartments at anterior, middle, and posterior levels in wild-type (control) and DPS mice. *, indicates P = 0.001 DPS vs. control. (H–K) TH-stained sections illustrating rostral (H and I) and caudal (J and K) levels through the substantia nigra and ventral tegmental area, taken from wild-type (H and J) and DPS (I and K) mice. The densocellular zone of the SNc is indicated by arrows in J and K (16). (Scale bars: A–F, 500 μm; H–K, 500 μm.) SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; VTA, ventral tegmental area.
Striatal fos Expression After Dopaminergic Stimulation in the Striatum of Adult DPS Mice.
To obtain an estimate of the excitability of striatal neurons in response to dopamine agonist stimulation (14), we treated adult wild-type and DPS mice with either saline or the D1/D2 dopamine receptor agonist apomorphine (5 mg/kg i.p.; n = 2 for each phenotype) and examined the patterns of FosB immunoreactivity in the striatum. Induction of FosB labeling was not observed in the saline-treated controls (data not shown). Apomorphine induced a marked increase in FosB expression in the striatum of both wild-type and DPS mice (Fig. S2 and Fig. S3). However, the induction of FosB was stronger in the DPS mice, particularly in the dorsolateral striatum (Fig. S2). Double immunofluorescence staining for FosB and TH showed that, in both DPS and wild-type mice, FosB-labeled nuclei were frequently found in the zones of lowered TH labeling (Figs. S2 C–H and S3 A–F). Initial counts of FosB-positive nuclei (Fig. S3G) suggested that the relatively augmented FosB induction in the DPS mouse occurred especially in striosomes at the midstriatal levels analyzed. Thus, it is likely there is dopaminergic hypersensitivity in the striatum of DPS mice, particularly in striosomes. Behaviorally, the apomorphine-treated DPS mice exhibited hyperactivity in open field conditions relative to controls, but variability was high, given the small number of animals available for testing.
Structural Preservation of the Nigrostriatal Pathway in Adult DPS Mice.
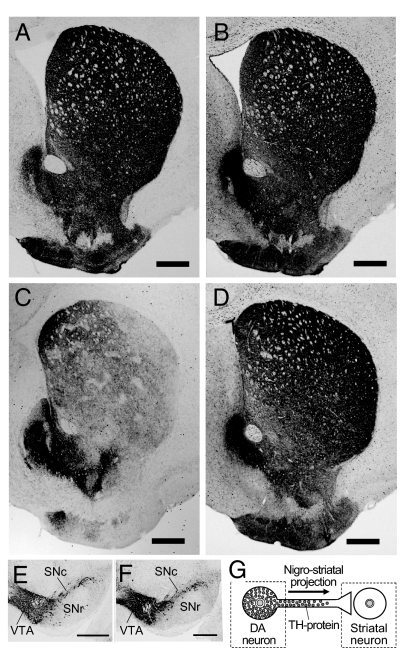
Despite the evident loss of TH staining in the striatum of the DPS mice, there was no obvious difference between the distribution of TH-labeled neurons in the midbrains of the DPS mice and their wild-type controls (Fig. 3 H and I); both the ventral tegmental area and substantia nigra pars compacta were densely stained in mice of both genotypes. Notably, the densocellular zone in the ventral tier of the nigral pars compacta could be identified in TH stains of both DPS and control midbrains (Fig. 3 J and K). This region is thought to be a main source of the nigrostriatal dopaminergic projections to striosomes (15, 16). Moreover, immunohistochemistry for aromatic l-amino acid decarboxylase (AADC), a dopaminergic marker for the nigrostriatal pathway that is normally colocalized with TH (13), demonstrated abundant staining of the striatum and of nigral neurons in the DPS mice (Fig. 4 A–F). These results suggest that in the DPS mice, the nigrostriatal pathway is present but has lost TH protein at the level of its nerve terminals in the striatum, particularly in striosomes and in caudal parts of the striatum (Fig. 4G).
Fig. 4.
Structural preservation of the nigrostriatal pathway in adult DPS mice. (A–D) Representative cross-sections from a wild-type mouse (A and B) and from a DPS mouse (C and D), stained for TH (A and C) and for AADC (B and D). (E and F) Sections through the midbrain stained for AADC, from wild-type (E) and DPS (F) mice. Note that the distribution of AADC immunostaining is normal in both the striatum and midbrain of the DPS mouse. (G) Schematic drawing of a nigrostriatal dopamine-containing neuron in the DPS mouse. TH protein (grayed circles) is highly expressed in the cell body but is depleted in the nerve terminal. (Scale bars: A–F, 500 μm.) SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; VTA, ventral tegmental area.
Abnormal Developmental Profile of TH Immunostaining in DPS Mice.
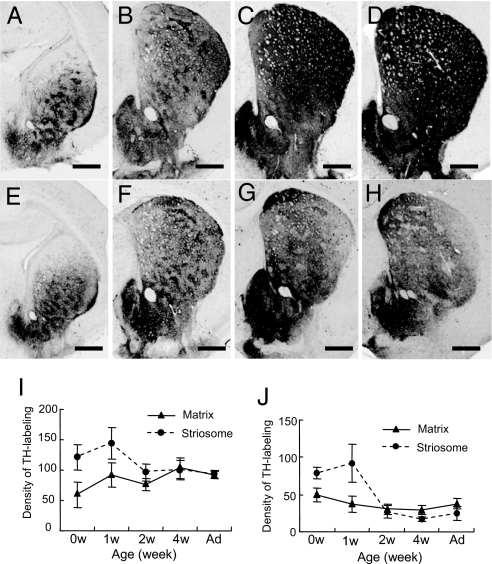
In agreement with the known pattern of TH expression in the mouse striatum (16, 17), in the wild-type control mice (Fig. 5 A–D and I), strong TH immunostaining first appeared in discrete “dopamine islands” corresponding to developing striosomes (18). With development, the level of TH-staining intensity in the matrix compartment increased to the levels found in the striosomes. The striosomal pattern of TH labeling became faint by postnatal week 2 and disappeared by postnatal week 4.
Fig. 5.
Postnatal development of TH protein expression in DPS and control mice. (A–H) TH-labeled striatal cross-sections at the midlevels through the caudoputamen of wild-type (A–D) and DPS (E–H) mice, illustrating TH-immunostaining patterns at birth (0 wk, A and E) and at 1 wk (B and F), 2 wk (C and G), and 4 wk (D and H) of age. (I and J) Measurements illustrating the developmental change in optical density of TH labeling in the matrix compartment (▴) and in striosomes (●) in wild-type (I) and DPS mice (J) at 0, 1, 2, and 4 wk and adulthood (Ad). (Scale bars: A–H, 500 μm.)
In the DPS mice (Fig. 5 E–H and J), TH-positive dopamine islands were also visible up to postnatal week 1, although their immunostaining intensity was slightly lower than that of the wild types. By postnatal week 2, however, the mean intensity of TH immunostaining in the striosomes had sharply decreased. Striosomes with lowered TH labeling were visible through most of the caudoputamen, along with low levels of TH immunostaining in the matrix. As shown in Fig. 5G, many striosomes now appeared as zones of lowered TH staining. By postnatal week 4, striosomes were severely depleted of TH labeling in the entire neostriatum, and this pattern remained through adulthood (Fig. 5H). As this striking decline in striosomal TH occurred, TH staining in the matrix compartment of the DPS striatum failed to develop. By week 4, the full posterior-to-anterior pattern of TH deficiency appeared, with very low levels of TH in both compartments caudally and with differentially low TH levels in striosomes rostrally (Fig. 5). Thus, in the DPS mice, the deficiency in TH in the caudoputamen resulted both from (i) a progressive postnatal loss of TH immunostaining that was present at birth, particularly in striosomes; and from (ii) a failure of increased TH immunostaining with maturation.
Discussion
Our findings demonstrate that DPS mice exhibit dystonic motor deficits potentially related to the phenotypes seen in DRD patients (6, 8, 19), including those in a patient with the PTS deficiency engineered in the DPS mice (6, 19). Our experiments further demonstrate that a loss of TH protein in the terminal regions of the nigrostriatal tract is a principal pathology of the basal ganglia in the DPS mice, as has been found in human DRD patients (9, 20, 21). These observations validate the DPS mouse as a model of DRD. The critical findings made possible by this DRD mouse model are that the loss of striatal TH in the model occurs mostly according to the striosome-matrix architecture of the striatum, with striosomes differentially affected, and that the onset of motor symptoms is temporally correlated with the onset of the striatal decline in TH.
DRD due to BH4 deficiency is considered to be a functional disorder of nigrostriatal dopaminergic neurons, because of the lack of evidence for degeneration of nigrostriatal fibers despite severe depletion of striatal TH and dopamine. Our findings in the DPS model are similar. Severe loss of TH immunostaining in the caudoputamen occurred with preservation of the nigrostriatal innervation as judged by AADC immunohistochemistry. Such structural preservation could partly underlie the dramatic and sustained response to relatively low doses of levodopa in DRD, contrasting with the situation in Parkinson's disease, in which a progressive degeneration of the nigral dopaminergic neurons occurs and high doses of levodopa are required for therapeutic effect. The mechanism for the selective reduction in striatal TH protein elicited by BH4 insufficiency in DRD is unknown. There is no apparent difference in TH mRNA levels in the brains of Pts−/− and Pts+/+ mice (12), suggesting that the steady-state level of TH protein may be regulated by stabilization, transportation or translation of the protein in the striatum, rather than by transcriptional control by BH4. One possibility is that the turnover rate of TH protein and/or BH4 recycling in the susceptible nerve terminals is faster than that in other regions, which could account for the tendency for there to be lower amounts of TH protein in the nerve terminals of the striatonigral pathway.
The pattern of loss of TH in the DPS mouse striatum is remarkably similar to that in the weaver mouse, both in the gradients of loss and in the striosomal predominance of TH loss at anterior striatal levels (17). In the weaver mouse, however, loss of nigral neurons accompanies the striatal deficit, whereas we found no obvious loss of nigral neurons in the DPS mouse, including in the putative striosome-projecting densocellular zone (17, 22). This result raises the possibility that striatal factors contribute in producing these patterns of dysfunction, whether accompanied by massive nigral cell body loss or not.
In the DPS mouse model of DRD, the loss of TH in the striatum follows an abnormal developmental pattern resulting in differential loss of TH in striosomes at rostral levels, where some TH-positive innervation survives and profound loss of TH in both compartments caudally. This pattern corresponds in remarkable detail to the pattern predicted by Hornykiewicz (11). Based on the patterns of loss of dopamine in dissected postmortem samples from a DRD patient, Hornykiewicz suggested that preferential loss of striosomes in the rostral striatum could be critical to producing dystonic symptoms, a prediction fully in accord with our findings in the DPS mouse model of DRD.
How could this pattern of loss contribute to dystonic symptoms? The loss of TH in the caudal striatum removes normal dopaminergic control over the matrisome-based direct and indirect pathways that lead toward motor-control circuits of the cortex and brainstem. TH is also lost in both striosomes and matrix at these caudal levels. In addition, however, striosomes are differentially depleted of TH rostrally, producing an imbalance between dopaminergic modulation of striosome and matrix compartments in the anterior striatum. Such an imbalance could be important. The regulation of movement by the striatum may depend not only on the balance of activity between the matrix-based direct and indirect pathways (23, 24), but also on a balance in activity between these pathways and the striosomal pathway (25). For example, an imbalance between compartmentally organized basal ganglia circuits has been suggested to contribute to the generation of repetitive behaviors including motor stereotypies and levodopa induced dyskinesias (14, 26). Our findings support the possibility that a differential striosome-matrix pattern of dopaminergic loss could contribute to the genesis of dystonia of the DRD type. Our preliminary results with apomorphine treatment of the DPS mice suggest that differential hypersensitivity to dopaminergic stimulation in the two compartments does exist at least in this model of DRD. According to this view, the striosomal compartment exerts critical control over movement by virtue of its interactions with the matrix compartment (Fig. 6), thereby providing experience-dependent motor or emotional control variables related to evaluative signaling (26–30).
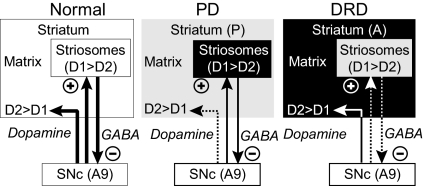
Fig. 6.
Diagram illustrating in highly schematic form patterns of loss in the striosome and matrix dopamine systems in a PD model (low-dose MPTP-treated primate) and in the mouse DRD model characterized here (DPS mouse) relative to the even distribution of TH obscured in control mice. Light gray indicates major loss of dopamine. The differential striosome-matrix pattern of dopamine deficiency in the PD model is in part complementary to that observed in the DRD model. A predominant loss of a dopaminergic marker is found in the matrix compartment in the PD model (posteriorly, P), but a predominant loss of a dopaminergic marker is found in striosomes in the DRD model (anteriorly, A). The DRD model suggests loss of dopamine content in striosomes could result, directly or indirectly, in altered activity of nigral dopaminergic neurons, which in turn regulate neural function of striatal output systems including the matrix-based direct and indirect pathways. DRD, dopa-responsive dystonia; D1, D1-dopamine receptor; D2, D2-dopamine receptor; SNc, substantia nigra pars compacta.
The loss of TH in the dorsal striatum of the DRD model mouse, with preservation of TH in the nucleus accumbens, parallels the pattern of dopamine loss in the striatum in PD as well as in DRD (11). The posterior-to-anterior gradient of loss in the DRD model also parallels the overall pattern of striatal dopamine deficiency in PD and the single DRD patient examined (11, 31). We could not securely compare the gradients of striatal TH loss in the mouse to those within the anterior striatum of the human cases, gradients that distinguish the PD and DRD patterns of depletion (11). However, the differential loss of striosomes rostrally in the DPS mouse was unequivocal.
It is not known whether there is differential vulnerability of dopamine in striosome and matrix compartments in PD. However, in a primate model of parkinsonism generated by exposure to low-dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), the dopamine-containing input to striosomes was found to be relatively spared in the caudal striatum, whereas dopaminergic inputs to the matrix were severely diminished (32). This pattern contrasts with the loss of TH in both compartments caudally and the accentuated TH loss in striosomes anteriorally in the DRD mouse.
This contrast raises the possibility that a predominant loss of dopaminergic activity in the matrix compartment at caudal (sensorimotor) levels of the striatum might result in paucity of movement, thereby resulting in hypokinetic parkinsonian symptoms in the PD model, whereas a differential loss of dopaminergic activity in striosome-based circuits originating at more rostral striatal levels might produce changes in the selection and release of behavioral alternatives, thereby promoting fixed dystonic posture in DRD. Differential degeneration of striosomes has been found in a postmortem analysis of the brains of DYT3 patients (33). Thus an important question to pursue is whether some dystonias are linked to a disproportionate loss of normal activity in the striosome compartment, and whether their hypokinetic or hyperkinetic character depends on the associated dopaminergic activities in the matrix compartment. At relatively early stages, levodopa therapy in DRD may produce a beneficial effect by restoring normal dopaminergic activity in striosomes, promoting reacquisition of a balance between striosome-based and matrix-based pathways.
An outstanding feature of DRD is that most cases are characterized by childhood-onset dystonia (34). Our findings on the DPS mouse model of DRD are of particular interest in this developmental context. In the DRD model, developing striosomes were TH-positive in the typical dopamine-island pattern at birth, but they lost TH labeling between postnatal weeks 1 and 2, when motor impairments in the mice were first detected. These findings support the possibility that DRD is a developmentally regulated disorder characterized by a predominant developmental decrease of TH (and thus of dopamine content) in the striosome compartment in advance of a severe caudal-to-rostral failure of the normal developmental increases in dopamine content across both striatal compartments.
Not only the total neural activity of pallidal neurons, but also their patterns of neural discharges, may be relevant to the genesis of dystonia (35). The DRD model mouse that we have characterized here is complicated in that gradients of loss in TH from posterior to anterior accompany the accentuated loss of TH in striosomes at the more anterior levels. Nevertheless, the model raises the possibility that a striosomal dopamine deficiency could alter the functional activities of matrix-based striatal output systems, contributing to the altered discharge pattern of pallidal neurons in DRD.
Methods
DPS mice and wild-type controls were examined in behavioral tests (tail suspension, open field, beam-walking) and in neuroanatomical studies of the nigrostriatal system as described fully in SI Text. All procedures were approved by the institutions of the authors (SI Text). For behavior, adult mice were used; for anatomy, both adult and 0- to 4-wk-old mice were studied. For anatomy, immunohistochemistry was performed on frozen sections from perfusion-fixed mice to demonstrate midbrain and striatal staining for TH, AADC, and MOR, and for MOR and FosB in mice treated with apomorphine 2 h before perfusion. Sections were analyzed by light microscopy, and quantitative measures were made by cell counting and densitometry.
Supplementary Material
Acknowledgments.
We thank Dr. David Standaert and Dr. Fu-Chin Liu for insightful comments on an earlier version of this manuscript and Noriko Ihira, Kazumichi Yamada, Patricia Harlan, and Emily Romano for technical assistance. This work was funded by grants-in-aid for Scientific Research (nos. 1500240 and 16590255); by a 21th Century COE (Center of Excellence) program grant (no. 16101J-1) from the Japan Ministry of Education, Science, Culture and Sports; and by the National Institutes of Health (MGH/MIT Parkinson's Disease Research Center Grant PS50 NS38372), the National Parkinson Foundation, and the Stanley H. and Sheila G. Sydney Fund (MIT).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806065105/DCSupplemental.
References
- 1.de Carvalho Aguiar PM, Ozelius LJ. Classification and genetics of dystonia. Lancet Neurol. 2002;1:316–325. doi: 10.1016/s1474-4422(02)00137-0. [DOI] [PubMed] [Google Scholar]
- 2.Grotzsch H, et al. Neuropathology of a case of dopa-responsive dystonia associated with a new genetic locus, DYT14. Neurology. 2002;58:1839–1842. doi: 10.1212/wnl.58.12.1839. [DOI] [PubMed] [Google Scholar]
- 3.Grimes DA, et al. A novel locus for inherited myoclonus-dystonia on 18p11. Neurology. 2002;59:1183–1186. doi: 10.1212/wnl.59.8.1183. [DOI] [PubMed] [Google Scholar]
- 4.Camargos S, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose H, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 6.Nomura Y, et al. Dystonias responding to levodopa and failure in biopterin metabolism. Adv Neurol. 1998;78:253–266. [PubMed] [Google Scholar]
- 7.Segawa M, Nomura Y, Nishiyama N. Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease) Ann Neurol. 2003;54(Suppl 6):S32–S45. doi: 10.1002/ana.10630. [DOI] [PubMed] [Google Scholar]
- 8.Hanihara T, et al. 6-Pyruvoyl-tetrahydropterin synthase deficiency with generalized dystonia and diurnal fluctuation of symptoms: a clinical and molecular study. Mov Disord. 1997;12:408–411. doi: 10.1002/mds.870120321. [DOI] [PubMed] [Google Scholar]
- 9.Rajput AH, et al. Dopa-responsive dystonia: pathological and biochemical observations in a case. Ann Neurol. 1994;35:396–402. doi: 10.1002/ana.410350405. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa Y. Update on dopa-responsive dystonia: locus heterogeneity and biochemical features. Adv Neurol. 2004;94:127–138. [PubMed] [Google Scholar]
- 11.Hornykiewicz O. In: Monographs in Neural Science. Segawa M, Nomura Y, editors. Basel: Karger; 1995. pp. 101–108. [Google Scholar]
- 12.Sumi-Ichinose C, et al. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin. A study from 6-pyruvoyltetrahydropterin synthase knockout mice. J Biol Chem. 2001;276:41150–41160. doi: 10.1074/jbc.M102237200. [DOI] [PubMed] [Google Scholar]
- 13.Sumi-Ichinose C, et al. Genetically rescued tetrahydrobiopterin-depleted mice survive with hyperphenylalaninemia and region-specific monoaminergic abnormalities. J Neurochem. 2005;95:703–714. doi: 10.1111/j.1471-4159.2005.03402.x. [DOI] [PubMed] [Google Scholar]
- 14.Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 15.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 16.Graybiel AM, Ohta K, Roffler-Tarlov S. Patterns of cell and fiber vulnerability in the mesostriatal system of the mutant mouse weaver. I. Gradients and compartments. J Neurosci. 1990;10:720–733. doi: 10.1523/JNEUROSCI.10-03-00720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roffler-Tarlov S, Graybiel AM. The postnatal development of the dopamine-containing innervation of dorsal and ventral striatum: Effects of the weaver gene. J Neurosci. 1987;7:2364–2372. [PMC free article] [PubMed] [Google Scholar]
- 18.Graybiel AM. Correspondence between the dopamine islands and striosomes of the mammalian striatum. Neuroscience. 1984;13:1157–1187. doi: 10.1016/0306-4522(84)90293-8. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa Y, Graf WD, Wong H, Shimadzu M, Kish SJ. Dopa-responsive dystonia simulating spastic paraplegia due to tyrosine hydroxylase (TH) gene mutations. Neurology. 2001;56:260–263. doi: 10.1212/wnl.56.2.260. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa Y. Genetics and biochemistry of dopa-responsive dystonia: significance of striatal tyrosine hydroxylase protein loss. Adv Neurol. 2003;91:401–410. [PubMed] [Google Scholar]
- 21.Furukawa Y, et al. Striatal biopterin and tyrosine hydroxylase protein reduction in dopa-responsive dystonia. Neurology. 1999;53:1032–1041. doi: 10.1212/wnl.53.5.1032. [DOI] [PubMed] [Google Scholar]
- 22.Gaspar P, Ben Jelloun N, Febvret A. Sparing of the dopaminergic neurons containing calbindin-D28k and of the dopaminergic mesocortical projections in weaver mutant mice. Neuroscience. 1994;61:293–305. doi: 10.1016/0306-4522(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 23.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–289. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 24.Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- 25.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 26.Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24:7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 29.Brown JE, Bullock D, Grossberg S. How the basal ganglia use parallel excitatory and inhibatory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci. 1999;19:10502–10511. doi: 10.1523/JNEUROSCI.19-23-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Kish SJ, Shannak K, Hornykiewicz O. Uneven patterns of dopamine loss in the striatum of patients with idiopathic Parkinson's disease - pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 32.Moratalla R, et al. Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1992;89:3859–3863. doi: 10.1073/pnas.89.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto S, et al. Functional anatomy of the basal ganglia in X-linked recessive dystonia-parkinsonism. Ann Neurol. 2005;58:7–17. doi: 10.1002/ana.20513. [DOI] [PubMed] [Google Scholar]
- 34.Nygaard TG, Marsden CD, Fahn S. Dopa-responsive dystonia: long-term treatment response and prognosis. Neurology. 1991;41:174–181. doi: 10.1212/wnl.41.2_part_1.174. [DOI] [PubMed] [Google Scholar]
- 35.Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131–140. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.