Abstract
A-Kinase Anchoring Proteins (AKAPs) ensure the fidelity of second messenger signaling events by directing protein kinases and phosphatases toward their preferred substrates. AKAP150 brings protein kinase A (PKA), the calcium/calmodulin dependent phosphatase PP2B and protein kinase C (PKC) to postsynaptic membranes where they facilitate the phosphorylation dependent modulation of certain ion channels. Immunofluorescence and electrophysiological recordings were combined with behavioral analyses to assess whether removal of AKAP150 by gene targeting in mice changes the signaling environment to affect excitatory and inhibitory neuronal processes. Mislocalization of PKA in AKAP150 null hippocampal neurons alters the bidirectional modulation of postsynaptic AMPA receptors with concomitant changes in synaptic transmission and memory retention. AKAP150 null mice also exhibit deficits in motor coordination and strength that are consistent with a role for the anchoring protein in the cerebellum. Loss of AKAP150 in sympathetic cervical ganglion (SCG) neurons reduces muscarinic suppression of inhibitory M currents and provides these animals with a measure of resistance to seizures induced by the non-selective muscarinic agonist pilocarpine. These studies argue that distinct AKAP150-enzyme complexes regulate context-dependent neuronal signaling events in vivo.
Keywords: AMPA, behavior, KCNQ, knockout
Sophisticated systems have evolved to manage the spatial and temporal organization of signal transduction pathways. A-Kinase Anchoring Proteins (AKAPs) target various protein kinases and phosphatases to subcellular environments where they control the phosphorylation state of neighboring substrates (1). Movement of enzymes in and out of multiprotein complexes contributes to the temporal regulation of signaling. Hence genetic manipulation of AKAP expression impacts the specificity and magnitude of cellular regulation within the context of the whole organism. This is particularly evident in the central nervous system where the elongated and branched morphology of neurons creates many intracellular compartments where AKAPs synchronize neuronal events (2–4).
AKAP79/150 is a family of three orthologs (human AKAP79, murine AKAP150, and bovine AKAP75) each initially defined on the basis of its ability to tether the type II PKA holoenzyme (4, 5). Additional binding partners were subsequently identified including PP2B and PKCs (6, 7). Thus, AKAP79/150 complexes can to respond to intracellular second messengers such as cAMP, calcium and phospholipids (7). Furthermore, the simultaneous anchoring of signal transduction and signal termination enzymes influences both forward and backward steps of a cellular event. For example, AKAP79/150 complexes can influence the phosphorylation and action of transmembrane proteins including G protein coupled receptors and adenylyl cyclases (8, 9). Loss of AKAP79/150 from heart cells contributes to the onset of angiotensin II-induced hypertension (10). Electrophysiological approaches have established a role for AKAP79/150 in the modulation of ion channels such as AMPA type glutamate receptors, L-type calcium channels and various potassium channels (11–13). This collective work supports the concept that AKAP79/150 assembles different customized combinations of binding partners (14, 15).
AKAPs have been implicated in the coordination of higher order neuronal events such as the regulation of synaptic strength and inhibition of neuronal excitability and auditory fear conditioning (16–18). Many of these studies have used non-specific reagents to disrupt PKA anchoring. Only a few studies have been able to ascribe the regulation of specific neuronal events to a particular AKAP. Mutation of AKAP150 to prevent PKA binding abolishes certain forms of hippocampal LTP and blocks phosphorylation of the L-type calcium channel (19, 20). Genetic ablation of another AKAP (WAVE-1) changes the morphology of dendritic spines. These mice have defects in neuronal outgrowth and spatial memory retention suggesting that WAVE-1 coordinates aspects of neuronal morphogenesis and the fidelity of synaptic connectivity (21).
We show that loss of AKAP150 is associated with an exclusion of the PKA holoenzyme from hippocampal dendritic spines, changes in synaptic transmission and deficits in memory retention. We identify behavioral defects associated with perturbed cerebellar function and a reduced ability to inhibit M-type potassium channels upon muscarinic stimulation in SCG neurons.
Results and Discussion
Generation of AKAP150 Knockout Mice.
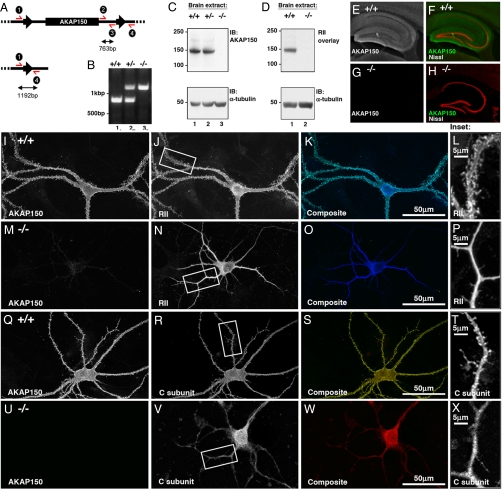
The AKAP150 coding region is contained within a single exon. Recombineering techniques were used to insert loxP recognition sequences on either side of the AKAP150 exon. The targeting vector was completed when a neomycin resistance cassette flanked by FRT recombination sequences was inserted downstream of the AKAP150 exon (Fig. 1A). This construct was transfected into mouse embryonic stem cells (ES) by electroporation. Neomycin-resistant cells containing both 5′ and 3′ loxP recombination sites were identified by PCR. Positive ES cell-lines were used to generate chimeric mice. Mice containing the AKAP150 transgene in germ cells were crossed with the FLPeR mouse strain to remove the neomycin resistance cassette. Deletion of AKAP150 was obtained when heterozygous (+/−) mice were crossed with a ubiquitous Cre deleter strain. Mating of +/− mice produced the expected numbers of homozygous null (−/−) progeny. Genotyping of the F2 offspring was performed by PCR. Primers annealing downstream of the AKAP150 exon, but upstream of the 3′ loxP sequence, produced a 763-bp band in wild-type (+/+) and +/− progeny (Fig. 1B, lanes 1 and 2). Primers annealing upstream of the 5′ loxP sequence and downstream of the 3′ loxP sequence exclusively produced an 1192-bp PCR product in −/− progeny (Fig. 1B, lane 3).
Fig. 1.
Generation of AKAP150 knockout mice. (A) Engineered mouse AKAP150 transgene before (Upper) and after (Lower) homologous recombination by Cre recombinase. Introduced 5′ and 3′ loxP recombination sequences flanking the AKAP150 exon indicated by black arrows. Genotyping oligonucleotides indicated in red. F2 offspring PCR used primers inside (2 and 3) and outside (1 and 4) of targeted genomic DNA. (B) F2 progeny: non-floxed allele (763bp band) or the floxed allele (1192bp band). PCR product from wild-type (+/+), heterozygous (+/−) and AKAP150 null littermates (−/−) are shown. DNA size markers are indicated. (C) Western blots of brain from +/+, +/− and −/− littermates using AKAP150 or α-tubulin antibody (loading control). Molecular weight markers indicated. (D) Autoradiograph of 32P-RII-overlay of from +/+ and −/− brain extracts. Loading control Western blot of α-tubulin. Molecular weight markers indicated. (E–H) Immunohistochemical localization of AKAP150 in coronal hippocampal sections from +/+ and −/− brains. Composite of AKAP150 (green) and Nissl (red) shown for +/+ (F) and −/− (H) mice. (I–X) Cultured hippocampal neurons from +/+ and AKAP150 −/− mice immunostained for AKAP150 (I, M,Q, and U) and the RII subunit of PKA (J and N) or the C subunit of PKA (R and V). Composite images of AKAP150 (green) and either RII (blue, K and O) or C subunit (red, S and W) are shown. Insets of PKA subunits (J, N, R, and V) enlarged to highlight dendritic morphology (L, P, T, and X).
Characterization of AKAP150 null phenotype was conducted on several levels. The anchoring protein was detected in brain extracts isolated from +/+ and +/− mice, but was absent from −/− mice (Fig. 1C, top immunoblot). Detection of α-tubulin was used as a loading control (Fig. 1C, Lower). Similar results were obtained upon 32P-RII overlay. A prominent RII binding band of ≈150 kDa was detected in +/+ brain extract but was not detected in corresponding samples from AKAP150 −/− mice (Fig. 1D). Prolonged exposure of the autoradiograph detected other RII binding bands in both genotypes that correspond to additional AKAPs that were identified by Western blot [supporting information (SI) Fig. S1]. It appears that there is no compensatory increase in the expression of other brain AKAPs occur as a consequence of the AKAP150 knockout (Fig. S1).
AKAP150 is expressed in a variety of brain regions (22). The anchoring protein was detected in several hippocampal structures including CA1, CA3, and dentate gyrus (Fig. 1 E and F). AKAP150 was not detected in coronal brain sections from −/− mice (Fig. 1 G and H). The brains of these animals had no obvious morphological abnormalities (data not shown). Fluorescent Nissl staining was used as a marker for hippocampal neurons (Fig. 1 F and H).
A defining property of AKAP150 is the ability to tether the type II PKA holoenzyme at the postsynaptic densities of neurons (5). Immunofluorescence techniques were used to compare the subcellular location of PKA subunits in cultured hippocampal neurons. The anchoring protein (green) was concentrated in the dendrites and spines of +/+ neurons (Fig. 1 I and K). A significant proportion of this signal overlapped with the pattern for RII (blue; Fig. 1 J and L). In contrast, RII staining was excluded from the spines in AKAP150 −/− neurons (Fig. 1 M–P). Parallel studies examined the subcellular distribution of AKAP150 (green) and the C subunit of PKA (red; Fig. 1 Q–X). There was significant overlap of AKAP150 and C subunit in the dendrites and spines of +/+ hippocampal neurons (red; Fig. 1 R–T). However, the majority of the C subunit was excluded from dendritic spines in AKAP150 −/− neurons (Fig. 1 U–X). Golgi staining revealed that the morphology and number of dendritic spines was similar in +/+ and −/− neurons (Fig. S2). These results show that loss of AKAP150 perturbs targeting of the PKA holoenzyme in a manner that favors accumulation of the kinase in the dendritic shaft. This may represent a redistribution of the kinase mediated through interaction with MAP2, a prominent AKAP found in the dendritic cytoskeleton (23).
The altered location of these PKA subunits in AKAP150 −/− neurons is shown in Insets (Fig. 1 L and P, T, X). This provides some of the most compelling cellular evidence to date that AKAPs control the location of the PKA holoenzyme in vivo. Therefore, we reasoned that AKAP150 null mice might have altered hippocampal function.
Electrophysiological and Behavioral Analyses Uncover Hippocampal Deficits in AKAP150 Knockout Mice.
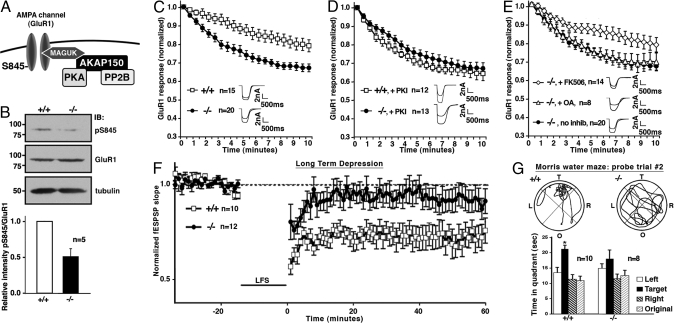
Experience-induced remodeling of hippocampal neural networks is essential for the higher-order cognitive processes of declarative memory, including spatial learning and memory (24). The movement of glutamate receptors in and out of synaptic membranes contributes substantially to network plasticity by regulating postsynaptic ion flow in response to the release of neurotransmitters (25). Several AKAP signaling complexes have been implicated in the modulation of hippocampal AMPA-type and NMDA-type glutamate receptors (3, 26). We have demonstrated that AKAP79/150 directs PKA and PP2B toward AMPA receptors to facilitate efficient channel regulation. PKA phosphorylation of Ser-845 on the GluR1 subunit of the AMPA receptor stabilizes this current. An AKAP150-anchored pool of PP2B opposes this process (11) (Fig. 2A). Immunoblot analysis with phosphopeptide antibodies detected phosphorylation of Ser-845 reduced by 51.2 ± 11% (n = 5) in AKAP150 −/− synaptosomal fractions when compared with +/+ controls (Fig. 2B). Whole-cell patch clamp analyses assessed the impact of AKAP150 loss on AMPA currents. Attenuation of glutamate-dependent AMPA currents was slight in +/+ neurons (Fig. 2C, white). However, agonist-dependent downregulation of the channel was pronounced in AKAP150 −/− neurons (Fig. 2C, black). Delivery of PKI 5–24 peptide, a PKA-specific inhibitor, induced similar agonist-dependent downregulation of AMPA channels in +/+ neurons (Fig. 2D, white) with no additional effect in AKAP150 knockout neurons (Fig. 2D, black). When these findings are considered in light of our immunocytochemistry results they support a model where AMPA currents are sustained when PKA is tethered at synapses via association with AKAP150.
Fig. 2.
Loss of AKAP150 modifies excitatory synaptic transmission. (A) Model molecular composition of an AKAP150-GluR1 complex. (B) Immunoblots of GluR1 subunit of AMPA channel detect serine 845 phosphorylation in +/+ or AKAP150 −/− mice. (Top) Ser-845 phosphorylation. (Middle) Total GluR1 protein. (Bottom) Tubulin control. Phosphate incorporation evaluated by densitometry (Bottom, n = 5). (C–E) Whole-cell electrophysiological recording of hippocampal AMPA currents after application of 100 μM glutamate. (C) Time courses of normalized AMPA currents from +/+ and AKAP150 −/− neurons presented for time = 0–10 min after treatment with glutamate. Representative traces at time = 0 and 5 min. (D) Time course of AMPA currents with 10 μM PKI (protein kinase A inhibitor peptide) included in pipette solution. (E) Pharmacological characterization of glutamate induced downregulation. Neurons treated with either 1 μM FK506, 100 nM okadaic acid or no inhibitor throughout the experiment. (no PKI). (F) Graph comparing the long-term depression (LTD) induced by low frequency stimulation (LFS, 1 Hz 900 pulses) of hippocampal SC-CA1 synapses in brain slices from +/+ or AKAP150 −/− mice. (G) Morris Water Maze evaluates spatial memory retention. (Upper) Representative trace of swim path during 2nd probe trial. The quadrants of the water maze are indicated: L = left, R = right, O = origin, and T = target. (Lower) Total time spent in each quadrant during the second probe trial presented for both +/+ and −/−. Error bars throughout figure indicate SEM.
Additional experiments in AKAP150 −/− neurons showed that agonist-dependent rundown of the channel was blocked upon application of the PP2B-selective inhibitor FK506 (Fig. 2E, diamonds). Regulation of AMPA currents was unaffected by the PP1/PP2A-selective phosphatase inhibitor okadaic acid (Fig. 2E, triangles). Congenital loss of the anchoring protein has a less pronounced effect on AMPA channel regulation than acute removal of AKAP150 by RNA interference (15). Ablation of the AKAP150 gene may trigger an adaptive response that promotes a subtle and compensatory elevation in PP2B activity within the vicinity of the channel. This seems plausible as PP2B (calcineurin) is abundant, representing ≈1% of total brain protein (27). Consequently, a modest redistribution of PP2B to the synapse could compensate for the loss of AKAP150. Alternatively, local regulation of AMPA channels in AKAP150 knockout mice may be performed by pools of PP2B tethered to other phosphatase-anchoring proteins such as Cain/cabin or MCIP1/2 (28, 29). However, another explanation is that PP2B activity is just dominant under these conditions.
Changes in synaptic efficiency are believed to contribute to memory storage (17). In the hippocampus, long-term depression (LTD) is a weakening of synaptic strength that is used as a cellular index for memory retention (30). LTD is a calcium-dependent process that is accompanied by dephosphorylation of postsynaptic PKA substrates including AMPA receptors (31, 32). PKA anchoring has been implicated in the induction of LTD, although the AKAP involved has not been identified (16). Thus, loss of AKAP150 could alter LTD. LTD was measured at SC-CA1 synapses in hippocampal slices using field recording technique (16). The induction of LTD by low frequency stimulation (LFS, 1Hz for 15 min) was markedly reduced in the −/− mice (Fig. 2F, black). This reduced synaptic depression could be explained by the lack of PKA in the spines of AKAP150 null neurons (Figs. 1 I–X). Loss of anchored kinase could limit the phosphorylation of postsynaptic substrates that are required to initiate LTD. No deficit was observed when AKAP150 −/− mice were evaluated for LTP (data not shown). This agrees with previous reports showing that LTP induced by a single tetanus of 100 Hz is normal in mice that express a PKA anchoring inhibitor transgene or AKAP150 knock-in strains that lack the ability to anchor PKA (17, 19).
Bear and colleagues used a cell-soluble PKA-anchoring inhibitor to show that global disruption of PKA targeting blunts the induction of LTD (16). The net effect is dephosphorylation of Ser-845 on GluR1 and internalization of the AMPA channel by endocytosis (26). This certainly tallies with the whole-cell electrophysiology recordings in Fig. 2F, where genetic removal of AKAP150 might favor a phosphatase-dominant environment that occludes induction of LTD. Interestingly, genetic removal of another neuronal anchoring protein, WAVE-1, produces a similar phenotype. However, the suppression of LTD in WAVE-1 knockout mice proceeds via a different mechanism where the sparse dendritic morphology of WAVE-1 null neurons may underlie changes in synaptic transmission (21). This is in contrast to AKAP150 −/− neurons, where Golgi stain analysis did not detect changes in spine morphology or numbers compared with +/+ (Fig. S2).
Changes in LTD are believed to underlie deficits in neural function that impair learning and memory. Therefore, three cognitive tests were initiated to monitor hippocampal performance in age matched +/+ and AKAP150 −/− mice. The Morris Water Maze evaluates spatial learning and memory. Initially, mice were trained over 2 days (6 trials per day) to swim to a visible platform (cued training). Both genotypes learned to locate the visible platform and exhibited similar swim velocities (+/+: 16 ± 0.4 cm/s n = 10 mice vs. −/−: 14.9 ± 0.5 cm/s n = 8 mice). During days 3 to 5, both genotypes were trained (6 trials/d) to locate a hidden platform that was submerged (acquisition). One h after the final acquisition session, the platform was removed and the mice were evaluated for spatial memory retention (probe trial). Neither genotype identified the target quadrant in the first probe trial (data not shown). On day 2 of the probe trial, the +/+ mice preferentially searched the target quadrant (Fig. 2G, Left) whereas AKAP150 −/− mice still swam around randomly (Fig. 2G Right). The Light–dark test assesses anxiety by measuring the percentage of time that a mouse chooses to occupy a darkened compartment over a 10-min period. Wild-type mice spent 59% ± 7 of the time (n = 12) in the dark, whereas AKAP150 −/− mice only spent 43% ± 6 of the time in the dark (n = 12). Collectively, these studies indicate that AKAP150 −/− mice harbor deficits in spatial memory retention and show reduced anxiety.
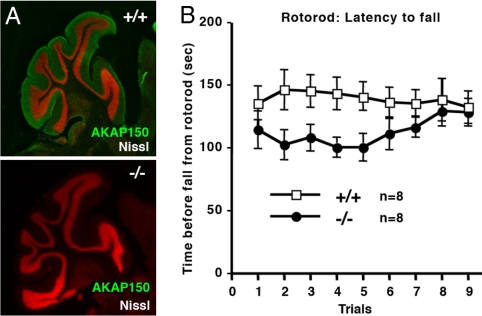
Due to the prominence of AKAP150 in the cerebellum (Fig. 3A), three behavioral tests investigated whether the −/− mice exhibited abnormal motor skills. Rotorod tests evaluate sensorimotor performance on a bar that revolves with steadily increasing speed. The latency of each mouse to fall was measured three times daily over a three-d period. AKAP150 −/− mice consistently performed more poorly than their +/+ littermates although they improved with training (n = 8; Fig. 3B). This inferred that AKAP150 −/− mice had deficits in motor coordination or strength. Therefore, both attributes were evaluated separately. The Inclined-screen evaluates coordination by recording the frequency of missteps while mice climb an upward sloping wire lattice over a five-min trial period. An average of 1.3 ± 0.4 (n = 8) forelimb missteps were recorded for +/+ mice (Fig. S3). The AKAP150 −/− cohort was less coordinated with 3.1 ± 0.9 (n = 8) recorded forelimb missteps. More pronounced deficiencies in hindlimb coordination were observed (+/+, 2.1 ± 0.5 missteps vs. −/−, 9.8 ± 2.4 missteps, n = 8; S3). Together the AKAP150 −/− mice were 3.8-fold more prone to missteps than their +/+ littermates (+/+, 3.4 ± 0.4, −/−, 12.9 ± 2). The wire-hanging test assesses muscular strength and endurance. Mice are suspended by their forelimbs from a wire (1 mm) until they release. The +/+ cohort performance was superior (70.4 ± 11 s before release; n = 8) to −/− mice (42.9 ± 7 s before release; n = 8, S3). Collectively, these behavioral tests indicate that AKAP150 −/− mice have impaired motor coordination and strength.
Fig. 3.
Loss of AKAP150 perturbs cerebellar-associated behavior. (A) Immunohistochemical localization of AKAP150 (green) in saggital cerebellar sections from wild-type +/+ (Upper) or AKAP150 −/− mice (Lower). Nissl (red) staining is also shown. (B) Cerebellar-associated behavior: Rotorod test. Time that a mouse maintained position on a rotating rod before falling is shown for +/+ (white) and −/− (black). Cohort daily averages with S.E.M. are presented.
Decreased M Channel Inhibition and Seizures in AKAP150 Knockout Mice.
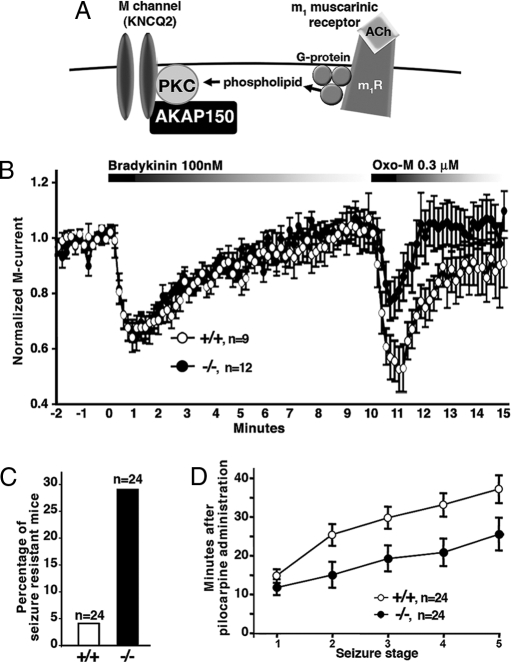
A number of studies have implicated AKAP79/150 in the regulation of neuronal potassium channels (12, 33). Muscarinic agonists suppress the “M current,” a K+ conductance that is prevalent in a variety of neurons (34). Genetic lesions in human M channel subunits have been linked to the onset of benign neonatal epilepsy. These mutations reduce channel activity and favor a hyperexcitable neuronal state (35). Classically, downregulation of this channel occurs via a Gq coupled pathway that evokes changes in phosphoinositide turnover (36, 37). However, an additional mechanism for M current downregulation involves AKAP79/150 anchored pools of PKC (12, Fig. 4A). Electrophysiological analysis of AKAP150 null mice evaluated the contribution of this alternative pathway to the regulation of M currents from cultured SCG neurons. Muscarinic (Oxo-M) suppression of the M current was blunted 40% ± 17 (n = 12) in AKAP150 −/− mice when compared with their +/+ littermates (n = 9; Fig. 4B). Control experiments confirmed bradykinin B2 receptor mediated suppression of the channel was similar in wild-type (n = 9) and AKAP150 knockout mice (n = 12; Fig. 4B). Bradykinin mediated suppression of the M current occurs via an AKAP150-independent mechanism that requires Gq and intracellular calcium (15). Although the muscarinic suppression of the M current is reduced in AKAP150 knockout mice, it is not completely lost. This remaining component most likely involves the depletion of PIP2 caused by PLC activation, which has been identified as major mechanism for the muscarinic suppression of the M current (37). Nonetheless, our data show that AKAP150 also contributes to the muscarinic suppression of the M current in vivo. Thus, Gq-coupled receptors can use at least three independent pathways to suppress the M current. These include depletion of PIP2, occupancy of the bradykinin receptor to elevate intracellular calcium, and an AKAP150-dependent pathway that involves PKC.
Fig. 4.
AKAP150 facilitates selective M current suppression in dissociated superior cervical ganglion (SCG) neurons. (A) Diagram of receptor-mediated G protein-coupled modulation of M channels. (B) Normalized M currents recorded from +/+ (white) and AKAP150 −/− (black) SCG neurons stimulated initially by bradykinin (100 nM) and secondarily by the agonist, Oxo-M (0.3 μM). Error bars indicate SEM. (C) Percentage of mice that did not respond by seizure to pilocarpine is shown for +/+ (white) or −/− (black). (D) Seizures severity was rated in pilocarpine responsive mice. The AKAP150 −/− mice that did respond to pilocarpine progressed more rapidly through the 5 seizure stages than −/− mice. Error bars indicate SEM.
M currents are essential in setting the resting membrane potential to control the excitability of neurons (38). Supraphysiolgical suppression of M currents with a non-subtype specific muscarinic agonist such as pilocarpine or transgenic expression of a dominant interfering M channel subunit that favors neuronal hyperexcitability has been implicated in the onset of seizures (39, 40). Further, support this concept comes from studies with m1 muscarinic receptor knockout mice showing that they are resistant to pilocarpine-induced seizures because the m1 receptor is not in place to transduce signals to M channel (39). Since AKAP150 contributes to the muscarinic suppression of this channel, we examined whether AKAP150 null mice were less prone to induced seizure. More AKAP150 −/− mice were resistant to seizure after peritoneal injection (300 mg/kg) of pilocarpine (Fig. 4C). Paradoxically, once induced, they progress more rapidly through the recognized stages of seizure (Fig. 4D). This apparent paradox may be explained by a two-step theory for pilocarpine seizure proposed by Solberg and Belkin (40) suggesting that muscarinic suppression of the M current permits excitatory neuronal evens to predominate (40). One aspect of this “hyperexcitatory condition” is the establishment of a glutamate-dependent “feed forward” mechanism that engages and activates the NMDA and AMPA-responsive channels to propagate the seizure. Since AKAP150 signaling complexes regulate both M channel and AMPA receptors, it is reasonable to assume that distinct AKAP150 complexes may coordinate events that temper seizure induction threshold and enhance seizure progression. Future studies are planned to test the contribution of NMDA receptors in this process.
In conclusion, we have shown that genetic removal of AKAP150 changes the local signaling environment to affect how second messenger-dependent kinases and phosphatases govern excitatory and inhibitory neuronal processes. In the hippocampus, mislocalization of PKA (and possibly PP2B) alters the bidirectional modulation of postsynaptic AMPA receptors with concomitant changes in synaptic transmission and memory retention. Likewise, the reduction in strength and motor coordination seen in the AKAP150 null mice is consistent with a role for the anchoring protein in the cerebellum. Finally, we found that loss of AKAP150 in SCG neurons lessens the muscarinic suppression of inhibitory M currents, and that some of these animals had an increased resistance to the drug pilocarpine, a strong muscarinic agonist. These studies provide functional validation for a model in which distinct AKAP150-enzyme complexes regulate context-dependent neuronal signaling events.
Methods
Animals.
Mouse colonies were kept at the OHSU Department of Comparative Medicine under IACUC-approved protocols. Mice were housed individually during behavioral tests (administered in order of increasing stress).
RII Overlay and Immunohistochemistry.
RII overlay assays and immunohistochemical analysis was performed using a method published in ref. 5 with modifications noted in SI Methods.
Cultured Neurons.
Primary hippocampal neurons were cultured from postnatal day 1 mouse embryos and used for 13–15 d in vitro (DIV), SCG neurons were isolated from 2-wk old mice and cultured as described in ref. 12. Modifications are noted in SI Methods.
Electrophysiological Measurements.
Whole-cell recording of isolated cells was done as described in ref. 15. Modifications are noted in SI Methods.
Behavioral Tests.
The Morris Water Maze, Rotorod, Inclined Screen, and Wire Hang behavioral tests were performed as described in ref. 21. Analysis of pilocarpine-induced seizure is described in SI Methods.
Supplementary Material
Acknowledgments.
We thank Lisa Dirling, Meg Cingel, Ted Benice, and Siegward Elsas for technical assistance. This work was supported in part by National Institutes of Health Grants GM48231 (to J.D.S.) and NNJ05HE63G, IIRG-05-14021, and MH77657 (to J.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805922105/DCSupplemental.
References
- 1.Wong W, Scott JD. AKAP Signalling complexes: Focal points in space and time. Nature Reviews Mol Cell biology. 2004;5:959–971. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 2.Rosenmund C, et al. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 3.Westphal RS, et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 4.Fraser ID, Scott JD. Modulation of ion channels: A“current” view of AKAPs. Neuron. 1999;23:423–426. doi: 10.1016/s0896-6273(00)80795-3. [DOI] [PubMed] [Google Scholar]
- 5.Carr DW, Stofko-Hahn RE, Fraser IDC, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins: Characterization of AKAP79. J Biol Chem. 1992;24:16816–16823. [PubMed] [Google Scholar]
- 6.Coghlan VM, et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 7.Klauck TM, et al. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 8.Fraser ID, et al. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 9.Bauman AL, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navedo MF, et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 11.Tavalin SJ, et al. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshi N, et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Kass RS. A-kinase anchoring proteins: Different partners, different dance. Nat Cell Bio. 2005;7:1050–1051. doi: 10.1038/ncb1105-1050. [DOI] [PubMed] [Google Scholar]
- 15.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7:1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder EM, et al. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie T, McDonough CB, Huang T, Nguyen PV, Abel T. Genetic disruption of protein kinase A anchoring reveals a role for compartmentalized kinase signaling in theta-burst long-term potentiation and spatial memory. J Neurosci. 2007;27:10278–10288. doi: 10.1523/JNEUROSCI.1602-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moita MA, Lamprecht R, Nader K, LeDoux JE. A-kinase anchoring proteins in amygdala are involved in auditory fear memory. Nat Neurosci. 2002;5:837–838. doi: 10.1038/nn901. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, et al. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall DD, et al. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 21.Soderling SH, et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostroveanu A, et al. A-kinase anchoring protein 150 in the mouse brain is concentrated in areas involved in learning and memory. Brain Res. 2007;1145:97–107. doi: 10.1016/j.brainres.2007.01.117. [DOI] [PubMed] [Google Scholar]
- 23.Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. JBC. 1982;257:3284–3290. [PubMed] [Google Scholar]
- 24.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 25.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 26.Colledge M, et al. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 27.Klee CB, Draetta GF, Hubbard MJ. Calcineurin. Adv Enzym. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- 28.Lai MM, Burnett PE, Wolosker H, Blackshaw S, Snyder SH. Cain, a novel physiologic protein inhibitor of calcineurin. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 29.Aramburu J, et al. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 30.Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci USA. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai S, et al. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J Biol Chem. 2007;282:22668–22677. doi: 10.1074/jbc.M703624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 35.Surti TS, Jan LY. A potassium channel, the M-channel, as a therapeutic target. Curr Opin Investig Drugs. 2005;6:704–711. [PubMed] [Google Scholar]
- 36.Zhang H, et al. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 37.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Scienc. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passmore GM, et al. KCNQ/M currents in sensory neurons: Significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton SE, et al. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solberg Y, Belkin M. The role of excitotoxicity in organophosphorous nerve agents central poisoning. Trends Pharmacol Sci. 1997;18:183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.