Abstract
Cognitively demanding tasks that evoke activation in the brain's central-executive network (CEN) have been consistently shown to evoke decreased activation (deactivation) in the default-mode network (DMN). The neural mechanisms underlying this switch between activation and deactivation of large-scale brain networks remain completely unknown. Here, we use functional magnetic resonance imaging (fMRI) to investigate the mechanisms underlying switching of brain networks in three different experiments. We first examined this switching process in an auditory event segmentation task. We observed significant activation of the CEN and deactivation of the DMN, along with activation of a third network comprising the right fronto-insular cortex (rFIC) and anterior cingulate cortex (ACC), when participants perceived salient auditory event boundaries. Using chronometric techniques and Granger causality analysis, we show that the rFIC-ACC network, and the rFIC, in particular, plays a critical and causal role in switching between the CEN and the DMN. We replicated this causal connectivity pattern in two additional experiments: (i) a visual attention “oddball” task and (ii) a task-free resting state. These results indicate that the rFIC is likely to play a major role in switching between distinct brain networks across task paradigms and stimulus modalities. Our findings have important implications for a unified view of network mechanisms underlying both exogenous and endogenous cognitive control.
Keywords: brain networks, cognitive control, insula, attention, prefrontal cortex
One distinguishing feature of the human brain, compared with brains lower on the phylogenetic ladder, is the amount of cognitive control available for selecting, switching, and attending to salient events in the environment. Recent research suggests that the human brain is intrinsically organized into distinct functional networks that support these processes (1–4). Analysis of resting-state functional connectivity, using both model-based and model-free approaches, has suggested the existence of at least three canonical networks: (i) a central-executive network (CEN), whose key nodes include the dorsolateral prefrontal cortex (DLPFC), and posterior parietal cortex (PPC); (ii) the default-mode network (DMN), which includes the ventromedial prefrontal cortex (VMPFC) and posterior cingulate cortex (PCC); and (iii) a salience network (SN), which includes the ventrolateral prefrontal cortex (VLPFC) and anterior insula (jointly referred to as the fronto-insular cortex; FIC) and the anterior cingulate cortex (ACC) (1, 2, 4, 5). During the performance of cognitively demanding tasks, the CEN and SN typically show increases in activation whereas the DMN shows decreases in activation (1, 2, 6). However, what remains unknown is the crucial issue of how the operation of these networks, identified in the resting state, relate to their function during cognitive information processing. Furthermore, the cognitive control mechanisms that mediate concurrent activation and deactivation within these large-scale brain networks during task performance are poorly understood.
In a recent meta-analysis, Dosenbach and colleagues hypothesized that several brain regions that overlap with the CEN and SN are important for multiple cognitive control functions, including initiation, maintenance, and adjustment of attention (7). However, no studies to date have directly assessed the temporal dynamics and causal interactions of specific nodes within the CEN, SN, and DMN. Converging evidence from a number of brain imaging studies across several task domains suggests that the FIC and ACC nodes of the SN, in particular, respond to the degree of subjective salience, whether cognitive, homeostatic, or emotional (4, 8–11). The CEN, on the other hand, is critical for the active maintenance and manipulation of information in working memory, and for judgment and decision making in the context of goal directed behavior (12–18). We therefore hypothesized a key role for the SN in the hierarchical initiation of cognitive control signals, specifically with respect to activation and deactivation in the CEN and DMN, and the dynamics of switching between these two networks.
We used three functional magnetic resonance imaging (fMRI) experiments to examine the interaction between the SN, CEN, and DMN, with particular interest in the role of the FIC/ACC in regulating these networks. In the first experiment, we scanned 18 participants as they listened with focused attention to classical music symphonies inside the scanner. We analyzed brain responses during the occurrence of “movement transitions:” salient, orienting events arising from transitions between adjacent “movements” in the music (19). To specifically elucidate the role of the FIC in driving network changes, we used chronometry and Granger Causality Analysis (GCA), to provide information about the dynamics and directionality of signaling in cortical circuits (20–22).
In the second experiment, we investigated the generality of network switching mechanisms involving the FIC by examining brain responses elicited during a visual “oddball” attention task (23). A third experiment examined whether the network switching mechanism could be observed during task-free resting state where there was no overt task and no behavioral response (4). Our motivation for examining the resting-state fMRI data was the recent finding, based on computer simulation of large-scale brain networks, that even in the absence of external stimuli, certain nodes can regulate other nodes and function as hubs (24). Our aim was to test the hypothesis that common network switching mechanisms apply across tasks with varying cognitive demands and differing stimulus modalities. If confirmed, our findings would provide insights into fundamental control mechanisms in the human brain.
Results
We describe findings from Experiment 1 in the first three sections. Convergent findings from Experiments 2 and 3 are described subsequently.
Activation of CEN and SN, and Deactivation of DMN During Auditory Event Segmentation.
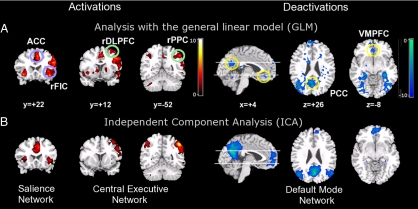
As reported previously (19), we found robust right-lateralized activation in the DLPFC, PPC, and FIC during “movement transitions” in the auditory event segmentation task. Here, we extend these findings to characterize network-specific responses in the CEN, DMN, and SN. Activations in the CEN and SN were found to be accompanied by robust deactivation in the DMN at the movement transition [Fig. 1A and General Linear Model Analysis in supporting information (SI) Materials and Methods]. To further confirm that these regions constitute coherent networks, rather than isolated regional responses, we performed independent component analysis (ICA) on the task data, which revealed the existence of statistically independent CEN, SN, and DMN (Fig. 1B, see also Table S1) [ICA is a model-free analysis technique that produces a set of spatially independent components and associated time courses for each subject (25)]. In the following two sections, we examine the putative causal mechanisms involved in switching between activation and deactivation in the context of the three networks, identified above, using a combination of mental chronometry and GCA (21, 22).
Fig. 1.
Activations in the Central-Executive and Salience Networks and deactivations in the Default-Mode Network during auditory event transitions. (A) Analysis with the General Linear Model (GLM) revealed regional activations (Left) in the right hemispheric FIC and ACC (blue circles); DLPFC and PPC (green circles) (coronal sections at y = +22, +12 and −52 mm) and deactivations (Right) in the VMPFC and PCC (sagittal section at x = +4 mm and axial sections at z = +26 and −8 mm, yellow circles) during event transitions. The scale for t-scores is shown along side. Activations height and extent thresholded at the P < 0.01 level (corrected). (B) Independent Component Analysis (ICA, a model-free analysis technique) provided converging evidence for spatially independent and distinct networks. From left to right: Salience Network (rFIC and ACC), Central-Executive Network (rDLPFC and rPPC), and Default-Mode Network (VMPFC and PCC). Activations height and extent thresholded at the P < 0.001 level (uncorrected). The ICA prunes out extraneous activation and deactivation clusters visible in the GLM analysis to reveal brain regions that constitute independent and tightly coupled networks.
Latency Analysis Reveals Early Activation of the rFIC Relative to the CEN and DMN.
First, we identified differences in the latency of the event-related fMRI responses across the entire brain using the method developed by Henson and colleagues (26). Briefly, this method provides a way to estimate the peak latency of the BOLD response at each voxel using the ratio of the derivative to canonical parameter estimates (see SI Materials and Methods for details). This analysis revealed that the event-related fMRI signal in the right FIC (rFIC) and ACC peaks earlier compared to the signal in the nodes of the CEN and DMN, indicating that the neural responses in the rFIC and ACC precede the CEN and DMN (see Fig. S1 and Table S2). To provide converging quantitative evidence, we estimated the onset latency of the blood oxygen level dependent (BOLD) response in these regions using the method of Sterzer and Kleinschmidt (27). Previous studies have used differences in the onset latency of the BOLD response as a measure of the difference in onset of the underlying neural activity (20, 21, 27). We first defined regions of interest (ROIs) in six key nodes of the SN, CEN, and DMN based on the peaks of the ICA clusters (see Materials and Methods); all subsequent analyses was confined to these six canonical nodes of the SN, CEN, and DMN (see also SI Text for a discussion on the choice of regions of interest and control analyses on regions not included in the main analysis). We extracted the mean time-course in each of these six nodes, and used a sixth-order Fourier model to fit the event related BOLD response for each subject and event, and averaged the fitted responses across events and subjects (see Fig. S2). Onset latencies were then computed as the time point at which the slope of the fitted response reached 10% of its maximum positive (or negative) slope in the initial ascending (or descending) segment. We found that the rFIC onsets significantly earlier than all of the nodes in the CEN and DMN (two-sample t-test, q <0.05; FDR correction for multiple comparisons) (Fig. 2, see also Table S3). These results confirm that activity in the rFIC onsets earlier compared to the activation in the CEN nodes, and deactivation in the DMN nodes.
Fig. 2.
Onset latencies of the event-related responses in the six key nodes of the SN (blue bars), CEN (green bars) and DMN (yellow bars) in the auditory event segmentation task. The rFIC onset significantly earlier than each of the nodes in the CEN and DMN (two-sample t-test, q <0.05, indicated by (*), FDR corrected for multiple comparisons). Error bars denote standard error of the mean (SEM) across subjects.
GCA Reveals that the rFIC Is a Causal Outflow Hub at the Junction of the CEN and DMN.
Finally, to elucidate the dynamic interactions between the three networks we applied GCA. Briefly, GCA detects causal interactions between brain regions by assessing the predictability of signal changes in one brain region based on the time-course of responses in another brain region (28). We performed GCA using a bivariate model (22) on the time-courses extracted from the six key regions used in the onset latency analysis. We used bootstrap techniques (29) to create null distributions of influence terms (F-values) and their differences (22). A causal connectivity graph was constructed using the thickness of connecting arrows to indicate the strengths of the causal influences (Fig. 3A, “raw” F-values normalized by the maximum F-value; raw F-values reported in Table S4). Only links that showed significant directed connectivity (influence terms) at the group-level (Mann-Whitney U test, P < 0.01; Bonferroni corrected for multiple comparisons) are shown (gray arrows, Fig. 3A); a subset of these links that showed a dominant direction of influence (difference of influence terms) are highlighted in red in the same figure (Mann-Whitney U test, P < 0.05, FDR corrected links shown in Table S4) (see SI Materials and Methods for details). GCA on the time-courses extracted from the key regions revealed statistically significant direct or indirect causal influences from the rFIC to all of the regions in the CEN and DMN (Fig. 3A). To quantify the causal interactions of each node of the network, we performed network analyses on key graph metrics (see Materials and Methods), and constructed a distribution of these metrics, across subjects (for each node). Network analysis on the causal flow network identified with GCA revealed that the rFIC had the highest number of causal outflow connections (out-degree), the lowest number of causal inflow connections (in-degree), and the shortest path length among all regions (means and standard errors of these metrics are reported in Table S5A). The rFIC also had a significantly higher net causal outflow (out-in degree) than all of the nodes of the CEN and DMN (two-sample t test, P < 0.05). Differences in (out-in) degree between the rFIC and the rDLPFC, rPPC, and PCC remained significant after FDR correction for multiple comparisons (q < 0.05) (Fig. 4A). Similarly, the rFIC had a significantly shorter path length than all of the other regions except the VMPFC (t test, P < 0.05); however, these differences did not remain significant after multiple comparison correction (data not shown). These results suggest that the rFIC is an outflow hub at the junction of the CEN and DMN.
Fig. 3.
Granger causality analysis (GCA) of the six key nodes of the Salience (blue nodes), Central-Executive (green nodes) and Default-Mode (yellow nodes) networks during (A) auditory event segmentation, (B) visual oddball attention task, and (C) task-free resting state. GCA revealed significant causal outflow from the rFIC across tasks and stimulus modalities. In each subfigure, the thickness of the connecting arrows between two regions corresponds to the strength of directed connection (F-value) normalized by the maximum F-value between any pair of regions for that task (“raw” F-values reported in Table S4). Only links that showed significant directed connectivity at the group-level (Mann-Whitney U test, P < 0.01; Bonferroni corrected for multiple comparisons) are shown (gray arrows); a subset of these links that showed a dominant directional influence (difference of influence term) are highlighted in red (Mann-Whitney U test, P < 0.05).
Fig. 4.
Net Granger causal outflow (out-in degree) of the key nodes of the Salience, Central-Executive, and Default-Mode Networks in the three experiments. Comparison of the net causal outflow (out-in degree) for the six key nodes of the Salience, Central-Executive, and Default-Mode networks as assessed by Granger causality analysis revealed that the rFIC has a significantly higher net causal outflow than the CEN and DMN regions across tasks (conventions as in Fig. 2). Specifically, the rFIC had a significantly higher net causal outflow than almost all of the other CEN and DMN regions for the auditory segmentation and resting-state tasks, and the rDLPFC for the visual oddball task (two-sample t-test, q < 0.05, indicated by (*), FDR corrected for multiple comparisons).
Converging Evidence from Two Additional fMRI Experiments.
To provide converging evidence for the rFIC as a causal outflow hub, we analyzed fMRI data from two other experiments using the same GCA and network analyses methods described above: (i) a visual “oddball” attention experiment, and (ii) a task-free resting state experiment (see also SI Materials and Methods). We found a pattern of significant causal outflow from the rFIC that was strikingly similar to the auditory event segmentation experiment (Fig. 3 B and C). We then constructed network metrics for these tasks using a procedure identical to the one used for the auditory segmentation task. In each case, the rFIC had the highest out-in degree and the shortest path length (Table S5 B and C). Again, the rFIC again had a significantly higher net causal outflow than several of the other nodes of the CEN and DMN (Fig. 4 B and C). Specifically, the rFIC had a significantly higher (out-in) degree than all of the other CEN and DMN nodes in the resting state, and the rDLPFC in the visual oddball task (two-sample t-test, q < 0.05, FDR correction for multiple comparisons). These converging results indicate that that the rFIC is a critical, causal outflow hub across task paradigms and stimulus modalities.
Discussion
ICA revealed the existence of statistically independent CEN, DMN, and SN during task performance, extending our recent discovery of similar networks in task-free, resting-state, conditions (4). Our analysis indicates that the rFIC, a key node of the SN, plays a critical and causal role in switching between the CEN and the DMN (we use the term “causal” here, and in the following sections in the sense implied by, and consistent with, latency analysis, GCA and network analysis). The striking similarity of significant causal outflow from the rFIC across tasks, involving different stimulus modalities, indicates a general role for the rFIC in switching between two key brain networks. Furthermore, our replication of this effect in the task-free resting state suggests that the rFIC is a network hub that can also initiate spontaneous switching between the CEN and DMN (24). Our findings help to provide a more unified perspective on exogenous and endogenous mechanisms underlying cognitive control.
In the SI Discussion, we suggest that these interactions are the result of neural, rather than vascular processes. Here, we focus on the neurobiological implications of our findings in the context of the three networks that we set out to examine; analyses of several other control regions (including the sensory and association cortices) that further clarify the crucial role of the FIC in the switching process are discussed in the SI Text.
FIC-ACC Network Is Neuroanatomically Uniquely Positioned to Generate Control Signals.
In primates, anatomical studies have revealed that the insular cortex is reciprocally connected to multiple sensory, motor, limbic, and association areas of the brain (30, 31). The FIC and ACC themselves share significant topographic reciprocal connectivity and form an anatomically tightly coupled network ideally placed to integrate information from several brain regions (9, 10, 32). Indeed, analysis of the auditory and visual experiments in our study found coactivation of these regions during task performance, as in many other studies involving cognitively demanding tasks (7). Previous neurophysiological and brain imaging studies have shown that the FIC-ACC complex moderates arousal during cognitively demanding tasks and that the rFIC, in particular, plays a critical role in the interoceptive awareness of both stimulus-induced and stimulus-independent changes in homeostatic states (9, 10). Furthermore, the FIC and ACC share a unique feature at the neuronal level: The human FIC-ACC network has a specialized class of neurons with distinctive anatomical and functional features that might facilitate the network switching process that we report here. The von Economo neurons (VENs) are specialized neurons exclusively localized to the FIC and ACC (33). Based on the dendritic architecture of the VENs, Allman and colleagues have proposed that “the function of the VENs may be to provide a rapid relay to other parts of the brain of a simple signal derived from information processed within FI and ACC.” (34). We propose that the VENs may, therefore, constitute the neuronal basis of control signals generated by the FIC and ACC in our study. Taken together, these findings suggest that the FIC and ACC, anchored within the SN, are uniquely positioned to initiate control signals that activate the CEN and deactivate the DMN.
Differential Roles of the rFIC, ACC, and Lateral Prefrontal Cortex in Initiating Control Signals.
Many previous studies of attentional and cognitive control have reported coactivation of the FIC and ACC (7, 23, 35, 36). The differential role of each of these regions has been poorly understood (37) as few studies have used chronometric techniques or causal analyses to dissociate the temporal and network dynamics of responses in these regions. We found that although onset latencies in the rFIC and ACC did not differ significantly, as might be expected from their being part of the same (salience) network, the FIC did have a powerful causal influence on the ACC (and correspondingly, higher net causal outflow than the ACC) in all three datasets (Figs. 3 and 4). Even under conditions in which the ACC plays an important role in cognitive control (23, 36), the rFIC may generate the signals to trigger hierarchical control and previous studies, including ours, may have missed detecting these effects. Our data further suggest that when the ACC is dysfunctional (38, 39), the FIC is well positioned to trigger alternate cognitive control mechanisms via the CEN. Our findings therefore help to clarify an important controversy regarding the primacy and uniqueness of control signals in the prefrontal cortex (39).
Brain regions in the right inferior frontal cortex, surrounding the FIC, have been implicated in a wide range of cognitive control mechanisms (40–42). For example, many of the paradigms involving response inhibition and inhibitory control have focused on ventrolateral regions (primarily within BA 47 and 45) within the right inferior frontal gyrus (43). However, the specific role of the right FIC has been less well studied perhaps because it is usually coactive with the lateral prefrontal cortex. A notable exception is a study by Dosenbach et al. (44) who used resting-state fMRI blocks, interspersed between task blocks, and graph theoretical analysis to underscore the distinctiveness of the FIC and its connectivity with the ACC. Further, a recent lesion study in humans has shown that the rFIC has an important role in cognitive control related to task switching. Using an oculomotor-switching task Hodgson and colleagues (45) showed that patients with lesions in the anterior rFIC were the most impaired in altering their behavior in accordance with the changing rules of the task. In normal healthy adults, functional brain imaging studies have suggested that the FIC and the ACC are together involved in a variety of cognitive control processes, including conflict and error monitoring, interference resolution, and response selection (23, 36, 40, 46–48). We hypothesize that in all these cases, the rFIC enables task-related information processing by initiating appropriate control signals to engage the ACC and the CEN. Our findings are inconsistent with the suggestion that the FIC-ACC provides stable ‘set-maintenance’ over entire task epochs whereas the fronto-parietal component initiates and adjusts control (49). In our view, it is the FIC-ACC-centered SN network that initiates key control signals in response to salient stimuli or events. As the lesion study by Hodgson and colleagues illustrates so dramatically, failure to generate these signals can have severe consequences for behavior. Our findings do not, however, preclude the possibility that after the FIC initiates changes in intra- and inter-network activity the CEN may carry out top-down important control functions either on its own or in association with the SN.
Our findings help to synthesize these and other extant findings in the literature into a common network dynamical framework and they suggest a causal, and potentially critical, role for the rFIC in cognitive control. We propose that one fundamental mechanism underlying such control is a transient signal from the rFIC, which engages the brain's attentional, working memory and higher-order control processes while disengaging other systems that are not task-relevant. We predict that disruptions to these processes may constitute a key aspect of psychopathology in several neurological and psychiatric disorders, including frontotemporal dementia, autism, and anxiety disorders (34, 50, 51). More generally, our study illustrates the power of a unified network approach—wherein we first specify intrinsic brain networks and then analyze interactions among anatomically discrete regions within these networks during cognitive information processing—for understanding fundamental aspects of human brain function and dysfunction.
Materials and Methods
Experimental Design.
We used data from three different experiments. The first experiment involved auditory event segmentation and detection of salient event boundaries in passages of music by the Baroque composer William Boyce. Eighteen right-handed participants (19–27 years of age, 8 females) with little or no musical training participated in the experiment. Participants listened to stimuli with focused attention inside the scanner over noise-reducing headphones. A follow-up behavioral study conducted outside the scanner ensured that subjects could accurately detect the occurrence of movement transitions when these occurred in the stimulus. Further details can be found in Sridharan et al. (19). The second experiment involved a visual oddball task. Thirteen subjects (24 ± 4.5 years of age, 8 females) participated in the experiment. Two hundred visual stimuli were presented for 150 ms with a 2,000-ms interval between stimulus onsets. Visual stimuli consisted of colored circles (either blue or green) and the frequency of the colored circles was counterbalanced across subjects (such that for half of them, green was the infrequent stimulus). Further details can be found in Crottaz-Herbette and Menon (23). The third experiment involved an eight minute resting state scan in which twenty-two subjects participated (19–21 years of age, 11 females). Subjects were instructed to rest while keeping their eyes closed and were requested to avoid moving during the scan (4).
fMRI Acquisition, analysis with the General Linear Model (GLM), Independent Component Analysis (ICA), the Calculation of Peak and Onset Latency differences, and Granger Causality Analysis (GCA) followed the procedure reported in a previously published experiment (19). Details can be found in the SI. Here, we describe methods specifically related to network analysis of causal interactions.
Region of Interest (ROI) Definition and Time Series Extraction.
ROI analysis was performed using the Marsbar software package (http://marsbar.sourceforge.net). Spherical ROIs were defined as the sets of voxels contained in 6–10-mm spheres centered on the peaks of activation clusters obtained from the ICA analysis (Table S1). These same ROIs were used throughout all of the subsequent analyses (onset latency, GCA, and network analyses). The mean time course in each ROI was extracted by averaging the time courses of all of the voxels contained in the ROI.
Granger Causality Analysis.
GCA was performed using the Causal Connectivity Toolbox (52), with modifications based on the methods proposed by Roebroeck et al. (22). GCA was performed on the timeseries extracted from ROIs to test for causal influences between ROIs taken pairwise using the difference of influence term (Fx→y − Fy→x) (22). We performed statistical inference on the causal connections using bootstrap analysis: An empirical null distribution of the difference of influence terms was estimated using block-randomized time series (22). For each subject, dominant (difference of influence) connections that passed a P = 0.05 significance level (bootstrap threshold) were used for computing the network metrics described next. For details on the construction of the causal connectivity graph (Fig. 3) refer to SI Materials and Methods.
Network Analysis.
To describe the interactions between brain regions in the causal network generated by GCA, we list the definition of the following metrics used in traditional graph-theoretic analyses (52):
Out-degree: Number of causal outflow connections from a node in the network to any other node.
In-degree: Number of causal in-flow connections to a node in the network from any other node
(Out − In) degree: Difference between out-degree and in-degree is a measure of the net causal outflow from a node.
Path length: Shortest path from a node to every other node in the network (normalized by the number of nodes minus one). Shorter path lengths indicate a more strongly interconnected or “hub-like” node.
For the present analysis, we constructed a distribution of these metrics, across subjects, for each node of the network. The mean value of these metrics (and their standard errors) across subjects are reported in Table S5. Path length was computed using Dijkstra's shortest path algorithm (53). A two-sample t-test was then applied on two key network metrics, the (out-in) degree and the path length, with FDR correction for multiple comparisons, to identify those nodes whose network metrics were significantly different from the other nodes.
Supplementary Material
Acknowledgments.
We thank Mike Greicius for useful discussions and Elena Rykhlevskaia and Catie Chang for their comments on a preliminary draft of this manuscript. We acknowledge two anonymous reviewers for their insightful comments and suggestions. This research was supported by a Stanford Graduate Fellowship to D.S. and by grants from the Natural Sciences and Engineering Research Council of Canada to D.J.L., the National Science Foundation (BCS-0449927) to V.M. and D.J.L., and the National Institutes of Health (HD047520, NS058899) to V.M.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800005105/DCSupplemental.
References
- 1.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golland Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- 4.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Chicago: Harcourt; 2000. [Google Scholar]
- 9.Craig AD. How do you feel? interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 10.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 11.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 13.Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: Differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage. 2004;21:340–351. doi: 10.1016/j.neuroimage.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller NG, Knight RT. The functional neuroanatomy of working memory: Contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 16.D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 19.Sridharan D, Levitin DJ, Chafe CH, Berger J, Menon V. Neural dynamics of event segmentation in music: Converging evidence for dissociable ventral and dorsal networks. Neuron. 2007;55:521–532. doi: 10.1016/j.neuron.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Menon RS, Luknowsky DC, Gati JS. Mental chronometry using latency-resolved functional MRI. Proc Natl Acad Sci USA. 1998;95:10902–10907. doi: 10.1073/pnas.95.18.10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Formisano E, Goebel R. Tracking cognitive processes with functional MRI mental chronometry. Curr Opin Neurobiol. 2003;13:174–181. doi: 10.1016/s0959-4388(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 22.Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 24.Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckmann CF, Smith SM. Probablistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 26.Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- 27.Sterzer P, Kleinschmidt A. A neural basis for inference in perceptual ambiguity. Proc Natl Acad Sci USA. 2007;104:323–328. doi: 10.1073/pnas.0609006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abler B, et al. Investigating directed influences between activated brain areas in a motor-response task using fMRI. Magn Reson Imaging. 2006;24:181–185. doi: 10.1016/j.mri.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Efron B, Tibshirani RJ. An introduction to the bootstrap (Monographs on statistics and applied probability #57) Boca Raton, Florida: CRC Press LLC; 1993. [Google Scholar]
- 30.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 31.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 32.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 33.Watson KK, Jones TK, Allman JM. Dendritic architecture of the von economo neurons. Neuroscience. 2006;141:1107–1112. doi: 10.1016/j.neuroscience.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 34.Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: A possible role for von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- 37.Milham MP, et al. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- 38.Baird A, et al. Cognitive functioning after medial frontal lobe damage including the anterior cingulate cortex: A preliminary investigation. Brain Cogn. 2006;60:166–175. doi: 10.1016/j.bandc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- 40.Nee TE, Wagner TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 42.Robbins TW. Shifting and stopping: Fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgson T, et al. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007;130:1525–1537. doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- 46.Roberts KL, Hall DA. Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- 47.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 48.Eichele T, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulus MP. Decision-making dysfunctions in psychiatry–altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- 51.Seeley WW, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seth AK. Causal connectivity of evolved neural networks during behavior. Network Comput Neural Sys. 2005;16:35–54. doi: 10.1080/09548980500238756. [DOI] [PubMed] [Google Scholar]
- 53.Dijkstra EW. A note on two problems in connection with graphs. Numerische Math. 1959;1:269–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.