Abstract
The adrenomedullin (AM) gene, adm, is widely expressed in the central nervous system (CNS) and several functions have been suggested for brain AM. Until now, a formal confirmation of these actions using genetic models has been elusive since the systemic adm knockout results in embryo lethality. We have built a conditional knockout mouse model using the Cre/loxP approach. When crossed with transgenic mice expressing the Cre recombinase under the tubulin Tα-1 promoter, we obtained animals with no AM expression in the CNS but normal levels in other organs. These animals lead normal lives and do not present any gross morphological defect. Specific areas of the brain of animals lacking CNS AM contain hyperpolymerized tubulin, a consequence of AM downregulation. Behavioral analysis shows that mice with no AM in their brain have impaired motor coordination and are hyperactive and overanxious when compared to their wild-type littermates. Treatment with methylphenidate, haloperidol, and diazepam did not show differences between genotypes. Circulating levels of adrenocorticotropic hormone and corticosterone were similar in knockout and wild-type mice. Animals with no brain AM were less resistant to hypobaric hypoxia than wild-type mice, demonstrating the neuroprotective function of AM in the CNS. In conclusion, AM exerts a beneficial action in the brain by maintaining homeostasis both under normal and stress conditions.
Adrenomedullin (AM) is a 52-aa regulatory peptide with structural homology to calcitonin gene-related peptide (CGRP) and amylin. The adm gene is located in mouse chromosome 7 and codes for a preprohormone which, after posttranslational modifications, generates 2 biologically active peptides, AM and proadrenomedullin N-terminal 20 peptide (PAMP). Expression of these peptides is widespread and several functions have been ascribed to these molecules, including vasodilatation, bronchodilatation, hormone secretion regulation, growth modulation, angiogenesis promotion, and antimicrobial activity, among others (1). The receptor system for AM consists of a heterodimer composed of calcitonin receptor-like receptor (CLR) and receptor activity modifying protein (RAMP) type 2 or 3 (2).
In the CNS, AM was initially found in the hypothalamus (3), but later on it became clear that this peptide is expressed throughout the whole brain (4). Components of the AM receptor system are also found in most areas of the brain (5), providing an anatomical basis for the implication of AM in brain physiology. Receptor autoradiography studies have shown that AM is able to bind to many areas of the brain, as well (6). Recently, a new member of the CGRP/AM peptide family, with a peptide sequence similar to AM but coded by a different gene, has been reported and named AM2 or intermedin. This peptide has similar actions and distribution in the brain to original AM (7).
Several observations suggest that AM could act as a neurotransmitter or neuromodulator. For instance, direct injection of AM in the CNS induces vasoconstriction (8), an increased heart rate, and production of nitric oxide (9). Electrophysiological studies in the area postrema showed that AM was implicated in spike production (10). Other authors suggest that AM may activate both GABAergic and cholinergic neurons through nitric oxide production and mechanisms dependent and independent of NMDA receptors (9). In addition, AM expression increases in the brain following ischemic and hypobaric insults (11, 12). In these situations, AM seems to play a protective role.
A formal demonstration of the functions of AM in the brain would imply a genetic model where expression of the peptide or the receptor has been eliminated. Several attempts have shown that regular knockouts for AM or its receptor result in embryo lethality, thus preventing studies of adult brain physiology (13–16). To circumvent this difficulty, we have generated a brain-specific conditional knockout model using Cre/loxP technology (17). Here we show that mice lacking AM in the brain show marked behavioral differences with their wild-type littermates, being hyperactive, less coordinated, and over-anxious. In addition, their survival is impaired when exposed to hypobaric insult.
Results
Generation of a Brain-Specific Conditional Knockout for AM.
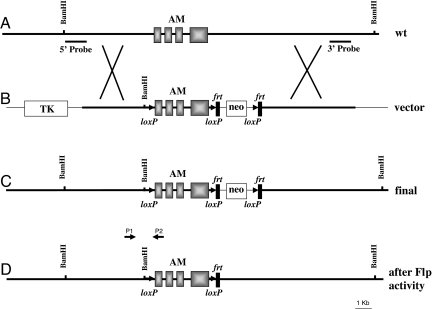
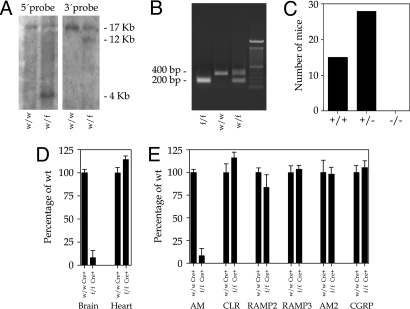
A targeting vector was generated from a 17-kb BamHI DNA fragment containing the complete adm gene (Fig. 1A). The linearized vector was introduced into embryonic stem (ES) cells and these cells were selected with G418 and FIAU. Surviving clones were analyzed by Southern blotting (Fig. 2A) to ascertain proper insertion (Fig. 1C) using an external and an internal probe (Fig. 1A). ES cells were injected into blastocysts and brought to term in surrogate mothers. These animals were crossed with transgenic mice expressing Flp to remove the pGKneo cassette (Fig. 1D). These “floxed” AM mice were crossed with transgenic mice expressing Cre under either an ubiquitous promoter or the tubulin Tα-1 promoter (18). This second promoter induces expression of Cre protein in neurons of the CNS during neurogenesis. Determination of the genotypes was performed by PCR with specific primers for both the floxed adm gene (Fig. 2B) and Cre [supporting information (SI) Table S1].
Fig. 1.
Gene targeting of adm. (A) Structure of the wild-type allele as found in mouse chromosome 7. The 4 exons of the adm gene are represented as solid boxes. Southern probes at the 5′ and 3′ ends of the construct are also indicated. (B) Targeting vector designed to insert loxP sequences (arrowheads) around the adm gene and the neomycin cassette (neo) surrounded by frt sequences (vertical rectangles). An additional BamHI site was added next to the first loxP to allow for identification. The TK gene was also added as a negative marker. A thinner line represents vector sequences. (C) Structure of the modified allele in the ES cells after recombination takes place. (D) Structure of the final allele, following the crossing with Flp expressing mice. P1 (AMD sense) and P2 (AMD antisense) are the primers for diagnostic PCR described in Table S1.
Fig. 2.
Characterization of the mice lacking brain AM. (A) Southern blot analysis of BamHI-digested ES cell DNA with the external 5′ probe (Left) and the internal 3′ probe (Right). The wild-type (w) allele renders a band of 17 kb whereas the mutant alleles (f) render 4- and 12-kb bands, respectively. (B) PCR analysis of tail DNA from 3-week-old mice with AMD primers (Table S1). After PCR, samples were digested with BamHI. The wild-type (w) allele produces a band of ≈400 bp whereas the mutant (f) is identified by a 200-bp band. Heterozygous mice (w/f) produce both bands. (C) Genotypes of offspring issued by the cross of 5 pairs of heterozygous mice for the ubiquitous deletion. No homozygous mutant mice (−/−) were produced. (D) Real-time PCR quantification of the levels of AM produced in the brain and heart of wild type (wt/wt Cre+) and brain conditional AM mutants (f/f Cre+). While a large diminution is appreciated in the mutant brain, the heart AM contents do not show any significant difference with the wild type. (E) Real-time PCR quantification of the levels of AM and AM-related molecules CLR, RAMPs, AM2, and CGRP in the brain of wild-type and mutant animals. The drastic reduction of AM in the mutant brain does not affect the expression of the related genes.
Characterization of the Knockout Animals.
Analysis of the ubiquitous adm knockout mice showed that the complete lack of AM from conception results in embryo lethality at midgestation, in agreement with previous reports (13–15). This can be clearly seen when analyzing the number of offspring from crosses between heterozygous parents (Fig. 2C). On the other hand, mice where Cre expression was restricted to the brain were born alive and did not have any gross morphological defect. Under standard conditions, these animals lead a normal life and reproduce normally. Expression studies using real-time RT-PCR demonstrated that the brain of knockout animals had a minimal expression of AM when compared to wild-type littermates whereas the AM levels in other organs were undistinguishable in both groups (Fig. 2D). We also studied whether molecules related with AM physiology suffered any change in the absence of cerebral AM. Neither CLR, the RAMPs, AM2, nor CGRP varied significantly their expression in the brain of knockout animals when compared to their wild-type siblings (Fig. 2E).
Morphological Changes.
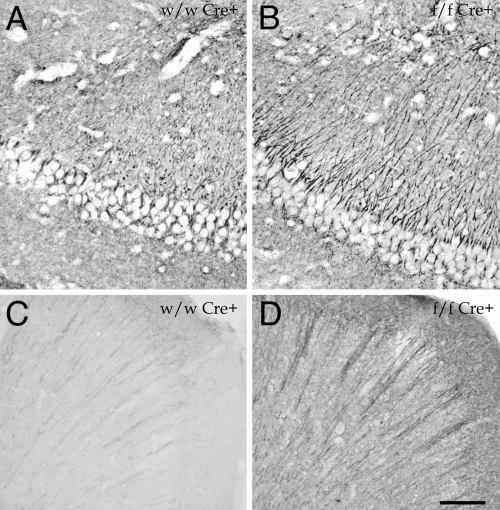
Histological analysis of the brain did not reveal any gross morphological change in the animals lacking AM. Recently we have described that AM and PAMP bind to the cytoskeleton and that reduction on the levels of these peptides leads to tubulin hyperpolymerization and to an increase on Glu-tubulin immunoreactivity (19). Application of the Glu-tubulin antibody to brain tissue sections showed that specific regions on the adm-null brains contained more hyperpolymerized tubulin than control animals. The areas where this effect was more prominent were the hyppocampus (Fig. 3 A and B), the cerebral cortex (Fig. 3 C and D), and the cerebellum.
Fig. 3.
Immunohistochemical staining for Glu-tubulin in the hippocampus (A and B) and frontal cortex (C and D) of wild type (A and C) and brain conditional AM mutants (B and D). Mutant animals show a higher staining intensity than normal mice. (Scale bar: A and B, 20 μm; C and D, 100 μm.)
Behavioral Analysis.
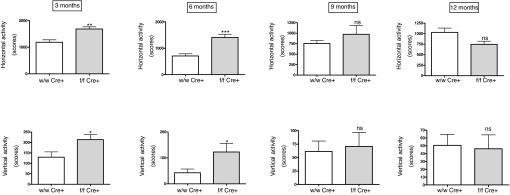
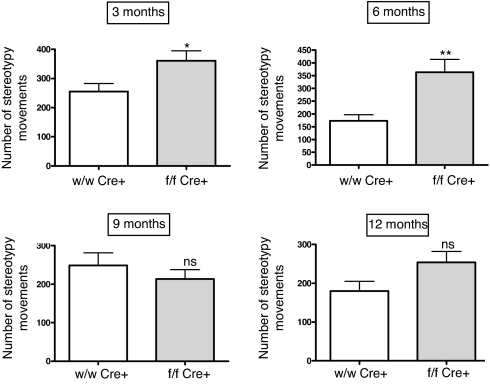
First, we used an activity cage to measure spontaneous locomotor activity. At 3 months of age, mice lacking brain AM had much higher levels of horizontal and vertical activity than their wild-type littermates (Fig. 4). The differences were similar in the first 5 min of the test (in which the mice explore the new environment) and in the following 5 min (at this time the animals are already used to the new place and engage in their regular routines). Activity cage measurements were performed with the same mice at 3, 6, 9, and 12 months of age. As the mice aged, their locomotor activity went down, and the differences between groups diminished, becoming indistinguishable at 9 months (Fig. 4). In all cases, locomotor parameters of the heterozygous mice were undistinguishable from their wild-type littermates (data not shown). There was also an increase on stereotypy counts in the knockout group for the first 6 months of extrauterine life (Fig. 5). In a similar way to the observations for general locomotion, after the animals became older the differences between genotypes disappeared (Fig. 5).
Fig. 4.
Activity cage measurements of horizontal (Upper) and vertical (Lower) activity in male mice at different ages. Mutant animals lacking brain AM (gray bars) are more active than their wild-type littermates (open bars) during the first 6 months of life. Bars represent the mean ± standard deviation of 10 mice. The whole experiment was repeated twice, and a representative example is presented here. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significant differences.
Fig. 5.
Stereotypy quantification as recorded in the activity cage in male mice at different ages. Mutant animals (f/f Cre+) present a higher number of stereotypies than wild-type littermates (wt/wt Cre+) for the first 6 months but the difference disappears in older mice. Bars represent the mean ± standard deviation of 10 mice. The whole experiment was repeated 3 times, and a representative example is presented here. *, P < 0.05; **, P < 0.01; ns, no significant differences.
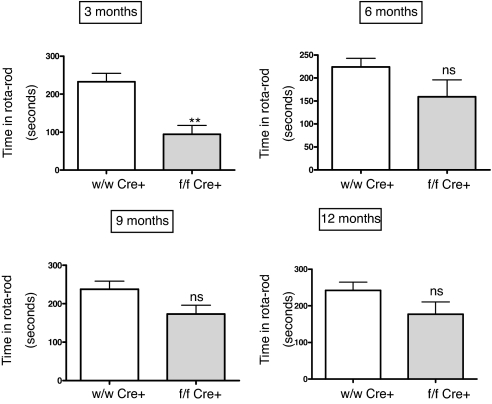
To test motor coordination and equilibrium, we performed a rota-rod test (Fig. 6). In this case, at 3 months of age the knockout mice had a poorer behavioral performance than their wild-type littermates, indicating impaired motor coordination. As with the previous test, significant differences were only seen in younger animals. As age increased, there was no change in the coordination of wild-type animals, but the knockout mice became more coordinated and the differences turned non-significant after 6 months of age (Fig. 6).
Fig. 6.
Rotarod measurements in male mice at different ages, expressed as the time in seconds the animal stayed in the rod. Mutant animals lacking brain AM (gray bars) are less coordinated than their wild-type littermates (open bars) at 3 months. Bars represent the mean ± standard deviation of 10 mice and 5 trials per animal. The whole experiment was repeated twice, and a representative example is presented here. **, P < 0.01; ns, no significant differences.
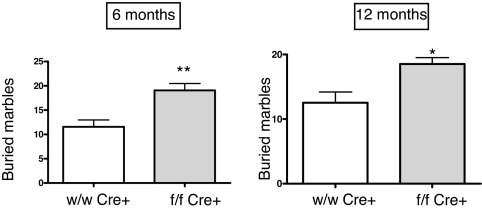
Anxiety levels were measured with a marble-burying assay. At six months, mice lacking AM in the brain buried 19.06 ± 1.39 marbles whereas the wild-type animals buried only 11.55 ± 1.42 (P < 0.01), indicating that animals lacking brain AM have a higher anxiety level. In this case, differences between genotypes were lower but still significant at 12 months (Fig. 7).
Fig. 7.
Anxiety assay based on the number of glass marbles buried by the mice in a 30 min period. Mutant animals lacking brain AM (gray bars) are more anxious than their wild-type littermates (open bars). Bars represent the mean ± standard deviation of 10 mice. The whole experiment was repeated twice, and a representative example is presented here. *, P < 0.05; **, P < 0.01.
Drug Treatments.
An initial interpretation of the behavioral data suggested that the animals lacking cerebral adm may be suffering a syndrome similar to the human attention deficit hyperactivity disorder (ADHD). Patients affected by ADHD are successfully treated with methylphenidate and other psychostimulants (20), which produce the paradoxical effect of bringing down the activity levels. To investigate whether our mice were sensitive to these drugs, we injected knockout and control mice with methylphenidate and measured their behavior on the activity cage at different times after treatment (Fig. S1 A and B). Contrary to what was expected, both groups of animals presented a significant increase in their spontaneous locomotor activities reaching a maximum at 30 min and diminishing progressively until going back to normal levels at 22 h.
The antipsychotic drug haloperidol is a dopamine receptor antagonist and was injected in both knockout and wild-type animals to check whether the dopaminergic system was involved in their behavioral differences. In both cases, a marked reduction of both horizontal and vertical activity was observed over a period of 180 min but no differences were found when comparing the genotypes (Fig. S1 C and D).
The anxiolytic drug diazepam, a GABAA receptor agonist, was used to check whether the differences in anxiety observed above were due to defects in the GABAergic system. As happens with haloperidol, a clear reduction in both horizontal and vertical activities was observed over a 180 min period following injection of diazepam. Nevertheless, the reductions were similar in both groups of animals (Fig. S1 E and F).
Determination of Stress Hormones.
To test whether the hypothalamus-pituitary-adrenal axis was involved in the excessive anxiety observed in mice lacking brain AM, we measured the levels of circulating adrenocorticotropic hormone (ACTH) and corticosterone in both genotypes at 3 months of age. No significant differences were found for either hormone.
Resistance to Hypobaric Stress.
Groups of 10 mice of each genotype were placed in a hypobaric chamber and exposed to increasing simulated altitude while continuously monitored. All males lacking AM in the brain died at 34,000 feet and within seconds of reaching that altitude, whereas wild-type animals died at 37,000 feet but over a period of 30 min.
Discussion
In this report, we have shown that deletion of brain adm in mice results in subtle morphological changes, behavior modifications, and lower survival when exposed to stressful conditions. Taken together, these results confirm the implication of the proAM peptides in brain physiology. We need to keep in mind that the adm gene generates a preprohormone that, after posttranslational modifications and maturation, produces 2 biologically active peptides, AM and PAMP (1). In our model, the whole gene is removed, thus the effects we have reported will be the result of the absence of both peptides.
Although AM2, also known as intermedin, has been localized in the same regions as AM and has been shown to perform similar activities (7), our study demonstrates that there are specific functions for AM that cannot be rescued by intrinsic expression of AM2. In the brain of our mutant mice, the levels of AM2 did not change with the absence of AM, and the knockout animals presented behavioral and survival defects even though they had normal levels of AM2. This lack of compensation has been also shown in other peptide families (21). In a similar way, we did not see any up-regulation of the components of the AM receptor, indicating that there is no compensatory response to the lack of brain AM.
The only morphological modifications we have seen in the adm-null brain were related to microtubule hyperpolymerization. Recent in vitro studies by our group have shown that tubulin hyperpolymerization mediated by lower levels of AM results in impaired cell motility and cell cycle defects (19). Since the activation of Cre expression by the tubulin Tα-1 promoter occurs early in brain development (at embryonic days 10–11) we can conclude that AM does not have a major function in defining brain architecture. Nevertheless, the hyperstabilized cytoskeleton of specific neurons may explain the subtle differences observed when comparing animals with and without brain AM. Several important diseases of the CNS involve microtubule dysfunction. One of the main molecules linked to the pathogenesis of Alzheimer's disease is the microtubule associated protein tau. When this protein is glycosylated and hyperphosphorylated it detaches from the microtubules and forms neurofibrilary tangles, whereas microtubule assembly gets inhibited (22). One of the most frequently mutated genes in Parkinson's disease is parkin, which codes for a microtubule stabilizing protein (23). The prion protein, PrP, also influences microtubule polymerization status, with the mutants related to familial Creutzfeldt-Jakob disease showing a much stronger inhibitive capacity on tubulin polymerization than the wild-type proteins (24). Most cases of early onset torsion dystonia (DYT1) are due to a mutation on the DYT1 gene which codes for a protein named torsinA, which in turn regulates cytoskeleton function (25). The results obtained in our study correlate especially well with a report of some neural side effects of the antineoplastic agent paclitaxel (taxol), which reduces tumor growth through a mechanism involving hyperpolymerization of the microtubules. Although the blood–brain barrier penetration for this drug is supposed to be low, several cases have been reported where patients receiving taxol developed a clinical state characterized by confusion, word-finding difficulty, and behavioral changes. The symptoms resolved spontaneously but reappeared with each subsequent infusion of the drug (26). Therefore our results indicate that changes in AM expression levels may be responsible for microtubule defects in the brain. It would be interesting to explore whether any of the above mentioned disorders correlates with changes in AM expression.
Our behavioral study indicates that mice with no AM in the brain are more active but less coordinated than their wild-type littermates. This behavior is consistent with lesions in the hippocampus and cerebellum (27). A number of genetically modified mouse models affecting hormones, receptors, transcription factors, or adhesion molecules show increased locomotion and/or impaired motor coordination (17), indicating that these are basic behaviors than can be affected through different pathways. Interestingly, our heterozygous mice behave exactly as wild-type animals, suggesting that even a partial presence of AM during brain development is enough to prevent damage.
Another characteristic of the mice lacking AM in the brain is their excessive anxiety levels. In several genetically modified models, a correlation between higher activity and higher anxiety has been described (28, 29). Several AM related peptides have been shown to modulate anxiety. For instance, intracerebroventricular administration of AM results in higher levels of corticosterone, CRH, and prolactin (30). In a similar way, s.c. injection of PAMP provoked an elevation on ACTH and corticosterone in the rat (31). In addition, several articles demonstrate that when humans or experimental animals are subjected to a diverse battery of stressors (restraint, dehydration, cold, disease, acute exercise, or psychological pressure) the common response is a sharp elevation on the levels of tissue and circulating AM (32–34). In our mice lacking adm in the brain, we observed a behavior consistent with higher basal stress levels when compared to their wild-type littermates. This clearly suggests that brain AM limits the magnitude of the stress response.
Another observation pointing to an exacerbated anxiety in mutant mice is the higher number of stereotypies observed in these animals. This phenomenon is usually linked to obsessive-compulsive disorders and gets modulated with the anxiety state of the animal (35).
Even though mice lacking brain AM present higher anxiety than their wild-type littermates, we did not find any difference in ACTH nor corticosterone levels when comparing both genotypes. Similar observations have been published on other modified organisms. For instance, when glycogen synthase kinase 3β was overexpressed in transgenic mice, increase locomotor activity was described without changes in HPA hormones (29).
In most of the behavioral tests, we have observed age-related changes with younger mice presenting more differences between genotypes than older animals. Although some genetic modifications result in higher differences with increasing age (36), it is not uncommon to find in the literature examples similar to our data (37, 38). This diminution of phenotypic differences with age may represent a compensatory mechanism that develops as the animals get older. Several studies have found changes in the expression of AM and its receptor through aging (39–41). These age-related changes may be responsible for the loss of phenotypic differences between genotypes.
The brain is very sensitive to hypoxia due to its high metabolic needs. In this scenario, the neuroprotective action of AM may be due to a series of mechanisms; these include vasodilatation, suppression of apoptosis and induction of angiogenesis, promotion of astrocyte migration and survival (42), and protection from oxidative stress (43). Our observation showing that deprivation of brain AM results in lower survival under hypoxic conditions points to a neuroprotective function for central AM and to an inefficiency for circulating AM to prevent injury.
Conditional knockout models provide excellent tools for the investigation of specific functions. Our results have been generated with a promoter that gets activated in neural development but other promoters would provide useful information on the physiological effects of AM on other organs. The usefulness of the floxed AM mouse as it is crossed with different Cre transgenic lines is warranted.
Methods
Targeting Vectors, Electroporation, and Selection.
For the adm gene, a replacement-type targeting vector was constructed from a genomic fragment obtained from a 129/SVJ mouse genomic library in Lambda FixII vector (Stratagene) (Fig. 1). The neo gene with the phosphoglycerate kinase 1 promoter (pGKneo) was used as a selectable marker; the pGK-thymidine kinase cassette was used as a negative selectable marker (44). Electroporation and selection were performed using the CJ7 ES cell line as described elsewhere (44). DNAs derived by G418/FIAU resistant ES clones were screened using a diagnostic BamHI restriction enzyme digestion using the 5′ and 3′ probes external to the targeting vector sequenced indicated in Fig. 1A. Recombinant clones containing the predicted 4-kb (for the 5′ probe) and 12-kb (for the 3′ probe) rearranged band were obtained at a frequency of 1/2.
Generation of Mutant Mice.
Three independent targeted ES cell clones for the adm gene injected into C57BL/6J blastocysts generated chimeras that transmitted the mutated allele to the progeny (45). Mice were bred in a specific, pathogen-free facility with food and water ad libitum. These animals were crossed with mice expressing Flp (FlpE, The Jackson Laboratory) and the elimination of the neo cassette was confirmed by PCR (Table S1). FlpE transgenic mice were originally generated in a B6SJL background. However, the original founder was backcrossed for at least 4 generations in a C57BL/6J background before using for specific crosses. The resulting animals were crossed until a homozygous colony where the adm gene was surrounded by loxP sequences (floxed) was created. The floxed adm mice were crossed with animals expressing Cre under either a ubiquitous promoter or the tubulin Tα-1 promoter (18). Cre expressing mice were generated in a mixed C57BL/6J-C3H/HeJ background and backcrossed 5 generations in a C57BL/6J background before being used for experimental crosses. All experiments were performed with littermates obtained from crosses of w/f Cre+ (heterozygous for the floxed allele and expressing Cre) parents.
Genotyping.
DNA was extracted from tail clips and characterization of the alleles for both AM and Cre was performed by PCR. Primer sequences are shown in Table S1.
Real-Time RT-PCR Determinations.
Total mRNA was extracted from different tissues of knockout and wild-type mice using RNeasy kits (Qiagen). The mRNA was reverse transcribed using SuperScript reverse transcriptase (Invitrogen). PCR was performed using the Chromo4 (MJ Research) thermocycler and software as described (12). All genes were normalized according to the 18S rRNA concentration of each sample. Primers are summarized in Table S1.
Immunohistochemistry.
Mice were fixed by transcardial perfusion with 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The brains were removed, cut into blocks, and postfixed for 3 h at room temperature. Free-floating sections were processed by the avidin-biotin peroxidase complex technique following standard methods (4) using rabbit anti-de-tyrosinated α-tubulin (glu-tubulin, Chemicon) as the primary antibody.
Activity Cage.
Locomotor activity testing was performed in a VersaMax activity cage (AccuScan Instruments). Vertical activity, horizontal activity, and stereotypy counts were measured for wild-type and knockout mice. Groups were compared by using Student's t test. Results were considered significant at P < 0.05.
Rota-Rod Test.
Motor coordination and equilibrium were analyzed in this test. The animals were placed with the four paws on a 2.5-cm-diameter bar, 25 cm above the floor, which was turning at 12 rpm. For each animal, the time of permanence on the bar up to 5 min was registered. The experiment was repeated 5 times per animal. Groups were compared by using Student's t test. Results were considered significant at P < 0.05.
Marble-Burying Assay.
The number of unfamiliar objects (in this case marbles) that are buried by a mouse in a specific period can be interpreted as a measure of the anxiety level of that animal. Mice were placed individually into a cage containing a 25-mm-deep layer of wooden chip bedding and 24 glass marbles with a diameter of 15 mm. The marbles were evenly spaced against the walls of the cage. Mice were left undisturbed for 30 min and the number of marbles that were at least two-thirds buried was recorded. Groups were compared by using Student's t test. Results were considered significant at P < 0.05.
Drug Treatments.
Methylphenidate was purchased from Laboratorios Rubio. All mice were tested on the activity cage before receiving the drug. Each mouse received 40 mg/kg methylphenidate diluted in 200 μl of PBS i.p. Activity cage parameters were measured for each mouse immediately before, and 30 min, 1 h, and 22 h after receiving the drug. Haloperidol was obtained from Almirall Prodesfarma. Mice received 0.1 mg/kg haloperidol i.p. Activity cage parameters were measured as above. Diazepam was purchased from Roche Farma. Each mouse was injected i.p. with 2 mg/kg diazepam as described. Activity cage parameters were measured as above.
Determination of Stress Hormones.
Blood serum was obtained from knockout and wild-type mice. ACTH and corticosterone were measured with specific enzyme immunoassay kits (Phoenix and Assay Designs, respectively), following manufacturers' protocols.
Hypobaric Treatment.
Altitude simulation was performed with a hypobaric chamber (Enviromental Tectonics International) type 10M which allows reduction of the barometric pressure and control of the temperature and relative humidity, as described (12). The ascent and descent rate was maintained <1,000 ft/min.
All procedures were carried out in accordance with the European Communities Council Directive (86/609/EEC) and reviewed by the Ethics Committee of the Instituto Cajal.
Supplementary Material
Acknowledgments.
This work was supported by Spanish Ministry of Science and Education Grants BFU2004–02838 and SAF2007–60010, Instituto de Salud Carlos III Grant RD06/0026/1001, and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (L.T. and F.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803174105/DCSupplemental.
References
- 1.Lopez J, Martinez A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int Rev Cytol. 2002;221:1–92. doi: 10.1016/s0074-7696(02)21010-4. [DOI] [PubMed] [Google Scholar]
- 2.McLatchie LM, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 3.Ueta Y, et al. Adrenomedullin-immunoreactive neurons in the paraventricular and supraoptic nuclei of the rat. Neurosci Lett. 1995;202:37–40. doi: 10.1016/0304-3940(95)12204-4. [DOI] [PubMed] [Google Scholar]
- 4.Serrano J, et al. Distribution of adrenomedullin-like immunoreactivity in the rat central nervous system by light and electron microscopy. Brain Res. 2000;853:245–268. doi: 10.1016/s0006-8993(99)02273-8. [DOI] [PubMed] [Google Scholar]
- 5.Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res. 2001;93:36–45. doi: 10.1016/s0169-328x(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 6.Juaneda C, Dumont Y, Chabot JG, Fournier A, Quirion R. Adrenomedullin receptor binding sites in rat brain and peripheral tissues. Eur J Pharmacol. 2003;474:165–174. doi: 10.1016/s0014-2999(03)02042-9. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, et al. Immunocytochemical localization of adrenomedullin 2/intermedin-like immunoreactivity in human hypothalamus, heart and kidney. Peptides. 2006;27:1383–1389. doi: 10.1016/j.peptides.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Samson WK, Murphy TC, Resch ZT. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am J Physiol. 1998;274:R1505–R1509. doi: 10.1152/ajpregu.1998.274.5.R1505. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Krukoff TL. Adrenomedullin in the rostral ventrolateral medulla increases arterial pressure and heart rate: roles of glutamate and nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R729–R734. doi: 10.1152/ajpregu.00188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen MA, Ferguson AV. In vitro recordings from area postrema neurons demonstrate responsiveness to adrenomedullin. Am J Physiol. 1996;270:R920–R925. doi: 10.1152/ajpregu.1996.270.4.R920. [DOI] [PubMed] [Google Scholar]
- 11.Serrano J, et al. Adrenomedullin expression is up-regulated by ischemia-reperfusion in the cerebral cortex of the adult rat. Neuroscience. 2002;109:717–731. doi: 10.1016/s0306-4522(01)00532-2. [DOI] [PubMed] [Google Scholar]
- 12.Serrano J, Fernandez AP, Sanchez J, Rodrigo J, Martinez A. Adrenomedullin expression is up-regulated by acute hypobaric hypoxia in the cerebral cortex of the adult rat. Brain Pathol. 2008;18:434–442. doi: 10.1111/j.1750-3639.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shindo T, et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- 15.Shimosawa T, et al. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation. 2002;105:106–111. doi: 10.1161/hc0102.101399. [DOI] [PubMed] [Google Scholar]
- 16.Dackor RT, et al. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol. 2006;26:2511–2518. doi: 10.1128/MCB.26.7.2511-2518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: Insights into brain function and diseases. Pharmacol Ther. 2007;113:619–634. doi: 10.1016/j.pharmthera.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Coppola V, et al. Ablation of TrkA function in the immune system causes B cell abnormalities. Development. 2004;131:5185–5195. doi: 10.1242/dev.01383. [DOI] [PubMed] [Google Scholar]
- 19.Sackett DL, et al. Intracellular proadrenomedullin-derived peptides decorate the microtubules and contribute to cytoskeleton function. Endocrinology. 2008;149:2888–2898. doi: 10.1210/en.2007-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King S, et al. A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health Technol Assess. 2006;10:1–162. doi: 10.3310/hta10230. [DOI] [PubMed] [Google Scholar]
- 21.Girard BA, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- 22.Alonso AC, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA. 2006;103:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng J. Microtubule: A common target for parkin and Parkinson's disease toxins. Neuroscientist. 2006;12:469–476. doi: 10.1177/1073858406293853. [DOI] [PubMed] [Google Scholar]
- 24.Dong CF, et al. The N-terminus of PrP is responsible for interacting with tubulin and fCJD related PrP mutants possess stronger inhibitive effect on microtubule assembly in vitro. Arch Biochem Biophys. 2008;470:83–92. doi: 10.1016/j.abb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. Neurobiol Dis. 2006;22:98–111. doi: 10.1016/j.nbd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Perry JR, Warner E. Transient encephalopathy after paclitaxel (Taxol) infusion. Neurology. 1996;46:1596–1599. doi: 10.1212/wnl.46.6.1596. [DOI] [PubMed] [Google Scholar]
- 27.Goddyn H, Leo S, Meert T, D'Hooge R. Differences in behavioural test battery performance between mice with hippocampal and cerebellar lesions. Behav. Brain Res. 2006;173:138–147. doi: 10.1016/j.bbr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 29.Prickaerts J, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor MM, Samson WK. A possible mechanism for the action of adrenomedullin in brain to stimulate stress hormone secretion. Endocrinology. 2004;145:4890–4896. doi: 10.1210/en.2004-0806. [DOI] [PubMed] [Google Scholar]
- 31.Malendowicz LK, Macchi C, Nowak M, Nussdorfer GG, Majchrzak M. Effects of proadrenomedullin N-terminal 20 peptide on the rat pituitary-adrenocortical axis under basal and stressful conditions. Int J Mol Med. 2006;18:285–287. [PubMed] [Google Scholar]
- 32.Shan J, Krukoff TL. Distribution of preproadrenomedullin mRNA in the rat central nervous system and its modulation by physiological stressors. J Comp Neurol. 2001;432:88–100. doi: 10.1002/cne.1090. [DOI] [PubMed] [Google Scholar]
- 33.Al-Ayadhi LY. Neurohormonal changes in medical students during academic stress. Ann Saudi Med. 2005;25:36–40. doi: 10.5144/0256-4947.2005.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang F, Wong MP, Hwang IS, Li YY. Ether stress increases adrenomedullin gene expression and levels in the rat adrenal. Horm Metab Res. 2005;37:585–588. doi: 10.1055/s-2005-870534. [DOI] [PubMed] [Google Scholar]
- 35.Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: Rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006;29:371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Navarro JA, et al. Mortality, oxidative stress and tau accumulation during ageing in parkin null mice. J Neurochem. 2007;103:98–114. doi: 10.1111/j.1471-4159.2007.04762.x. [DOI] [PubMed] [Google Scholar]
- 37.Acevedo SF, Ohtsu H, Benice TS, Rizk-Jackson A, Raber J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc−/−) mice. Brain Res. 2006;1071:113–123. doi: 10.1016/j.brainres.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto K, et al. Sterol regulatory element binding protein (SREBP)-1 expression in brain is affected by age but not by hormones or metabolic changes. Brain Res. 2006;1081:19–27. doi: 10.1016/j.brainres.2006.01.081. [DOI] [PubMed] [Google Scholar]
- 39.Dackor R, Fritz-Six K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem. 2007;282:18094–18099. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- 40.Li YY, Wai-Sum O, Tang F. Effect of aging on the expression of adrenomedullin and its receptor component proteins in the male reproductive system of the rat. J Gerontol A Biol Sci Med Sci. 2007;62:1346–1351. doi: 10.1093/gerona/62.12.1346. [DOI] [PubMed] [Google Scholar]
- 41.Hwang IS, Fung ML, Liong EC, Tipoe GL, Tang F. Age-related changes in adrenomedullin expression and hypoxia-inducible factor-1 activity in the rat lung and their responses to hypoxia. J Gerontol A Biol Sci Med Sci. 2007;62:41–49. doi: 10.1093/gerona/62.1.41. [DOI] [PubMed] [Google Scholar]
- 42.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Postischemic infusion of adrenomedullin protects against ischemic stroke by inhibiting apoptosis and promoting angiogenesis. Exp Neurol. 2006;197:521–530. doi: 10.1016/j.expneurol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Kis B, Busija DW, Yamashita H, Ueta Y. Adrenomedullin protects rat cerebral endothelial cells from oxidant damage in vitro. Regul Pept. 2005;130:27–34. doi: 10.1016/j.regpep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Tessarollo L. Manipulating mouse embryonic stem cells. Methods Mol Biol. 2001;158:47–63. doi: 10.1385/1-59259-220-1:47. [DOI] [PubMed] [Google Scholar]
- 45.Bonin A, Reid SW, Tessarollo L. Isolation, microinjection, and transfer of mouse blastocysts. Methods Mol Biol. 2001;158:121–134. doi: 10.1385/1-59259-220-1:121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.