Abstract
Evolutionary changes in the size of the cerebral cortex, a columnar structure, often occur through the addition or subtraction of columnar modules with the same number of neurons underneath a unit area of cortical surface. This view is based on the work of Rockel et al. [Rockel AJ, Hiorns RW, Powell TP (1980) The basic uniformity in structure of the neocortex. Brain 103:221–244], who found a steady number of approximately 110 neurons underneath a surface area of 750 μm2 (147,000 underneath 1 mm2) of the cerebral cortex of five species from different mammalian orders. These results have since been either corroborated or disputed by different groups. Here, we show that the number of neurons underneath 1 mm2 of the cerebral cortical surface of nine primate species and the closely related Tupaia sp. is not constant and varies by three times across species. We found that cortical thickness is not inversely proportional to neuronal density across species and that total cortical surface area increases more slowly than, rather than linearly with, the number of neurons underneath it. The number of neurons beneath a unit area of cortical surface varies linearly with neuronal density, a parameter that is neither related to cortical size nor total number of neurons. Our finding of a variable number of neurons underneath a unit area of the cerebral cortex across primate species indicates that models of cortical organization cannot assume that cortical columns in different primates consist of invariant numbers of neurons.
Keywords: allometry, brain size, primates, number of neurons, cortical surface
The expansion of a cerebral cortex that varies in surface area by more than five orders of magnitude across mammals (1) is considered a key event in mammalian brain evolution (2, 3), even though evolution is not always associated with increased brain or cortical size (4). Given that the cerebral cortex is a columnar structure (2, 5, 6), the most accepted view of cortical expansion proposes that this is achieved through the addition of columnar modules (7, 8). This view is so pervasive that the cortical surface area, rather than cortical volume, has often been used as a measure of the information processing capacity of the brain (3, 9).
Moreover, the columnar modules that build the cerebral cortex have been assumed to be composed of a constant number of neurons underneath a unit area of cortical surface, since Rockel et al. reported that there is a constant number of ≈147 thousand neurons beneath 1 mm2 of cortical surface of various mammalian species (10). In that study, the authors used two-dimensional counts of neurons in sections of the cerebral cortex from mouse, rat, cat, macaque, and man, and concluded that a uniform number of neurons beneath 1 mm2 is present across species. The notion of numerical uniformity, although supported by data obtained through light microscopy analysis in the mouse (11), has since been disputed by separate research studies within (12, 13) and across mammalian orders (14). Despite the evidence to the contrary, most models of cortical evolution and connectivity still assume that cortical columns hold a constant number of neurons across species (7, 8, 15, 16).
To test whether the number of neurons underneath a given unit of cerebral cortex is invariant across primate species, we used the Isotropic Fractionator (17) to determine the total number of neurons in one hemisphere of the cerebral cortex of nine primate species, ranging 18× in cortical gray matter mass from Callithrix to Papio, and in the closely related Tupaia spp. (18). Next we examined whether this number varied with total cortical surface area, gray matter thickness, gray matter mass, and neuronal density. We also examined predictions of the models of cortical expansion based on the addition of columns of constant numbers of neurons, such as the expected inverse proportionality between cortical thickness and neuronal density (10). Our results agree with stereological data from other mammalian orders that indicate the number of neurons beneath a surface unit of cerebral cortex is not constant but rather covaries with neuronal density (12–14).
Results
Across the nine primate species examined and in the closely related Tupaia sp., we found that the average number of neurons beneath one square millimeter of cerebral cortex (N/A; calculated as the ratio between the total number of neurons in the cerebral cortex, N, and the total cortical surface area, A) varied threefold from 42,710/mm2 in Macaca fascicularis to 129,670/mm2 in Saimiri sp. (Table 1), with an average of 83,945 neurons/mm2 across species. Despite this variation, these values as a whole were not significantly different from the N/A of 97,351/mm2 that was predicted by Rockel et al. (10) from which a nominal value of 147,000/mm2 was corrected for 18% shrinkage in each of two dimensions (P = 0.1903, one sample t test). Because exclusion of Tupaia sp. from the analyses did not modify the results (data not shown), all comparisons henceforth include all 10 species studied.
Table 1.
Average number of neurons beneath 1 mm2 of cerebral cortex surface
| Species | M, g | A, mm2 | T, mm | N, millions | Dneur, thousands/mg | N/A, thousands |
|---|---|---|---|---|---|---|
| Tupaia glis (n = 2) CV | 0.515 ± 0.063 | 497 ± 158 | 1.089 ± 0.021 | 21.95 ± 1.60 | 38.16 ± 7.42 | 47.09 ± 18.18 |
| 0.122 | 0.318 | 0.019 | 0.073 | 0.194 | 0.386 | |
| Callithrix jacchus (n = 3) CV | 2.042 ± 0.120 | 1534 ± 273 | 1.310 ± 0.235 | 120.33 ± 43.30 | 54.24 ± 23.34 | 78.00 ± 22.70 |
| 0.059 | 0.178 | 0.179 | 0.360 | 0.430 | 0.291 | |
| Otolemur garnetti (n = 2) CV | 2.556 ± 0.129 | 1745 ± 331 | 1.462 ± 0.196 | 88.50 ± 14.75 | 31.90 ± 7.26 | 50.83 ± 1.18 |
| 0.050 | 0.190 | 0.134 | 0.167 | 0.228 | 0.023 | |
| Aotus trivirgatus (n = 3) CV | 3.698 ± 0.663 | 2214 ± 213 | 1.499 ± 0.039 | 200.32 ± 67.34 | 47.96 ± 15.34 | 92.96 ± 39.07 |
| 0.179 | 0.096 | 0.026 | 0.336 | 0.320 | 0.420 | |
| Callimico goeldi (n = 1) | 3.872 | 1953 | 1.600 | 178.77 | 38.55 | 91.53 |
| Saimiri sciureus (n = 3) CV | 6.996 ± 0.356 | 5250 ± 1332 | 1.465 ± 0.023 | 645.73 ± 43.74 | 80.92 ± 9.68 | 129.67 ± 39.62 |
| 0.051 | 0.254 | 0.016 | 0.068 | 0.120 | 0.306 | |
| Macaca fascicularis (n = 1) | 10.459 | 9381 | 1.413 | 400.74 | 32.94 | 42.71 |
| Macaca radiata (n = 1) | 15.493 | 8441 | 1.572 | 829.60 | 43.81 | 98.28 |
| Cebus apella (n = 2) CV | 15.820 ± 4.982 | 7653 ± 29 | 1.767 ± 0.147 | 930.67 ± 507.78 | 51.08 ± 15.44 | 121.73 ± 66.80 |
| 0.315 | 0.004 | 0.083 | 0.546 | 0.302 | 0.549 | |
| Papio sp. (n = 2) CV | 36.334 ± 8.233 | 16689 ± 3052 | 2.034 ± 0.028 | 1420.34 ± 18.07 | 33.73 ± 7.23 | 86.65 ± 16.93 |
| 0.226 | 0.183 | 0.014 | 0.013 | 0.214 | 0.195 | |
| Variation | 70.6× | 29.2× | 1.9× | 60.7× | 2.4× | 3.0× |
All values are average ± standard deviation and refer to one cerebral cortical hemisphere only. M, grey matter mass; A, surface area; T, grey matter thickness; N, number of neurons in the grey matter; Dneur, neuronal density; N/A, average number of neurons underneath 1 mm2 of the cortical surface; CV, coefficient of variation (standard deviation/average).
One prediction of models of cortical expansion based on the addition of uniform columns of cerebral cortex is that total surface area (A) should increase linearly with the number of cortical neurons (N) across species. In contrast, we found that A increased as a power function of N with an exponent of 0.805 (P < 0.0001; Fig. 1a), which is significantly smaller than 1.0 (95% confidence interval, 0.614–0.996). Across species, a larger cortical mass (M) correlated with a larger cortical surface by a power function of A with an exponent of 1.123 (P < 0.0001; Fig. 1b), and with a larger number of neurons by a power function of N with an exponent of 0.934 (P < 0.0001, Fig. 1c). Similar relationships are obtained if cortical volume (V) is used instead of M (data not shown). These exponents indicate that cortical surface (A) increases slightly slower than cortical mass (M), and, thus, also more slowly than the number of neurons in the cortex (N).
Fig. 1.
Coordinate increase in cerebral cortical mass, surface area, and number of neurons of the different primate species and the tree shrew. Points represent the average values for each of the 10 species analyzed. (a) Cortical surface area increases more slowly than the number of neurons in the cortex as a power function of exponent 0.805. (b) Cortical mass increases slightly faster than cortical surface as a power function with exponent 1.123. (c) Cortical mass increases as a power function of cortical number of neurons with exponent 0.934.
N/A = D × T: Predictions Are Not Supported by Evidence.
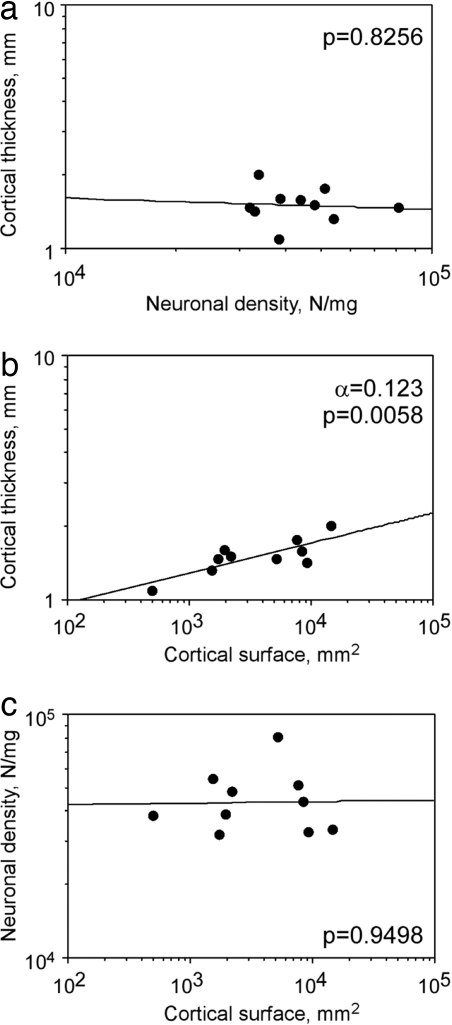
The number of neurons (N) in the cerebral cortex can be expressed as the product of surface area (A), cortical thickness (T), and cortical neuronal density (D), such that N = A × T × D. Given this mathematical relationship, for the ratio N/A to remain constant across species, it is necessary that D and T be inversely correlated, such that the product of D × T also remains constant and qual to N/A. We found, on the contrary, that neuronal density D did not correlate with cortical thickness T across species (ρ = 0.067, P = 0.8415, Spearman rank correlation; Fig. 2a). T increased concertedly with A as a power function of exponent 0.123 (P = 0.058, Fig. 2b). The small exponent implies that larger cortices increase only slightly in thickness. For instance, Papio sp., with a 34× larger cortical surface than Tupaia sp., had an only 1.9× thicker gray matter (Table 1).
Fig. 2.
Cortical thickness covaries with cortical surface area but not with neuronal density. Points represent the average values for each of the 10 species analyzed. (a) Cortical thickness does not correlate with neuronal density in the cerebral cortex. (b) Cortical thickness increases slowly as a power function of cortical surface. (c) Neuronal density in the cortex does not covary with cortical surface area. P values of the plotted power functions are indicated.
Although cortical surface area (A) and cortical thickness (T) were correlated, variations in neuronal density in the cerebral cortex were not related to A (ρ = −0.127, P = 0.7026, Spearman rank correlation; Fig. 2c). As found previously for a subgroup of these primate species (19), variations in neuronal density also were not related to cortical mass (M) (ρ = −0.006, P = 0.9855, Spearman rank correlation; data not shown).
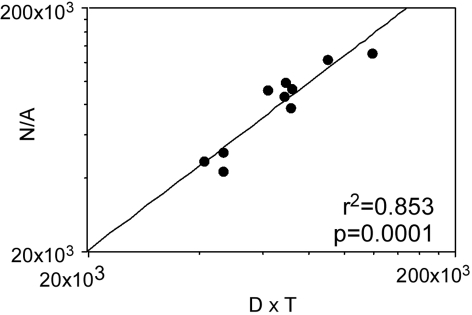
One consequence of the lack of an inverse correlation between neuronal density and cortical thickness is that the product D × T is not constant as would be expected, which agrees with our finding that neither is N/A. Indeed, the product D × T can be expressed as a linear function of the ratio N/A across both species averages, as expected from the equation N = A × D × T (r2 = 0.853, P = 0.0001 and r2 = 0.743, P < 0.0001; Fig. 3).
Fig. 3.
The average number of neurons beneath the cortical surface (N/A) varies across species as a linear function of the product of neuronal density and cortical thickness (D × T) as expected from the equation N = A × D × T. Points represent the average values for each of the 10 species analyzed.
What Makes N/A Vary?
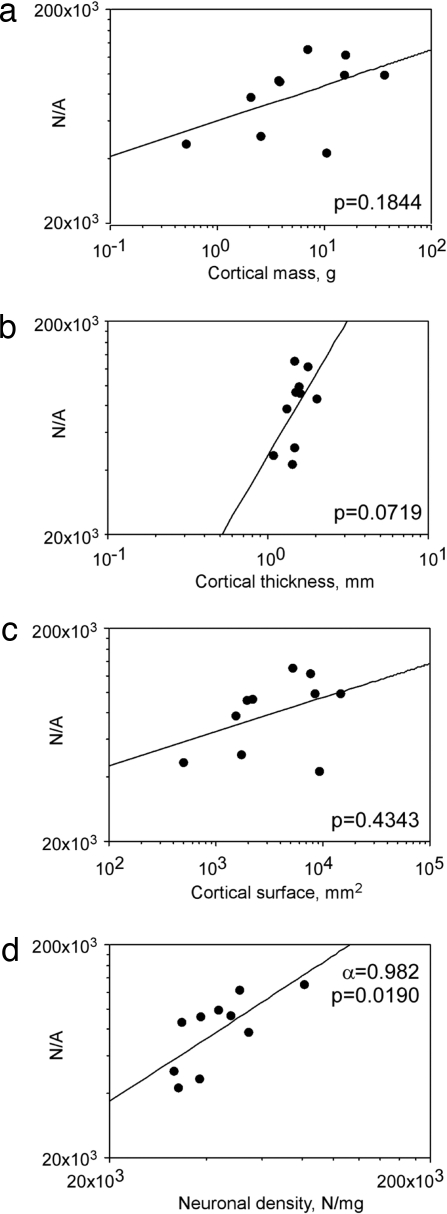
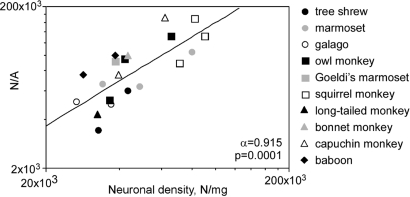
The threefold variation in the number of neurons per unit area of cortex observed across species correlated with variations in density of neurons in the different species (ρ = 0.733, P = 0.0278, Spearman rank correlation; Fig. 4d). The interspecific variation in N/A was not related to variations in cortical mass, volume, thickness, or surface (P = 0.1844, 0.2674, 0.0719, and 0.4343, respectively, Spearman rank correlation; Fig. 4 a–c). The correlation between N/A and D was stronger when all individuals were analyzed (ρ = 0.759, P = 0.0009, Spearman rank correlation) and can be expressed as a power function of exponent 0.915, close to 1.0 (Fig. 5). This indicates that the large variation found in N/A (Table 1) can be attributed to variations in D.
Fig. 4.
The average number of neurons beneath the cortical surface (N/A) varies across species as a power function of neuronal density (d), but not of cortical mass (a), cortical thickness (b), or cortical surface area (c). Points represent the average values for each of the 10 species analyzed. P values for the plotted power functions are indicated.
Fig. 5.
The number of neurons beneath the cortical surface (N/A) varies across individuals as a power function of exponent 0.915 of neuronal density. Each point represents the values for each of 20 individuals of the 10 species analyzed.
We found that N/A can vary by as little as 2% across Otolemur sp. individuals and as much as 42% across Aotus sp. specimens (Table 1, CV). Remarkably, although N/A and D were correlated across individuals, the intraspecific variability (CV) of N/A was not related to CVs for the other parameters (Spearman correlation, all P values >0.1). The nonuniformity of N/A across species can, therefore, be safely considered not to be an artifact of variability within the individual species analyzed, but rather a real finding that is related to variations in neuronal density across species.
Factor Analysis.
To establish the factors that contribute to variations in the composition of the gray matter of the cerebral cortex, we subjected our data to factor analysis. A principal component analysis applied to N, A, D, T, and M yields two factors (P < 0.0001), one composed of N, A, T, and M (which together explained 72.0% and 73.5% of data variance across individuals and across species, respectively) and another consisting of D alone (which explained 21.2% and 20.6% of the variance among individuals and among species, respectively). This finding further implies that variation in the ratio N/A result from variations in neuronal density both across species and individuals.
Discussion
Our finding of a nonuniform number of neurons beneath a cortical surface unit across species, which is in line with observations from several other groups (12–14), challenges the notion of uniformity in the number of neurons contained in a cortical column, a notion that is widely used (15) or implied in models of cerebral cortical evolution, development, and connectivity. A variable number of neurons underneath 1 mm2 of cerebral cortical surface does not invalidate the essence of models such as the radial unit hypothesis (8), which considers that cortical expansion in evolution happened through the increase in the number of neuronal precursors, which in turn increased “the number of columnar units without changing the number of neurons within each unit significantly” (20), despite expectations that small changes in cortical thickness depended on the number of cells in each radial unit. However, the numerical nonuniformity does call for an examination of how connectivity models are numerically impacted, if the cortical unit is no longer considered constant in number of neurons across species.
Previous Evidence of Cerebral Cortex Nonuniformity.
We are not the first to find results that do not support the putative uniformity of the cerebral cortex across species. According to the original study (10), the number of neurons beneath 1 mm2 of cortical surface is ≈147,000 in the mouse, rat, cat, macaque monkey, and man, or 97,351 when corrected for shrinkage. Although one study (11) found that the number of neurons under a given cortical surface in mice is constant and close to the number estimated by Rockel et al. (10), and the density of neurons increases with decreasing cortical thickness, studies on other mammalian orders have found numbers that contradict the predicted numerical uniformity of the cerebral cortex. Across insectivores, for instance, the number of neurons beneath 1 mm2 was found to decrease from close to 70,000 to <40,000 together with increasing brain size and decreasing neuronal density (13). One study across several mammalian orders found that the number of neurons beneath 1 mm2 of cerebral cortex varies from 20,000 to >160,000 (14). A more recent study found that the number of neurons beneath 1 mm2 of the cerebral cortex decreases from >50,000 to <20,000 across delphinid species with increasing brain size (12)—and, therefore, probably decreases with decreasing neuronal density.
In line with these previous findings, our present results show that the average number of neurons found beneath 1 mm2 of the cerebral cortical surface varied across primate species together with neuronal density, as in insectivores (13), even though neuronal density did not covary with cortical mass across primate species (19). This variation is in sharp contrast to the claim by Rockel et al. that the cortex is a uniform structure across mammalian species, built with a constant number of neurons <1 mm2 of cortical surface (10), a claim that is widely used to support models of cortical connectivity (15, 16) and developmental theories of cortical evolution that assume that cortex increases or decreases in size by adding or subtracting columnar units of modules of a fixed number of neurons (7).
Additionally, we have previously found that, in rodents, neuronal density in the cerebral cortex decreases as a power function of increasing cortical mass with an exponent of −0.444 (21), such that the neuronal density of the cerebral cortex is 10 times smaller in the capybara than in the mouse. Given that cortical thickness does not increase by a corresponding 10 times from mouse to capybara (S.H.-H., unpublished observation), this decrease in density is much faster than would be compatible with a constant number of neurons per unit area of cortical surface across species. Therefore, we predict that, in rodents and in primates, insectivores (13), and delphinids (12), the number of neurons per unit area of cortical surface decreases together with neuronal density. This correlation is expected whenever changes in neuronal density are not accompanied by an inversely proportional change in cortical thickness such that N/A = D × T. With similar thicknesses, the cortex with the larger density of neurons should also have a larger number of neurons beneath a unit area of cortical surface.
Possible Alternatives.
Here, we applied the isotropic fractionator (17), a nonstereological method, to determine the average number of neurons beneath a unit area of cortical surface, determined as the ratio between the total number of neurons in the cerebral cortex and the total surface area of the structure. Although this approach ignores regional differences across cortical areas by pooling them together and, therefore, does not address specializations in N/A, which are known to occur in striate cortex (10), it is a suitable approach to address the issue of a constant average number of neurons beneath the cortical surface, a central tenet to many models of cortical connectivity and evolution (7, 15, 16).
The estimation of N/A for the entire cerebral cortex opens the possibility that the inclusion of striate cortex (V1), a structure with a particularly high neuronal density (10), might bias the average number of neurons beneath the surface, making it appear variable across species. However, we believe that this is an unlikely possibility for two reasons. First, even if the striate cortex is considered to represent up to 20% of the entire cerebral cortex in primates (22) and to have a neuronal density that is two times higher than density in the remaining cortex (10), it would contain 30% of all brain neurons and, therefore, account for a variation of only up to 30% in the average number of neurons per unit surface. This number is much smaller than the 3× variation that we find across species.
Second, the relative size of the striate visual cortex varies little with neocortical size among the primates analyzed here (23), comprising 12% of the entire neocortex in macaque monkeys and 16% in marmosets (21). This small variation suggests that, everything else being equal, a 33% larger relative size of the striate cortex alone should impact the average number of neurons in the cortex by only 33%, a factor again much smaller than the 3× variation. Moreover, our finding that the number of neurons per unit surface area was not related to absolute cortex size, regardless of surface, mass, or volume size, indicates that the variation in N/A is not significantly influenced by the smaller relative size of a visual cortex expected in larger cortices. Additionally, although the striate cortexes of the diurnal marmoset and squirrel monkey have been found to occupy a somewhat larger proportion of neocortex than the nocturnal owl monkey and galago (24), our finding of large numbers of neurons per surface area in the owl monkey, small numbers in M. fascicularis, and variable numbers in the other diurnal species in our sample, argue against a correlation between N/A and nocturnal versus diurnal behavior.
Our estimates of N/A included the allocortex, which might have particularly low values of N/A because of the small number of layers and, thus, might also bias the average number of neurons beneath the surface. However, given that the allocortex occupied a small percentage of total cortical volume and decreased with increasing cortex size from 7% in Callithrix sp. to 3% in Macaca sp. (25), it is unlikely that the inclusion of the allocortex accounted for the 3× variation in N/A.
Another possible explanation for the 3× variation in the N/A ratio across species would be that an equivalent error in the estimation of neuronal densities occurred. This variable depends on two parameters measured directly with our method: cortical mass and the percentage of nuclei expressing the neuronal marker NeuN (26). Cortical mass, however, varied typically by <10% within species, and although the percentage of neuronal nuclei varied 2× (between 27.8 and 55.49; data not shown) across species, it was found to be stable within primate species (19). Thus, the 2.4× variation in neuronal density found across species was most likely a true biological variation that does impact N/A and not experimental error.
Finally, among the species examined, we found the highest number of neurons beneath a unit area of cortical surface in the closely related Saimiri sp. and Cebus sp. (27), two species, along with Callithrix sp., that also exhibit the highest neuronal densities. This finding raises the possibility that a particularly high N/A resulting from increased neuronal density in the cerebral cortex may be a phylogenetic characteristic of these two species. A comparative analysis of independent contrasts of the cellular composition of the brain of the current species is currently underway to address this issue.
Implications for Models of Cortical Evolution and Connectivity.
It will be interesting to examine how developmental models of cortical evolution are impacted by considering the cerebral cortex to be a nonuniform structure. For instance, our finding that the number of neurons beneath a unit area of cortical surface covaries with neuronal density suggests that evolutionary changes in neuronal size, which should result in altered neuronal density when not compensated by changes in cortical thickness, are a phenomenon that must be considered in developmental models of cortical evolution, in addition to simple expansion of the neuronal precursor population and in the number of asymmetric divisions of these precursors. The ensuing variation up to three times in the number of neurons arranged in a radial column may conceivably result in a varying percentage of neurons that project axons into the white matter across species. This differs from the constant percentage often presumed in models of connectivity (15) and may impact local connectivity within cortical columns. Additionally, the surface diameter of functional columns would be expected to vary across species according to variations in N/A and neuronal density. These important implications of our present findings can be addressed in future studies.
Finally, the contrast between our main finding and that of Rockel et al. (10) constitutes yet another demonstration that inferences made from a few species across mammalian orders are not necessarily valid when applied to a range of species within orders (19, 21) and may, therefore, be misleading about the actual allometric relationships that apply to building brains in evolution.
Methods
Animals.
We examined one of the cortical hemispheres of tree shrews (Tupaia belangeri, n = 2), galagos (Otolemur garnetti, n = 2), marmosets (Callithrix jacchus, n = 3), owl monkeys (Aotus trivirgatus, n = 3), squirrel monkeys (Saimiri sciureus, n = 3), capuchin monkeys (Cebus apella, n = 2), baboons (Papio sp., n = 2), Goeldi's marmosets (Callimico goeldii, n = 1), long-tailed macaque monkeys (Macaca fascicularis, n = 1), and bonnet macaque monkeys (Macaca radiate, n = 1). One adult capuchin monkey (Cebus apella) was obtained from the colony at the Biophysics Institute at the Federal University of Rio de Janeiro, and the other was donated by the primate colony of the Centro de Primatologia in Belém, State of Pará, which also provided one Callimico sp. cerebral hemisphere. All other animals were obtained from colonies at the Department of Psychology at Vanderbilt University and were young adults at the time of the experiments.
Dissection.
All animals were killed by a lethal injection of sodium pentobarbital, weighed, and perfused transcardially with 0.9% PBS followed by 4% phosphate-buffered paraformaldehyde. Brains were removed from the skull, weighed, and post-fixed for 2 weeks to 18 months by immersion in 4% phosphate-buffered paraformaldehyde. Only one cerebral hemisphere of each animal (13 right hemispheres and 7 left hemispheres) was available for analysis.
Cerebral Cortical Reconstruction.
After fixation, one cerebral hemisphere of each animal was embedded in agar and cut entirely into 2 mm thick serial coronal sections with the aid of a hand-operated device that advanced the knife in steps of 1 mm. Although the device does not guarantee equal thickness across sections, the procedure does ensure that the average section thickness is 2 mm, which is sufficient for the determination of total surface area. The anterior surface of all sections was digitalized with a 1,200 dpi desktop scanner. The cerebral cortex in all animals was defined as all cortical regions lateral to the olfactory tract, including the hippocampus (except in baboons) and piriform cortex. The pial surface perimeter of each section of the cerebral cortex was measured from the digitalized image using Canvas X software (ACD Systems). The sum of all perimeters was then multiplied by 2 mm to estimate the total surface area (A) of that cortical hemisphere. For each section, we also measured the coronal area of the gray matter. Gray matter volume (V) was estimated as the sum of the coronal gray matter area of all sections multiplied by 2 mm. The average thickness (T) of the cortical hemisphere was estimated as the average ratio between total coronal area of the gray matter and surface perimeter in all sections that included white matter.
Isotropic Fractionator.
Once all sections were scanned, the gray matter in the coronal sections was dissected from the underlying white matter under a stereoscope and weighed to determine cortical mass (M). Total numbers of neuronal cells in the gray and white matter of each cortical hemisphere were estimated separately as described previously using the Isotropic Fractionator method (17) and considered jointly as the total number of cortical neurons (N) to avoid loss of gray matter neurons because of imprecision in the dissection. The number of neurons found in the white matter represented 7–18% of all neurons found in the cortex of the individuals examined (data not shown). Briefly, the tissue was turned into an isotropic suspension of isolated nuclei of known, defined volume and kept homogeneous by agitation. The total number of nuclei in suspension and, therefore, the total number of cells in the original tissue was estimated by determining the density of nuclei in small aliquots stained with the fluorescent DNA marker DAPI (4′-6-diamidino-2-phenylindole dihydrochloride; Molecular Probes), using a Neubauer chamber to count sample aliquots under the microscope. For each cortex, typically four independent samples were taken from the homogenate and counted. The CV of the four samples of each tissue was usually <0.10 for 16 of 20 animals, and the other four animals had a CV = 0.11, which is compatible with values of CV obtained in stereological estimates. Once the total cell number was known, the proportion of neurons in each sample was determined by immunocytochemical detection of the Neuronal Nuclear antigen (NeuN; Millipore) that is expressed in all nuclei of most neuronal cell types but not in nonneuronal cells (25). Estimates of the proportion of NeuN-positive nuclei are considered reliable because the coefficient of variation among animals of the same species is typically <0.15. Neuronal density (D) was calculated by dividing the number of cortical neurons (N) by cortical mass (M). Similar values were obtained by dividing N by cortical volume (V) (data not shown).
Data Analysis.
All statistical analyses and regressions were performed in Statview (SAS), using the average values obtained from the individuals of each species. Correlations between variables were calculated using the Spearman correlation coefficient. If a significance criterion of P < 0.05 was reached, regressions of the data to linear and power functions were calculated.
Acknowledgments.
Thanks to Alexandre Valotta and Esper Cavalheiro for donating the hemispheres of one Cebus and one Callimico. This work was supported by FAPERJ-Jovem Cientista and CNPq–Edital Universal (S.H.-H.), FAPERJ-Bolsa de Bancada, CNPq–Bolsa Prêmio, Edital Universal and Pronex (R.L.), and National Eye Institute Grant EY02686 (to J.H.K.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 12099.
References
- 1.Hofman M. On the evolution and geometry of the brain in mammals. Prog Neurobiol. 1989;32:137–158. doi: 10.1016/0301-0082(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 2.Mountcastle VB. In: The Mindful Brain. Schmitt FO, editor. Cambridge, MA: MIT Press; 1978. pp. 7–50. [Google Scholar]
- 3.Hofman MA. Neuronal correlates of corticalization in mammals: a theory. J Theor Biol. 1985;112:77–95. doi: 10.1016/s0022-5193(85)80117-x. [DOI] [PubMed] [Google Scholar]
- 4.Catania KC, Lyon DC, Mock OB, Kaas JH. Cortical organization in shrews: Evidence from five species. J Comp Neurol. 1999;410:55–72. [PubMed] [Google Scholar]
- 5.Szentágothai J. In: The Neurosciences, Fourth Study Program. Schmitt FO, Worden FG, editors. Cambridge, MA: MIT Press; 1979. pp. 399–415. [Google Scholar]
- 6.Douglas RJ, Martin KAC. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 7.Prothero J. Cortical scaling in mammals: A repeating units model. J Hirnforschung. 1997;2:195–207. [PubMed] [Google Scholar]
- 8.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 9.Sultan F. Analysis of mammalian brain architecture. Nature. 2002;415:133–134. doi: 10.1038/415133b. [DOI] [PubMed] [Google Scholar]
- 10.Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- 11.Schüz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol. 1989;286:442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- 12.Poth C, Fung C, Güntürkün SH, Ridgwat SH, Oelschläger HHA. Neuron numbers in sensory cortices of five delphinids compared to a physeterid, the pygmy sperm whale. Brain Res Bull. 2005;66:357–360. doi: 10.1016/j.brainresbull.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Stolzenburg JU, Reichenbach A, Neumann M. Size and density of glial and neuronal cells within the cerebral neocortex of various insectivorian species. Glia. 1989;2:78–84. doi: 10.1002/glia.440020203. [DOI] [PubMed] [Google Scholar]
- 14.Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: A stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci USA. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Changizi MA, Shimojo S. Parcellation and area–area connectivity as a function of neocortex size. Brain Behav Evol. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- 17.Herculano-Houzel S, Lent R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy WJ, Pevzner PA, O'Brien SJ. Mammalian phylogenomics comes of age. Trends Genet. 2004;20:631–639. doi: 10.1016/j.tig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 21.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA. 2006;103:12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frahm HD, Stephan H, Baron G. Comparison of brain structure volumes in insectivore and primates. V. Area striata. J Hirnforsch. 1984;25:537–557. [PubMed] [Google Scholar]
- 23.Kaskan PM, et al. Peripheral variability and central constancy in mammalian visual system evolution. Proc Biol Sci. 2005;272:91–100. doi: 10.1098/rspb.2004.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: Areal, modular, and retinotopic patterns. Vis Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- 25.Stephan H, Bauchot R, Andy O. Data on size of the brain and of various brain parts in Insectivores and Primates. In: Nobeck CR, Montagna W, editors. The Primate Brain. New York: Appleton–Century–Crofts; 1970. pp. 289–297. [Google Scholar]
- 26.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 27.Purvis A. A composite estimate of primate phylogeny. Phil Trans R Soc London Ser B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]