Abstract
Striatal dopamine plays a major role in the regulation of motor coordination and in the processing of salient information. We used voltammetry to monitor dopamine-release evoked by electrical stimulation in striatal slices, where interneurons continuously release acetylcholine. Use of the α6-selective antagonist α-conotoxin MII[E11A] and α4 knockout mice enabled identification of two populations of dopaminergic fibers. The first population had a low action potential threshold, and action potential-evoked dopamine-release from these fibers was modulated by α6. The second population had a higher action potential threshold, and only α4(non-α6) modulated action potential-evoked dopamine-release. Striatal dopaminergic neurons fire in both tonic and phasic patterns. When stimuli were applied in a train to mimic phasic firing, more dopamine-released was observed in α4 knockout vs. wildtype mice. Furthermore, block of α4(non-α6), but not of α6, increased dopamine release evoked by a train. These results indicate that there are different classes of striatal dopaminergic fibers that express different subtypes of nicotinic receptors.
Keywords: voltammetry, nicotine, knockout mice, alpha-conotoxin, striatum
Introduction
Nicotinic acetylcholine receptors (nAChRs) are a family of ligand-gated cation channels with numerous and diverse members. Each is a pentamer composed of α and β subunits (Anand et al. 1991; Cooper et al. 1991). Diversity of nAChRs results from the large number of types of subunits; nine α isoforms (α2 through 10) and three β isoforms (β2 through 4) have been cloned from vertebrate tissues (Lindstrom 1996; Dani and Bertrand 2007). Upon agonist binding, a pore associated with the receptor opens allowing flux of Na+, K+, and Ca++ (Lindstrom 1996; Dani and Bertrand 2007). During prolonged exposure to ACh, nAChRs can become desensitized and therefore are not functional in the continued presence of agonists.
Nigrostriatal (NS) dopaminergic (DA-ergic) neurons have cell bodies in the substantia nigra pars compacta and termini in the striatum. This pathway participates in the regulation of voluntary motor coordination, and selective loss of NS DA-ergic neurons results in Parkinson's disease (PD). Studies suggest that nicotine may protect against the loss of these DA neurons (Quik and Jeyarasasingam 2000; Costa et al. 2001; Dajas et al. 2001; Quik and Kulak 2002; Quik et al. 2006; Quik and McIntosh 2006; Quik et al. 2007), and nicotine and other nAChR agonists have been suggested as drug therapies for treating PD in view of their ability to modulate NS DA-release (Quik and McIntosh 2006; Janhunen and Ahtee 2007; Singh et al. 2007).
In addition, NS DA plays a major role in maintaining established stimulus-response habits associated with drug addiction (Ito et al. 2002; Everitt and Robbins 2005; Vanderschuren et al. 2005), positive reinforcement associated with learned behavior (Schultz 1998; Reynolds et al. 2001; Wickens et al. 2003), and processing salient and unpredictable information (Schultz 2002; Cragg 2006). NS DA-ergic neurons are characterized by consistent low-frequency firing. This standard firing pattern is interrupted by phasic bursting activity (high-frequency firing) that accompanies salient, novel or intense stimuli (Schultz 1986; Hyland et al. 2002; Schultz 2002; Rice and Cragg 2004; Zhang and Sulzer 2004; Cragg 2006; Heien and Wightman 2006).
NS DA-release is modulated by presynaptic nAChRs (Clarke et al. 1985; Grady et al. 1992; Kulak et al. 1997; Grady et al. 2002; Champtiaux et al. 2003; Luetje 2004; Dani and Bertrand 2007). Tonically active interneurons in striatum provide the ACh that activates nAChRs on these NS DA-ergic nerve terminals (Bennett et al. 2000; Zhou et al. 2002). Studies of mammalian striatal slices with fast-scan cyclic voltammetry (FSCV) have revealed that block or desensitization of these presynaptic nAChRs results in decreased DA-release electrically evoked with single pulses or low frequency trains of pulses (Zhou et al. 2001; Rice and Cragg 2004; Zhang and Sulzer 2004). In contrast, DA-release evoked by high frequency trains of pulses is enhanced by block or desensitization of nAChRs (Rice and Cragg 2004; Zhang and Sulzer 2004).
In vitro experiments show that nicotine elicits [3H]DA release from mammalian striatal synaptosomes (Kulak et al. 2001; Grady et al. 2002; Salminen et al. 2004; Azam and McIntosh 2005). Studies using nAChR subunit knockout (KO) mice, subunit-specific ligands and/or subunit-specific antibodies show that at least three subtypes of nAChRs present on NS DA-ergic terminals modulate DA-release: α4β2*, α6β2β3, α4α6β2β3 (Grady et al. 1992; Kulak et al. 2001; Champtiaux et al. 2002; Grady et al. 2002; Zoli et al. 2002; Cui et al. 2003; Marubio et al. 2003; Salminen et al. 2004; Azam and McIntosh 2005; Gotti et al. 2005). However, these reports do not indicate the role of each subtype in the differential modulation of DA-release by low- versus high-frequency firing of the terminals. In the present study, we addressed this question using an α6-selective antagonist, namely α-conotoxin MII[E11A] (McIntosh et al. 2004; Salminen et al. 2005), in conjunction with FSCV in striatal slices of wild-type (WT) and α4 KO mice.
Materials and Methods
Brain sectioning
C57/BL6/J WT and nAChR α4 KO mice (Ross et al. 2000) aged 2 to 6 months (obtained from the Institute for Behavioral Genetics, Univ. of Colorado, Boulder, CO. USA) were anesthetized by i.p. injection of 100 μl of the following cocktail: ketamine (100 mg/ml), xylazine (100 mg/ml), and acepromazine (10 mg/ml). Fully anesthetized mice were killed by cervical dislocation, decapitated and their brains removed. All animal protocols were approved by the University of Utah Institutional Animal Care and Use Committee and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals). The brains were chilled for ten minutes in ice cold assay buffer (125 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM CaCl2, 1.3 mM MgCl2, 10 mM glucose, continuously bubbled with 95% O2/5% CO2. Four hundred micron horizontal sections were prepared from the brains in ice cold assay buffer using a Vibratome 3000 (Vibratome Co., St. Louis MO, USA). Sections containing striatum were incubated in assay buffer at room temperature for 1 to 3 hours. Sections were then transferred to a recording chamber for FSCV and perfused at a rate of 0.7 to 1.5 ml/min with assay buffer maintained at 30 °C by an in-line solution heater and automatic temperature controller (SsH-27B and TC-324B, respectively, from Warner Instruments, Inc., Hamden, CT, USA).
Preparation of carbon fiber microelectrodes
Carbon fiber microelectrodes (CFEs) were made by inserting a 2K, 10 micron diameter carbon fiber (P55s, Amoco Polymers, Greenville, SC, USA, gift of John Dani), previously cleaned in acetone, into a 1.2 mm thin-walled glass capillary (World Precision Instruments, Sarasota, FL, USA). Fiber-insertion was assisted by applying a slight vacuum to the distal end of the capillary. The fiber-filled capillary was pulled by a conventional vertical microelectrode puller adjusted such that the tip of the glass embraced the carbon fiber, which extended beyond the glass, with little to no gap between the carbon fiber and surrounding glass. The junction between the fiber and glass tip was sealed with a small amount of Sylgard (Dow Corning Corp, Midland, MI, USA) and cured overnight in a 90 °C oven. The protruding fiber was trimmed to an exposed length of 50 to 200 microns, such that when used (see below), the background current was between 100 and 1000 nA.
Fast-scan cyclic voltammetry (FSCV)
FSCV was performed with a Chem-Clamp amplifier (Dagan Corp. Minneapolis, MN, USA). The waveform of the potential applied to the CFE (relative to a Ag/AgCl reference electrode) was W-shaped, consisting of four concatenated linear ramps: from 0 to −400 to 1000 to −400 to 0 mV, each with 300 mV/ms slopes (total duration of 12 ms). This waveform was applied at a frequency of 10 Hz. The current signal was low pass filtered at 10 kHz and digitized at 50 kHz. The voltage waveform was generated and the current signals were acquired by a PCI-6052E board (National Instruments, Austin, TX ) that was controlled by Tar Heel Electrochemistry Suite software (Heien Instruments, Champaign, Il, USA). The CFE was continuously subjected to the potential-waveform and equilibrated in 30 °C assay buffer for at least 30 minutes before use.
The stimulating electrode was constructed from a 0.5 MΩ tungsten bipolar electrode (76 mm length, 3 μm thick insulation) purchased with the following custom modifications: tips were straight and blunted to 200 microns exposed length and located 100 microns apart (World Precision Instruments, Sarasota, FL, USA). The stimulating electrode was placed approximately 150 microns from the CFE with poles on either side of the CFE, the tip of the latter was placed about 50 microns beneath the surface of the slice at the dorsal lateral striatum.
DA-release was evoked by a one ms biphasic constant current stimulus of 80 to 800 μA applied every 5 minutes with an AC-coupled Analog Isolator 2200 (AM Systems, Inc., Carlsborg, WA, USA) connected to a National Instruments PCI-6711E board controlled by the aforementioned Tar Heel Electrochemical Suite software. Two stimulus strengths were used: i) a sub-maximal stimulus that produced a response that was 60% of the maximum obtainable (level-60 stimulus), and ii) a supra-maximal stimulus that produced a maximum response (level-100 stimulus). When trains of pulses were use, each train consisted of seven level-100 stimuli at a frequency of 100 Hz (as per Rice and Cragg, 2004 and Zhang and Sulzer, 2004), with an inter-train interval of 5 minutes. The train was over well before the response peaked, so the peak of the response is the summed response to all pulses in the train. DA-release in response to a train was directly compared to that evoked by a single pulse (see Figures 3 and 4A) by obtaining a single pulse-response every 5 minutes for 15 to 20 minutes before the train was applied, averaging 3 to 4 of these single pulse-responses and using the average to normalize the train-response. An average of 4 train-responses was used to represent the response to train for each slice.
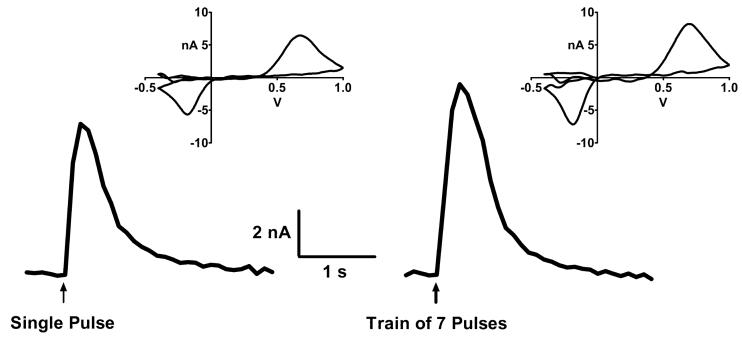
Figure 3.
Representative traces of DA-released in a striatal slice from a WT mouse in response to a single electrical stimulus (left) or a stimulus consisting of a train of 7 pulses presented at a frequency of 100 Hz (right). The same supramaximal stimulus pulse was used in both cases. The train was over well before the response peaked, so the peak of the response is the summed response to all pulses in the train. Insets depict the voltammogram captured at the peak of each trace.
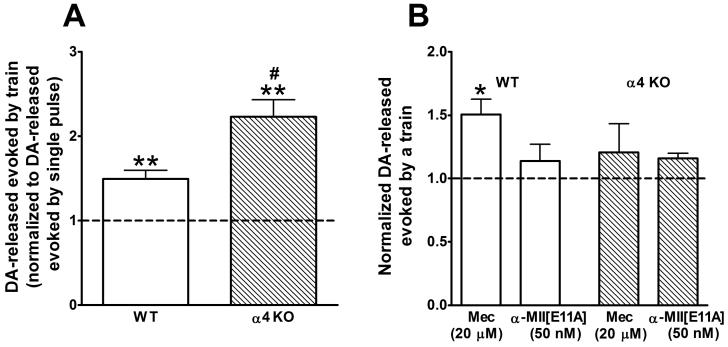
Figure 4.
A. DA-release evoked by a 100 Hz train of 7 pulses in slices from WT (white bars) and α4 KO (hatched bars) mice. Bars represent level of DA-release ± SEM relative to that evoked by a single pulse; thus, the average response to a single pulse is 1, denoted by the dotted line. Relative to the single pulse response, the train induced a statistically significant larger response (**p < 0.01, determined by paired t-tests). Furthermore, the train induced increase for α4 KO slices was significantly larger than that for WT slices, (#p < 0.05, determined by Student's t-test). n = 8 (WT) and 6 (KO) slices from 7 and 6 mice, respectively. B. Effect of nAChR antagonists on DA-release evoked by 100 Hz trains of 7 pulses in slices from WT (white bars) and α4 KO (hatched bars) mice. Bars represent average relative responses ± SEM in the presence of saturating concentrations of mecamylamine (Mec, a non-selective nAChR antagonist, 20 μM) or α-MII[E11A] (a selective α6* antagonist, 50 nM). n = 3 slices, each from a different mouse except for α-MII[E11A] in WT where n = 5 slices from 4 mice. Responses measured before slices were exposed to antagonist are set as 1 and denoted by the dotted line. Significant antagonist-induced increases are shown by *p < 0.05, determined by paired t-tests.
Stimulus delivery and CFE waveform timing were synchronized by the aforementioned Tar Heel Electrochemistry Suite software, which was also used to analyze the data. The current signal was digitally low-pass filtered at 2 kHz off-line, and an average of 10 current traces obtained one second before the stimulus was used for digital background subtraction; peak oxidation currents for DA occurred at approximately 600 mV. CFE's were calibrated by exposure to 0.5 to 10 μM DA. One nA of peak current corresponded to 0.5 to 1 μM DA.
Stable baseline responses were obtained for at least 60 minutes prior to drug application. The average of four responses obtained just prior to drug application served as the control response, and the average of four responses obtained ∼14 min after initiation of drug application was used to represent the response in the presence of drug. DA-release in the presence of drug is presented as an average percentage of control ± S.E.M. (Figures 2 and 4B). Statistical differences were tested using paired t-tests when controls were internal, and unpaired t-tests were used when comparisons were made between experiments.
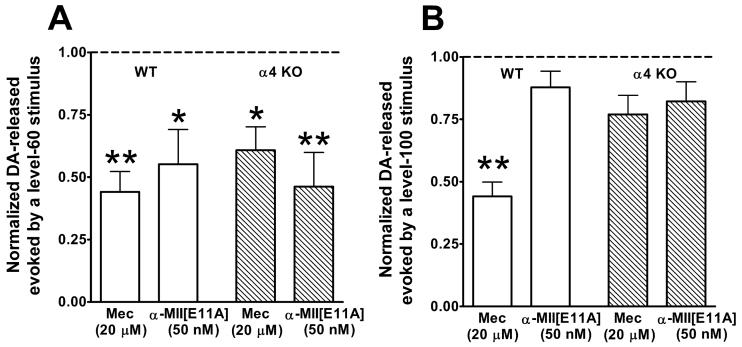
Figure 2.
DA-release evoked by single level-60 (A) or level-100 (B) stimulus. Stimulus levels used to evoke DA-release were as described in Fig. 1. Shown are average responses ± SEM in the presence of mecamylamine (Mec, a non-selective nAChR antagonist) or α-MII[E11A] (a selective α6* antagonist). Each antagonist was applied at a saturating concentration (20 μM or 50 nM, as indicated). n = 3 to 5 slices, each from a different mouse. Responses in WT slices are depicted as white bars and responses in α4 KO slices are shown as hatched bars. As described in Methods, the responses in the presence of antagonist are normalized to the average of four responses recorded just before slices were exposed to antagonist; thus, in both A and B, responses obtained before slices were exposed to antagonist are set at 1 and denoted by the dotted line. For each slice, four responses were recorded at 5 minute intervals starting ∼14 minutes following exposure of the slice to antagonist, and their average was used to represent the response in the presence of antagonist. Statistically significant antagonist-induced decreases are shown by *p < 0.05 or **p < 0.01, determined by paired t-tests.
Materials
Ketamine hydrochloride was from Abbott Laboratories, North Chicago, Il. Acepromazine was from Vedco, Inc., St. Joseph, MO, USA, and Xylazine was from Akorn, Inc., Decatur, Il, USA. Dopamine hydrochloride, mecamylamine hydrochloride and reagents in the assay buffer were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). α-conotoxin MII[E11A] was synthesized as previously described (McIntosh et al., 2004). Stocks of drugs were stored frozen and dissolved or diluted in the assay buffer just prior to use.
Results
Modulation of DA-release evoked by single-pulse stimuli is dependent both on nAChR subtype and stimulus strength
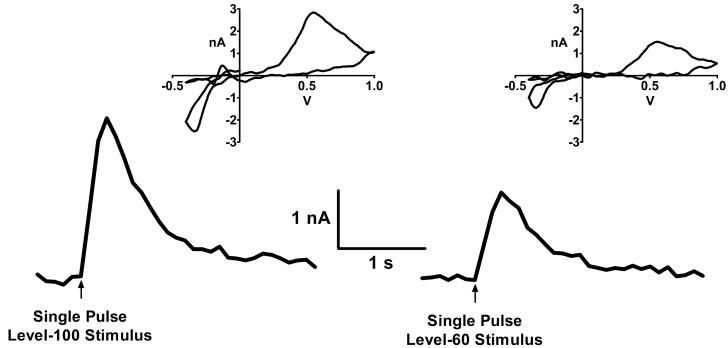
We initially examined the effects of stimulus strength and inhibition of specific nAChR subtypes on single-pulse evoked DA-release in striatum. Figure 1 shows representative traces corresponding to DA-release evoked by a level-60 stimulus (i.e., a stimulus strength that elicited responses that were 60% of maximal) and by a level-100 stimulus (i.e., a stimulus strength that was supramaximal resulting in maximal, or 100%, responses). Both traces were recorded within 20 minutes of each other from the same WT mouse striatal slice preparation. The voltammogram captured at the peak of each trace (Fig. 1 inset) has the waveform characteristic of DA (Kawagoe et al. 1993). No response was obtained when Ca++ was absent from the bathing medium (not illustrated) indicating that DA-release was synaptically mediated.
Figure 1.
Representative traces of DA-released in a striatal slice from a WT mouse in response to electrical stimulation. Release of DA was measured by fast scan cyclic voltammetry as described in Materials and Methods. Left, DA-release evoked by a supramaximal stimulus (level-100 stimulus). Right, response to a level-60 stimulus; i.e., stimulus was adjusted so release was 60% that produced by a supramaximal stimulus. Insets depict the voltammogram captured at the peak of each trace.
The effects of saturating concentrations of the α6-selective antagonist, α-MII[E11A] (50 nM; (McIntosh et al. 2004), and the non-selective nAChR antagonist, mecamylamine (20 μM), on DA-release at each stimulus strength were compared in striatal slices from WT and α4 KO mice. The release evoked by a level-60 stimulus was blocked ∼50% in both WT and α4 KO slices by both α-MII[E11A] and mecamylamine (Figure 2A). In contrast, DA-release evoked by a level-100 stimulus in wild-type slices was blocked ∼50% by mecamylamine while α-MII[E11A] had no significant effect; in addition, neither antagonist significantly blocked DA-release evoked by a level-100 stimulus in slices from α4 KO mice (Figure 2B).
The observation that mecamylamine and α-MII[E11A] both inhibited DA-release, evoked by a level-60 stimulus, to the same extent in WT mice implies that block of α6* nAChRs is mainly responsible for this inhibition. This interpretation is supported by data showing that mecamylamine and α-MII[E11A] each inhibit DA-release in α4 KO slices to the same extent as in WT slices (Figure 2A). One explanation for these findings is that low-stimulus strength (level-60) stimuli activate DA-ergic axons that express only α6* and not α4(nonα6)* nAChRs. This explanation is consistent with the presence of two kinds of DA-ergic fibers terminating in the striatum, as detailed in Discussion.
Mecamylamine decreased DA-release evoked by a high-stimulus strength (level-100) pulse in the WT striatum (Figure 2B) to the same degree that it decreased DA-release evoked by a single low stimulus strength (level-60) pulse (Figure 2A). However, there was no significant change in DA-release evoked by a high-stimulus strength pulse in the presence of α-MII[E11A] in the WT striatum or in the presence of either antagonist in the α4 KO striatum (Figure 2B). These data imply that block of α4(nonα6)*, but not α6*, nAChRs results in decreased DA-release evoked by a level-100 stimulus. These results are opposite that seen with a level-60 stimulus and consistent with the possibility of two types of DA-ergic fibers terminating in striatum that differ in the nAChR subtypes they express.
DA-release exhibits repetitive stimulus-induced depression
Figure 3 compares representative records of DA-release evoked by a single stimulus and a 100 Hz train of 7 stimuli in the same WT striatal slice preparation. The strength for both the single pulse stimulus and all pulses of the train were the same, level-100 (i.e., supramaximal; see Methods). There is only a ∼50% increase in the amount of DA-released by the train compared to that released by the single stimulus. The increase in DA-release evoked by the train of 7 pulses in this example is smaller than what would be expected from the sum of 2, much less 7, pulses and suggests a repetitive-stimulus induced depression of DA-release in mouse striatum. This is consistent with results obtained by others in guinea pig striatum (Rice and Cragg 2004) and mouse nucleus accumbens (Zhang and Sulzer 2004).
The repetitive stimulus-induced depression is affected by activity of α4-containing nAChRs
We used α4 KO mice to examine the role of the α4 nAChR subunit in repetitive stimulus-induced depression of striatal DA-release. For these experiments, we used level-100 stimulus strengths throughout. In Figure 4A, the amount of DA-release evoked by a train of 7 pulses, normalized to DA-release evoked by a single pulse, is shown for WT and α4 KO slices. In preparations from both strains of mice, DA-release evoked by the train was greater than that evoked by a single pulse. Nevertheless, just as in the experiment illustrated in Figure 3, the 7-pulse train induced increase in DA-release was considerably less than 7-times the DA-release induced by a single pulse. This was true for both the WT and the α4 KO. However, the train-induced increase in the α4 KO slices was significantly larger than that in the WT. These results indicate that the α4 nAChR subunit plays a role in repetitive stimulus-induced depression of striatal DA-release. In other words, absence of the α4 subunit partially alleviates repetitive stimulus-induced depression.
Block of α4 (non-α6)*, but not α6*, nAChRs results in increased DA-release evoked by a train of pulses
To further assess the roles of nAChR subtypes on train-induced DA-release, we examined the effects of specific nAChR antagonists on striatal DA-release evoked by the 100 Hz train of 7 pulses. Figure 4B depicts the effects of α-MII[E11A] and mecamylamine on train-induced DA-release in WT and α4 KO striatal slices. In contrast to Figure 4A, where the amount DA-release evoked by a train was normalized to the amount of DA-release evoked by a single pulse, Figure 4B shows average DA-release evoked by a train in the presence of a nAChR antagonist normalized to DA-release evoked by the train in the absence of any antagonist. A significant increase of ∼50% in DA-release was seen in the presence of mecamylamine in the WT slices. In contrast, there was no significant change in the amount of DA-release in the presence of α-MII[E11A] in WT slices or in the presence of either antagonist in α4 KO slices. These results indicate that the increase in train evoked DA-release in the presence of mecamylamine in WT slices was due to block of α4(nonα6)*, but not α6*, nAChRs. A summary of the results from Figures 2 and 4B is shown in Table 1.
Table 1.
Summary of results from Figures 2 and 4B. Values indicate approximate percent decreases (down arrows) or increases (up arrows) in DA-release, respectively.
| Single Pulse | Train of 7 Pulses | |||
|---|---|---|---|---|
| Mouse | Treatment | Level-60 stimulus | Level-100 stimulus | Level-100 stimulus |
| WT | α-MII[E11A] | ∼50↓* | ∼13↓ | ∼13↑ |
| WT | Mec | ∼55↓* | ∼55↓* | ∼50↑* |
| α4 KO | α-MII[E11A] | ∼55↓* | ∼18↓ | ∼15↑ |
| α4 KO | Mec | ∼40↓* | ∼25↓ | ∼20↑ |
Indicates statistical significance.
Discussion
The present study is the first that uses FSCV to show distinct populations of DA-ergic fibers in striatum. In addition, these results are the first to indicate that populations of striatal DA-ergic fibers are heterogenous based on nAChR subtype expression. These findings have implications for Parkinson's Disease (PD), a disorder which results from loss of nigrostriatal DA-ergic neurons. In both the MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine) animal model and in human PD, loss of DA-ergic neurons results in a differential time-dependent loss of specific nAChR subtypes (Quik et al. 2003; Bordia et al. 2007a).
We also present evidence for the existence of two populations of striatal DA-ergic fibers that differ in nAChR subtype expression and action potential threshold. Several distinct lines of published results support this hypothesis. First, subpopulations of DA-ergic fibers are found in mammalian striatum that differ morphologically in terms of axon diameter, terminal branching pattern, and number and size of varicosities (Gerfen et al. 1987a). In addition, pharmacological differences in striatal DA-ergic fibers are observed with populations differentially expressing calbindinD (Gerfen et al. 1987b), μ-opioid receptors (Gerfen et al. 1987a) and autoreceptors (Shepard and German 1988). Furthermore, striatal DA-ergic fibers are electrophysiologically heterogenous in terms of firing patterns and rates and conduction velocities (Shepard and German 1988; Grace and Onn 1989). Finally, striatal DA-ergic fiber populations have anatomical distinctions based on regions of origin; i.e., A8, A9 or A10 (Gerfen et al. 1987a; Langer and Graybiel 1989; Langer et al. 1991).
In this report, we propose that there are the two DA-ergic fiber populations as follows: type-A fibers that express α6* nAChRs and are activated at low stimulus strengths and type-B fibers that express α4(nonα6)* nAChRs and are activated only at higher stimulus strengths. Block of α6* only decreases DA-release evoked by a pulse of low, but not high, stimulus strength (see Figure 2). These results suggest that blocking α6* nAChRs increases the action-potential threshold of type-A fibers as illustrated in Figure 5A. In this model, as stimulus strength increases, the fraction of type-A fibers that are activated increase until a point is reached beyond which all type-A fibers are activated (plateau in Fig. 5Aiv). We propose that block of α6* nAChRs increases action-potential threshold of type-A fibers, resulting in a lower fraction of activated type-A fibers at low stimulus strengths; i.e., block of α6* shifts the stimulus-response curve to the right. This in turn will result in decreased DA-release at a given low stimulus strength. In contrast, at strong stimulus strengths that are more than adequate to activate all type-A fibers, blocking α6* nAChRs will have no effect on the fraction of fibers activated, and thus will have no effect on DA-release. There is precedence for this phenomenon in peripheral unmylinated C-fiber axons. In these fibers, nAChR agonists reduced the stimulus-strength necessary to generate action potentials (Lang et al. 2003).
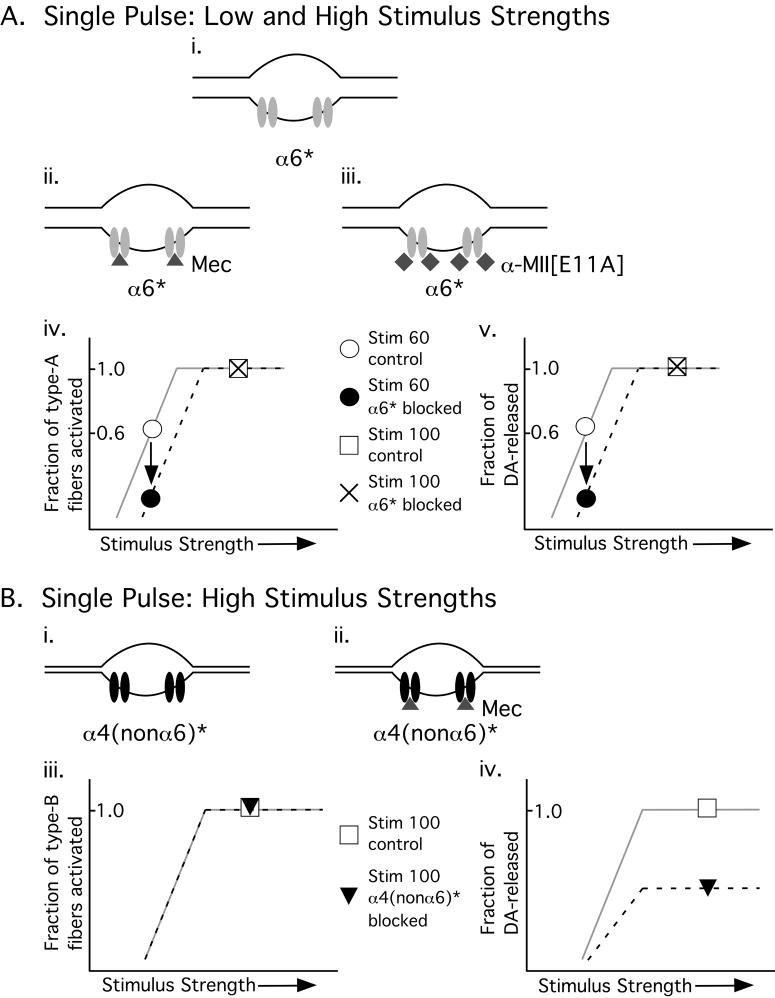
Figure 5.
Proposed model with two fiber types to explain the results. Type-A fibers have a large diameter (Ai - iii) and are therefore activated by low (as well as high) stimulus strengths (Aiv, v) while type-B fibers have a smaller diameter (Bi, ii) and require higher stimulus strengths to be activated. Type-A fibers express α6* nAChRs (light gray ovals in Ai, Aii and Aiii). These receptors can be blocked by both the pore blocker mecamylamine (Mec, gray triangles, Aii) and the competitive antagonist α-MII[E11A] (gray diamonds, Aiii). In contrast, type-B fibers express α4(nonα6)* nAChRs (black ovals in Bi and Bii). α4(nonα6)* nAChRs can be blocked by mecamylamine (Mec, gray triangles, Bii) but not α-MII[E11A]. Graphs represent fraction of type-A fibers activated (Aiv) and fraction of DA-released (Av) as a function of stimulus strength. The control condition where nothing is blocked is represented by a solid light gray line and the condition where α6* is blocked, by a dashed black line. Specific points are: open circle, control level-60 stimulus; closed circle, level 60 stimulus with α6* blocked; open square, control level-100 stimulus; and X, level-100 stimulus with α6* blocked. The graph in Aiv shows that block of α6* nAChRs increases the threshold for generation of action-potentials of type-A fibers, a mechanism proposed to explain why block of α6*nAChRs decreases DA release evoked by a level-60, but not level-100 stimulus (Av). Therefore, Av is the same as Aiv except for their ordinates. Biii and Biv show fraction of type-B fibers activated and fraction of DA-released as a function of stimulus strength. Specific points are: open square, control level-100 stimulus and closed triangle, level-100 stimulus with α4(nonα6)* blocked. In Biii, the solid light gray line representing control conditions overlaps the black dashed line representing conditions in which α4(nonα6)* is blocked, indicating that block of α4(nonα6)* receptors does not alter action-potential threshold of type-B fibers. On the other hand, Biv depicts that block of α4(nonα6)* decreases DA-released by ∼50%. The graphs in B show that ACh modulates DA-release from, but not threshold of, type-B fibers while the graphs in A show that ACh modulates threshold of type-A fibers thereby affecting DA-release.
Block of α4(nonα6)* nAChRs expressed on type-B fibers results in decreased DA-release evoked by a single pulse of high stimulus strength (Figure 2B and model in Figure 5B; also see (Exley et al. 2007). We hypothesize that this decrease in DA-release may be due to block of the increased conductance of the presynaptic membrane induced by the activity of α4(nonα6)* nAChRs. Previous work showed distinct colocalization of specific nAChR subtypes with specific voltage-gated calcium channels (VGCCs) on DA-ergic terminals in striatum (Kulak et al. 2001). That report also showed that activation of nAChRs on DA-ergic terminals results in membrane depolarization that in turn activates colocalized VGCCs and ultimately results in DA-release. Therefore, blocking α4(nonα6)* nAChRs on DA-ergic terminals may decrease DA-release evoked by a single pulse of high stimulus strength by decreasing endogenous ACh-induced membrane depolarization.
In addition, the present study shows that the α4 nAChR subunit is involved in repetitive stimulation-induced depression in mouse striatum. Previous studies in guinea pig striatum (Rice and Cragg 2004) and in mouse nucleus accumbens (Zhang and Sulzer 2004) showed that nAChRs in general are involved in frequency-dependent modulation of DA-release, and that a non-selective block of nAChRs alleviates high-frequency repetitive stimulation-induced depression of DA-release. Our study of mouse striatum extends these previous studies by showing that the α4 nAChR subunit plays a role in repetitive stimulation-induced depression since there is less repetitive stimulation induced-depression in the α4 KO mouse (Figure 4A). Furthermore, block of α4(nonα6)* nAChRs, but not α6* nAChRs, resulted in increased DA-release evoked by a train of pulses (Figure 4B; also see Exley et al. 2007). In both cases, the α4 nAChR subunit is implicated in increased DA-release evoked by a train of pulses. Figure 4B indicates that there is no further increase in DA-release evoked by a train of pulses when α6* nAChRs are blocked in both WT and α4 KO slices. That is, there is no increase in train-evoked DA-release in WT slices in the presence of α-MII[E11A] or in the α4 KO striatal slices in the presence of α-MII[E11A] or mecamylamine. The only increase in train-evoked DA-release was observed with mecamylamine in WT slices. Previous studies have shown that a non-selective block of nAChRs in guinea pig striatum (Rice and Cragg 2004) and in mouse nucleus accumbens (Zhang and Sulzer 2004) results in a relative increase in DA-release evoked by train of pulses. The current study is the first to indicate that α4(nonα6)* nAChRs but not α6* nAChRs underlie this phenomenon.
In conclusion, this study shows differential roles of nAChR subtypes present on DA-ergic terminals in striatum in modulating DA-release. Whether α4(nonα6)* or α6* nAChRs modulate DA-release is dependent on the electrical stimulus strength used to evoke DA-release. This is consistent with the existence of two populations of DA-ergic fibers in striatum, type-A and type-B, that differ both in nAChR subtype expression and in action-potential threshold. We propose a model wherein block of α6* nAChRs increases action-potential threshold for a subset of striatal DA-ergic fibers. Such modulation of action-potential threshold may have relevance with regard to branch-point failure of action-potential propagation in the striatal DA-ergic terminal arbors. Further studies using other methods, such as monitoring of action potentials per se, are required to directly test this hypothesis.
Finally, it is notable that there is a time-dependent subtype-selective loss of nAChRs in PD (Quik et al. 2003; Bordia et al. 2007b). That is, as the disease progresses, α6* nAChRs decline before α4(nonα6)* nAChRs do (Bordia et al. 2007b). This may reflect the preferential loss of one population of DA-ergic fibers early in the disease followed by the eventual loss of the other population. Although the events that trigger PD are not known, this study raises the possibility that certain DA-ergic fibers may be more susceptible than others to nigrostriatal damage.
Acknowledgements
This work was supported by NIH MH 53631 (JMM and ELM) and GM 48677 (DY). Production of the null mutant mice was supported by animal resources grant DA015663 from NIDA (to Allan C. Collins). We thank Sean Christensen for peptide folding and John Dani and Lifen Zhang for inviting us to their laboratory, instructing us in FSCV, and providing us with carbon fibers. We also thank the John Drago and the Institute for Behavioral Genetics, University of Colorado, Boulder, CO for providing us with the α4 nAChR null mutant mice.
Abbreviations used
- ACh
acetylcholine
- DA
dopamine
- PD
Parkinson's Disease
- nAChR
nicotinic acetylcholine receptor
- NS
nigrostriatal
- KO
knockout
- FSCV
fast-scan cyclic voltammetry
- WT
wildtype
- CFE
carbon fiber microelectrode
- Mec
mecamylamine
- MPTP
1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine
- VGCC
voltage-gated calcium channels
References
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991;266:11192–11198. [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Effect of novel alpha-conotoxins on nicotine-stimulated [3H]dopamine release from rat striatal synaptosomes. J Pharmacol Exp Ther. 2005;312:231–237. doi: 10.1124/jpet.104.071456. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol Pharmacol. 2007a;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of {alpha}6{beta}2* nAChRs in mouse, monkey and Parkinson's disease striatum. Mol Pharmacol. 2007b doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain Res. 2001;888:336–342. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas F, Costa G, Abin-Carriquiry JA, McGregor R, Urbanavicius J. Involvement of nicotinic acetylcholine receptors in the protection of dopamine terminals in experimental parkinsonism. Funct Neurol. 2001;16:113–123. [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. alpha6-Containing Nicotinic Acetylcholine Receptors Dominate the Nicotine Control of Dopamine Neurotransmission in Nucleus Accumbens. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987a;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Thibault J. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci. 1987b;7:3935–3944. doi: 10.1523/JNEUROSCI.07-12-03935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol Disord Drug Targets. 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: Implications for drug development. Neurosci Biobehav Rev. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JM, McIntosh JM, Yoshikami D, Olivera BM. Nicotine-evoked transmitter release from synaptosomes: functional association of specific presynaptic acetylcholine receptors and voltage-gated calcium channels. J Neurochem. 2001;77:1581–1589. doi: 10.1046/j.1471-4159.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Lang PM, Burgstahler R, Sippel W, Irnich D, Schlotter-Weigel B, Grafe P. Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human C-fiber axons by in vitro studies. J Neurophysiol. 2003;90:3295–3303. doi: 10.1152/jn.00512.2003. [DOI] [PubMed] [Google Scholar]
- Langer LF, Graybiel AM. Distinct nigrostriatal projection systems innervate striosomes and matrix in the primate striatum. Brain Res. 1989;498:344–350. doi: 10.1016/0006-8993(89)91114-1. [DOI] [PubMed] [Google Scholar]
- Langer LF, Jimenez-Castellanos J, Graybiel AM. The substantia nigra and its relations with the striatum in the monkey. Prog Brain Res. 1991;87:81–99. doi: 10.1016/s0079-6123(08)63048-4. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Neuronal nicotinic acetylcholine receptors. Ion Channels. 1996;4:377–450. doi: 10.1007/978-1-4899-1775-1_10. [DOI] [PubMed] [Google Scholar]
- Luetje CW. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol Pharmacol. 2004;65:1333–1335. doi: 10.1124/mol.65.6.1333. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Quik M, Jeyarasasingam G. Nicotinic receptors and Parkinson's disease. Eur J Pharmacol. 2000;393:223–230. doi: 10.1016/s0014-2999(99)00888-2. [DOI] [PubMed] [Google Scholar]
- Quik M, Kulak JM. Nicotine and nicotinic receptors; relevance to Parkinson's disease. Neurotoxicology. 2002;23:581–594. doi: 10.1016/s0161-813x(02)00036-0. [DOI] [PubMed] [Google Scholar]
- Quik M, McIntosh JM. Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson's disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- Quik M, O'Neill M, Perez XA. Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci. 2007;28:229–235. doi: 10.1016/j.tips.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006;26:4681–4689. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, McIntosh JM, Collins AC, Grady SR. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–1179. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-Conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Shepard PD, German DC. Electrophysiological and pharmacological evidence for the existence of distinct subpopulations of nigrostriatal dopaminergic neuron in the rat. Neuroscience. 1988;27:537–546. doi: 10.1016/0306-4522(88)90287-4. [DOI] [PubMed] [Google Scholar]
- Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson's disease. Prog Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]