Abstract
Background
The choroid plexus (CP) is an epithelial and vascular structure in the ventricular system of the brain that is a critical part of the blood-brain barrier. The CP has two primary functions, 1) to produce and regulate components of the cerebral spinal fluid, and 2) to inhibit entry into the brain of exogenous substances. Despite its importance in neurobiology, little is known about how this structure forms.
Methodology and Principal Findings
Here we show that the transposon-mediated enhancer trap zebrafish line EtMn16 expresses green fluorescent protein within a population of cells that migrate toward the midline and coalesce to form the definitive CP. We further demonstrate the development of the integral vascular network of the definitive CP. Utilizing pharmacologic pan-notch inhibition and specific morpholino-mediated knockdown, we demonstrate a requirement for Notch signaling in choroid plexus development. We identify three Notch signaling pathway members as mediating this effect, notch1b, deltaA, and deltaD.
Conclusions and Significance
This work is the first to identify the zebrafish choroid plexus and to characterize its epithelial and vasculature integration. This study, in the context of other comparative anatomical studies, strongly indicates a conserved mechanism for development of the CP. Finally, we characterize a requirement for Notch signaling in the developing CP. This establishes the zebrafish CP as an important new system for the determination of key signaling pathways in the formation of this essential component of the vertebrate brain.
Introduction
The choroid plexus (CP) is a set of vital structures in the brain central to the formation, regulation and protection of the cerebral spinal fluid (CSF). Development and physiology of the CP has implications for many central nervous system pathologies and for the targeting of pharmaceutical treatment to the central nervous system [1], [2]. In humans and other mammals, the CP consists of four independent structures, one in each of the four ventricles of the brain [1]. On the cellular level, the structure can be divided into three separate compartments, a vascular core, a stroma, and a layer of polarized epithelia [1]. The polarized epithelial cells surround the stroma and vascular core and exist as a monolayer, with their basal surface facing the stroma, and their apical surface extending microvilli and cilia into the CSF-filled ventricles [3].
Classic studies in human embryology place the onset of CP development at 6 weeks gestation and continuing past birth [1]. Comparative studies in mice place development of the CP beginning at embryonic day 11 [4]. In humans, the first of the choroid plexuses to develop is the fourth ventricle CP (4vCP), or its orthologue the myelencephalic choroid plexus (mCP), in other organisms including telosts [1], [5]. However, despite its importance in neurobiology, little is known about how this important structure forms.
We use the transgenic line EtMn16, which express GFP in a subset of definitive mCP epithelial cells, to characterize the development of the zebrafish mCP in vivo. The subset of the mCP primordial epithelia are initially found in a diffuse pattern along the dorsal roof of the fourth ventricle and coalesce at the midline to form the definitive mCP. Upon coalescence, the dorsal longitudinal vein (DLV) provides the small vessel bed that invades the epithelium of the mCP. Time-dependent inhibition of Notch signaling defines a requirement for this pathway in CP development. Morpholino-based ligand/receptor inhibition studies map this critical signaling event to the notch1b receptor and the two candidate ligands, dla and dld. Together, this study provides the first description of CP development in vivo and demonstrates a role for Notch signaling in mCP development.
Results
Identification of the zebrafish CP
EtMn16 is a GFP expressing transposon-mediated enhancer trap line identified as part of an ongoing genomic annotation project [6]. At 30 hours post fertilization (hpf), cells of the fin bud, a symmetric set of cells that extend processes medially in the ventral hindbrain and spinal column, and the otic vesicles express GFP (Fig. 1A–C). At 4 days post fertilization (dpf), we observe two additional structures expressing GFP. The first structure appears at the dorsal-most point of the fourth ventricle on the midline (Fig. 1D, arrowhead). The second structure is situated anterior to the eyes at the dorsal-most point of the third ventricle on the midline (Fig. 1D, arrow). Based on comparative anatomical studies in multiple organisms including telosts [1], we attribute the anterior GFP-expressing cells as those of the diencephalic choroid plexus (dCP), and the posterior GFP-expressing cells as those of the myelencephalic choroid plexus (mCP) [Fig. 1E (lateral section), Fig. 1F (transverse section)].
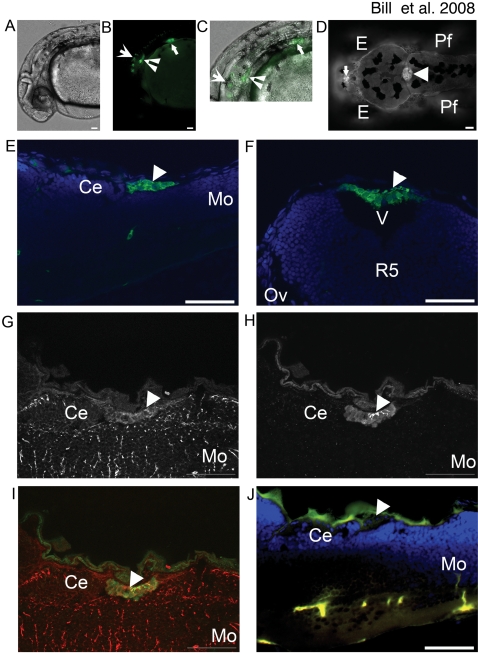
Figure 1. EtMn16 co-localizes with Gfap in mCP epithelia.
GFP is expressed in multiple anatomical locations in EtMn16 larvae (A–D). At 30 hpf (A–C)(lateral orientation), GFP is expressed in cells of the otic vesicle (inverted arrowhead), the pectoral fin (closed arrow), ventral hindbrain and spinal cord (open arrow) brightfield image (A), fluorescent image (B), and merged image (C). In addition to cells of the ventral hindbrain, at 4 dpf (D)(dorsal orientation), two additional structures express GFP, the diencephalic CP (dCP) (closed arrow), and the myelencephalic CP (mCP) (closed arrowhead). The mCP lies posterior to the cerebellum (Ce) [(5 dpf, 6 µm longitudinal cryosection) (E)], within the ventricle (v) dorsal to the fifth rhombomere (r5)[5 dpf, 6 µm transverse cryosection (F)]. Cells of the mCP express Gfap (G) and colocalize with GFP expressing cells (H), merged image (I) and are not seen in the negative control lacking antibodies for Gfap and GFP (J). Abbreviations: eye (E), pectoral fin (Pf), cerebellum (Ce), and medulla oblongata (Mo). All images except F are oriented anterior to the left; F is oriented dorsal to the top. Scale bar is 50 µm.
Glial fibrillary acidic protein (Gfap) is an early transient marker of the CP epithelia [7]. We used immunohistochemistry on 6 µm longitudinal cryosections of 5 dpf mCP with a monoclonal antibody (zrf-1) directed against zebrafish Gfap [8]. We observe Gfap expression in the epithelial cells of the mCP (Fig. 1G) as defined by colocalization of Gfap (red) and GFP (Fig. 1H, merged 1J). This colocalization of Gfap and GFP-expressing cells in EtMn16 larvae in conjunction with anatomical analyses indicates EtMn16 labels the forming zebrafish mCP.
Development of the mCP as visualized in the EtMn16 Larvae
CP formation was imaged in vivo using EtMn16 zebrafish larvae. GFP-expressing presumptive mCP are first observed in the roof plate of the fourth ventricle at 2.5 dpf (Fig. 2B). At 3 and 4 dpf, the cells progressively coalesce (Fig. 2C,D) toward the midline forming a tight rounded structure by 5 dpf (Fig. 2E). A subset of the cells within the mCP do not express GFP at this time (Fig. 2G, arrowhead). The structure remains visibly unchanged at 6 dpf, indicating the labeling of the definitive zebrafish CP (Fig. 2F).
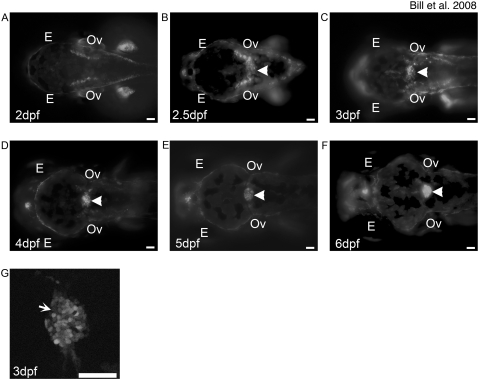
Figure 2. Development of the mCP as defined by EtMn16 larvae.
Fluorescent images of dorsal-oriented live zebrafish taken at 2 dpf (A), 2.5 dpf (B), 3 dpf (C), 4 dpf (D), 5 dpf (E), and 6 dpf (F); arrowhead indicates mCP. GFP-expressing cells first appear diffusely across the roof plate of the fourth ventricle (B), as defined by the level of the otic vesicles (Ov). Cells migrate toward the midline and finish coalescence by 4 dpf. The expression in this structure remains static through 6 dpf. Not all cells of the mCP express GFP (G, arrow). In all images, anterior is to the left, and scale bar is 50 µm. Abbreviations: eye (E), otic vesicle (Ov).
The vasculature of the mCP is supplied by the DLV
The CP is tightly associated with vasculature in mammalian systems [9] as part of the blood brain barrier. We visualized functional blood vessel integration in the CP using EtMn16/Tg(gata1:dsRed) double transgenic fish, with the latter transgene expressing DsRed in the circulating red blood cells [10]. Blood vessel identities were established utilizing the zebrafish vasculature map [11]. At 5 dpf, the mCP is at the bifurcation of the Dorsal Longitudinal Vein (DLV) as this vessel progresses into the Posterior Cerebral Vein (PCeV and PCeV′, prime indicates opposing bilateral side) (Fig. 3A–C). The bifurcation occurs shortly after the entry into the mCP domain. Three-dimensional renderings of the blood flowing through the DLV and mCP demonstrate that the vein is tightly associated with the CP epithelia (Fig. 3D–F). In addition, the DLV makes a slight dorsal bend just before the bifurcation, locating this vessel on the dorsal side of the mCP opposite the ventricle (Fig. 3G–H). The DLV then flattens as it joins the PCeV and PCeV′, transiting the mCP. After the PCeV and PCeV′ exit the mCP, these vessels again turn ventrally.
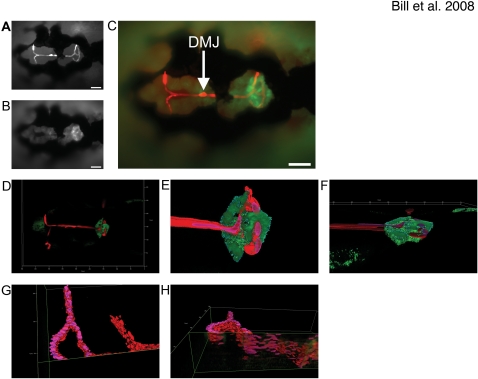
Figure 3. The Dorsal Longitudinal Vein supplies the mCP with its vascular network.
The Dorsal Longitudinal Vein (DLV) closely associates with the mCP epithelia as defined by doubly transgenic EtMn16/Tg(gata1:dsRed) dorsally-mounted 5 dpf live zebrafish, epithelia express GFP (A) and red blood cells express DsRed (B) merged image (C) (scale bars are 50 µm). The vessels, as defined by blood flow, directly contact the dorsal surface of the mCP. Low magnification of a 5 dpf dorsally mounted live EtMn16/Tg(gata1:dsRed), high magnification three-dimensional renders of DLV/PCeV junctions and TCB of zebrafish shown in D (E,F). Panel F is rotated 80° into the plane to show association (dorsal facing the top of the image). The DLV bends dorsally slightly before the bifurcation, traverses the mCP epithelial domain, and then turns ventrally as it transitions to the PCeV and PCeV′. Panels G and I are three-dimensional renders of the DLV, PCeV and TCB [(G,I – 85° rotation into the plane)(oriented with anterior to the right)]. The mCP has been digitally removed, and the DLV has been bisected utilizing the clipping tools of Image4D in order to define the vascular structure. Scale is as indicated on each render.
We further characterized mCP vasculature by describing the development of the DLV in more detail utilizing the Tg(fli1:eGFP) zebrafish transgenic line that labels vascular endothelial cells with GFP [12]. The DLV begins development between 40–48 hpf by sprouting from the Medial Cerebral Vein (MCeV and MCeV′)(Fig. 4B). The DLV extends by angiogenesis extending a growth cone like structure (Fig. 4C), until it reaches a hypothesized decision point (Fig. 4D). At this time, blood can be seen within the lumenized vessel, but remains static as it lacks a functional vascular outlet (data not shown). The vessels of the PCeV and PCeV′ migrate dorsomedially to meet the DLV (Fig. 4D), appearing to be attracted to the locale independent of the DLV (Fig. S1E). The DLV then develops preferentially to one of the PceV's where this vessel will connect and establish blood flow (Fig. 4E). A branch will then proceed toward the other PCeV and connect (Fig. 4F). The connection of the DLV and PCeV's approximately corresponds to the initial point at which GFP-expressing cells are observed in the EtMn16 line. At around the same time, the MCeV-DLV junction fuses with the Mesencephalic Vein (MsV) forming the Dorsal Midline Junction (DMJ) (Fig. 4E–G). Concurrently, both the MsV and the MCeV supply the DLV with blood, with the MsV becoming the primary supply by 120 hpf (data not shown). Lastly, a cross-vein will form between the DLV-PCeV junction and the DLV-PCeV′ junction crossing the mCP between 3 and 5 dpf (Fig. 4G,H). We label this previously unannotated cross-vein, the trans-choroid plexus branch (TCB) as it transits the mCP. Further elaboration of the small vessels that supply the mCP continues past 5 dpf. This network is highly variable with vessels extending between the DLV and TCB (Fig. 4H and Fig. S1).
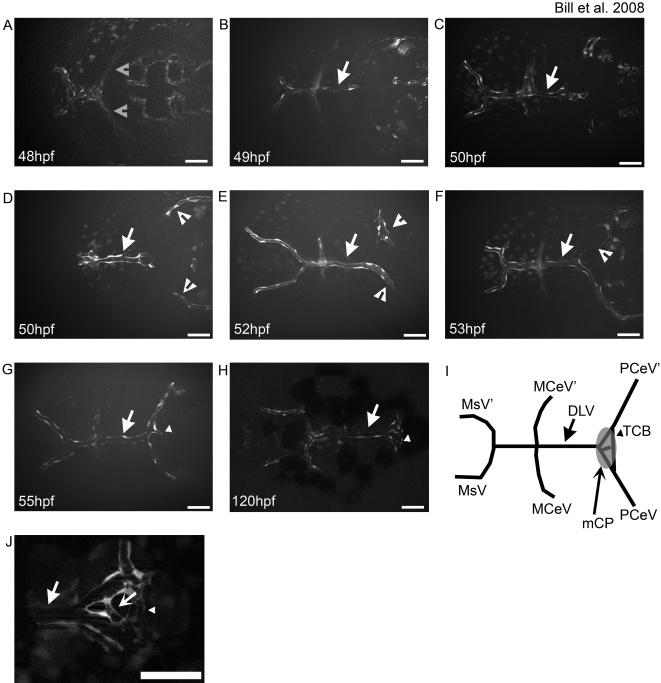
Figure 4. The DLV develops via angiogenic sprouting and supplies the mCP.
The development of the integral vasculature in the mCP was examined in living zebrafish larvae. The DLV (arrow in all panels) sprouts from the MCeV/MCeV′ (gray open arrowheads) via angiogenic sprouting between 40 hpf (A) and 48 hpf (B), and develops via growth cone-like filopodial extensions (C). The PCeV and PCeV′ (white open arrowheads) grow dorsomedially to meet the DLV in the roof of the fourth ventricle (D). Fusion occurs between the DLV (arrow) and PCeV or PCeV′ (E), followed by extension to the symmetric partner (PCeV or PCeV′) (F). Once connected, the DLV branches once more (G, small arrowhead) to form the trans-choroid plexus branch (TCB) (small arrowhead). By 120 hpf, the main vasculature of the mCP is in place (H). A diagrammatic representation of the final structure, with naming of the vessels and a superimposed mCP for comparison is shown (I). Small connecting vessels (concave arrow) connecting the DLV and TCB elaboration to form the mCP (J). Abbreviations: mesencephalic vein (MsV & MsV′), middle cerebral vessel (MCeV and MCeV′), dorsal longitudinal vein (DLV), posterior cerebral vein (PCeV and PCeV′) and myelencephalic choroid plexus (mCP). All images are oriented anterior to the left, and scale bars are 50 µm.
The Notch signaling pathway in mCP development
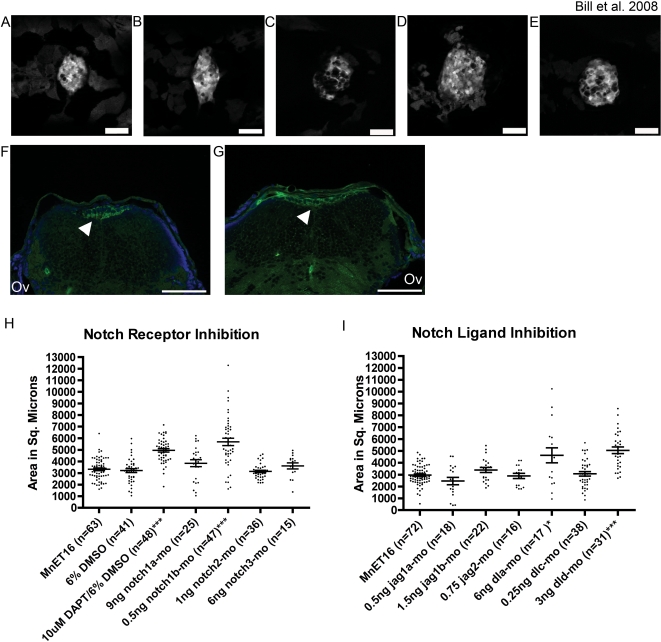
Multiple Notch ligands are expressed within the developing mammalian CP [13], with no known requirement for Notch signaling yet described. We used the gamma secretase inhibitor N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester (DAPT) [14] for a temporal-specific knockdown of Notch signaling during CP formation. DAPT was applied to embryos at 50 hpf to avoid indirect effects caused by early embryologic patterning defects. DAPT treatment resulted in a dramatic increase in mCP size in EtMn16 larvae [from a mean of 3200 µm±160 s.e.m. in vehicle-treated EtMn16 to a mean size of 5000 µm±150 s.e.m. (p = 1.98*10−11) in DAPT-treated larvae (Fig. 5H)]. This appears to be due to a lateral expansion as the mCP remains a monolayer (Fig. 5F).
Figure 5. Notch signaling is required for proper development of the myelencephalic choroid plexus.
Pan Notch Inhibition with DAPT (B, dorsal-mounted live larvae and G, transverse section) results in an increase in the mCP epithelial domain compared to vehicle-treated control larvae (A, dorsal mounted live larvae and F, transverse section). This increase in domain size is due to lateral spreading as the mCP remains as a monolayer (G, DAPT-treated versus F, vehicle treated). Further analysis showed that this effect is mediated by inhibition of notch1b (D, 5 dpf live larvae) dla, and dld (E, 5 dpf live larvae). Inhibition of notch1a (C, 5 dpf live larvae) did not significantly alter the size of the mCP epithelial domain but did effect overt structure. Panels A–F are dorsal views with anterior to the left, and panels G and H are 6 µm cryosections labeled with an antibody against GFP (green) and DAPI (blue) staining the nuclei. Quantitative measurements show the distribution mCP sizes in individual fish. Measurements are shown for Notch receptor inhibition by DAPT and morpholino experiments (H), and for Notch ligand inhibition by morpholinos (I) Each point in the histograms represents a measurement of a live larval zebrafish mCP. The mean±s.e.m. is indicated by the line and error bars respectively. Significant effects on mCP size are observed for 10 µM DAPT, notch1b, dla, and dld knockdown (* p = 0.02 and *** p<0.0001). For a full list of mean, s.e.m., and p-values, see table S2. Abbreviations: eye (E), pectoral fin (Pf), and otic vesicle (Ov). Arrows indicate mCP. Scale bars are 50 µm.
As DAPT is a pan-Notch signaling inhibitor, we sought to specifically identify the individual Notch receptor and its ligand(s) involved in mCP formation. We conducted a reverse genetic screen utilizing morpholino oligonucleotides previously designed and validated against ten known zebrafish Notch pathway members [15]–[19]. We first screened morpholinos targeting four Notch receptors: notch1a, notch1b, notch2, and notch3 (Fig. 5H). Treatment with 0.5 ng of notch1b-targeted morpholino leads to expansion of the mCP epithelia (mean 5700 µm±320 s.e.m. p = 3.8*10−9) (Fig. 5D) compared to wild type (3300 µm±120 s.e.m.)(Fig. 5A). We then screened morpholinos targeting Notch ligands: jagged1a, jagged1b, jagged2, deltaA (dla), and deltaD (dld) (Fig. 5I). The mCP in dld-targeted morphant larvae is significantly larger (5100 µm±280 s.e.m) compared to untreated EtMn16 controls (3000 µm±95 s.e.m.)(p = 1.9*10−8) (Fig. 5E). Knockdown of dla also increases the mCP size significantly (4600 µm±630 s.e.m. p = 0.02), but this effect is much less consistent than the effect of dld (Fig. 5I). In addition, while the size of the mCP epithelial domain is not significantly different in notch1a-targeted morphants compared to controls, there is a visible effect on the mCP structure in morphant larvae (Fig. 5C) with a loss of a cohesive mCP epithelial sheet. Therefore, we conclude that knockdown of notch1b, dla, and dld significantly expands the domain occupied by the mCP epithelia. Together, these analyses implicate a novel Notch pathway critical role in CP formation.
Discussion
Identification of the CP as visualized in the EtMn16 larvae
We demonstrate that the GFP-expressing cells of EtMn16 are dCP and mCP epithelium. The mCP in zebrafish is located within the fourth ventricle posterior to the cerebellum (Fig. 1E,F), consistent with the location of the 4vCP/mCP in mammals and other teleosts [1], [20]. The mCP of zebrafish expresses Gfap, a transient fetal marker for human 4vCP [7]. This is the first description of the CP in zebrafish.
Development of the mCP epithelia
We describe EtMn16 GFP-expressing cells that migrate laterally along the roof plate of the fourth ventricle, coalesce and become a subset of the definitive mCP epithelia. Garcia-Lecea and coworkers describe a second enhancer trap SqET33-E20 (Gateways) transgenic zebrafish that express GFP within cells that migrate along the anterior posterior axis at the midline, coalesce and form a distinct subset of definitive mCP epithelia [21]. These SqET33-E20-labeled cells are temporally and spatially separated from the cells we describe marked by EtMn16. The EtMn16 transgenic line labels cells highly reminiscent of cell lineages described by Hunter and Dymecki that migrate out of the dorsal rhombomeric lip and directly populate the mouse mCP [22]. We conclude that these migrating cells found in fish and mice are orthologous and the resulting developmental model represents a likely shared and conserved mechanism for CP development in vertebrates.
The vasculature of the mCP is supplied by the DLV
We characterize the mCP-associated vasculature in zebrafish. The DLV supplies the blood to the mCP, bifurcating just inside the margin of the mCP epithelia, and forming junctions with both the PCeV and PCeV′ (Fig. 3A–F). The DLV bends dorsally just before crossing the mCP margin and is tightly associated with the dorsal surface of the mCP epithelial monolayer (Fig. 3G,H). The DLV sprouts from the MCeV and MCeV′ between 40–48 hpf via angiogenesis. The DLV reaches a hypothesized decision point at ∼50 hpf, and the PCeV and PCeV′ grow dorsomedially to meet it. The trans-choroid plexus branch (TCB) develops between the DLV-PCeV junction and the DLV-PCeV′ junction between 55–120 hpf. This forms a variable structure that overlays the mCP epithelia, and produces a network of small vessels that connect the bifurcated DLV and TCB. This is the first description of the development of the vasculature associated with the mCP and has many similarities to the development of mammalian mCP development [9]. Future studies will be required to identify whether these small vessels that cross between the DLV and TCB are orthologous to the fenestrated capillaries of mammals.
Synthesis of a model for zebrafish mCP development
By comparing the developmental time points at which the DLV and mCP epithelia develop (Fig. 2, Fig. 4) [21], we can precisely order developmental events in the mCP. Garcia-Lecea and coworkers describe a subset of mCP epithelial precursor cells that are initially spread out at the dorsal midline from rhombomere 2–6 that coalesce between 48–72 hpf and express GFP in their SqET33-E20 line [21]. The DLV and PCeV develop to the location associated with the mCP synchronously with the GFP-expressing cells of SqET33-E20. Intriguingly, the developmental cue is independent of the vasculature (Fig. S1E) suggesting an attractant that promotes growth to the fourth ventricle roof plate. Previous work has suggested the existence of such an organizer in mouse [23]–[26]. After the DLV has completed junction formation with the PCeV and PCeV′, the subset of EtMn16 defined mCP migrate laterally coalescing on the roof of the fourth ventricle, by 5 dpf. This model is the first to integrate both the cellular migration of the mCP epithelial precursors with that of the development of the vasculature and lays the groundwork for further vascular studies. For example, the zebrafish should be a very tractable model to identify, the dorsal roof organizer and the factors that localize both vasculature and epithelial development to the fourth ventricle roof plate.
The Notch signaling pathway is required for mCP development
We undertook a small-scale targeted chemical and morpholino-mediated screen to investigate the role of Notch signaling in mCP development. The pan-Notch signaling chemical inhibitor (DAPT), when exposed to 50 hpf EtMn16 embryos increased the size of the developing mCP epithelia. To specify those members of the Notch pathway that are involved, we utilized previously validated Notch and Notch ligand targeting morpholinos to investigate the individual effect of several notch receptors and ligands. The notch1b, dla, and dld-targeted mopholinos increase the size of the mCP similar to the DAPT treatment, while other Notch and Notch ligand-targeting morpholinos did not significantly alter mCP size. It should be noted, however, that while other morpholinos were unable to change CP size, there may have been other effects on the structure as exemplified by a notch1a knockdown mCP (Fig. 5c) that has an increase in GFP-negative surface area within the mCP structure. Previous reports on Notch signaling in the mammalian CP have shown that multiple Notch ligands are expressed in the CP [13], a novel Notch2-mediated ligand independent role for adult rat CP integrity [27], and that expression of a constitutively activated Notch1 protein causes an increase in the proliferative potential of the CP precursor cells by activating mitosis in normally quiescent cells [22]. This study presented here identifies an endogenous requirement for notch1b in development of the mCP and a functional role for any Notch ligand (dla and dld) in the mCP. Future studies will be required to determine the relationship of Notch1a, Dla, and Dld, the localization of these proteins, and the nature of the expanded mCP including the role of the vasculature in determining the domain occupied by the CP.
Conclusion
We provide the first description of the zebrafish CP by characterizing a transgenic line (EtMn16) that expresses GFP in the zebrafish dCP and mCP epithelia. We observe a tight integration between the mCP and its vascular supply, primarily the DLV and TCB. We provide a model that integrates development of the epithelial and vascular components of the mCP. In addition, we demonstrate a functional role for endogenous Notch signaling in mCP epithelia. We identify a Notch receptor and two Notch ligands involved in the lateral expansion of the mCP epithelial layer. This model provides a unique system in which to rapidly expand the knowledge of the molecular signaling pathways that contribute to CP development.
Methods
Fish Strain, Care and Use
Fish are housed in the University of Minnesota Zebrafish Core facility under standard conditions [28] in accordance with IACUC-approved protocols. Lines propagated for this study include: Tg(fli-1:eGFP)[12] (ZIRC), Tg(gata-1:dsRed)[10] (a kind gift from Dr. Len Zon), and EtMn16 (see below). Double transgenics were developed by paired matings and include: Tg(fli-1:eGFP)/Tg(gata-1:dsRed) and Tg(gata-1:dsRed/EtMn16). Wild type zebrafish are obtained from Segrest Farms. Timed bulk matings were used to obtain one-cell embryos for injections.
Creation of the EtMn16 line
EtMn16 is a product of a Sleeping Beauty (SB) transposon mediated-enhancer trap screen previously described [6]. In brief, a weak EF1α enhancer element upstream of zebrafish optimized GFP (GM2) was placed in between the SB inverted-direct repeats (pT2/S2EF1a-GM2). Co-injection of this construct with SB transposase mRNA was performed into 1–2 cell embryos and propagated as described [29], [30].
Immunohistochemisty
Six micron cryosections were obtained on a Leica CM3050S cryostat via previously developed techniques [31]. Antibodies used include zrf-1[8], [32] (1∶5, ZIRC), anti-GFP G1544 (1∶200, Sigma), and Alexafluor conjugated goat anti-mouse and goat anti-rabbit (1∶2000, Invitrogen). Slides were mounted with Vectashield Mounting Medium with DAPI (Vector Laboratories).
Microinjection of morpholinos
Zebrafish were collected and injected as described previously [33]. Morpholino oligonucleotides were obtained from GeneTools LLC. To investigate Notch signaling during CP formation, we used published oligonucleotides against Notch, Jagged and Delta transcripts from the zebrafish (Table S1) [15]–[19]. For each morpholino, we confirmed that there was no significant sequence overlap between oligonucleotides targeting these highly related proteins (Table S1). The notch1b-targeted morpholino was co-injected with tp53-targeted morpholino (1.5×) to verify effects were not due to off-targeting [34].
Notch inhibitor treatment
We used the gamma-secretase inhibitor DAPT (Sigma) to inhibit all Notch signaling in zebrafish [14]. To identify the window of Notch sensitivity, we added DAPT at several different developmental time-points (data not shown). We determined treatment at 50 hours post fertilization was optimal. Fish were dechorinated and placed in medium Petri dishes with 6ml of embryo medium. 100 µM DAPT (suspended in DMSO) was added at various doses as described previously [35]. 6% DMSO in embryo medium (v/v) was used as a vehicle control.
Microscopy and Imaging
All images were taken on a Zeiss Axioplan 2 with Apotome utilizing the Axiocam mRM (Zeiss). For brightfield microscopy, fish were imaged under DIC optics. For imaging CP development, zebrafish were mounted in 1% low melting point and anesthetized using tricane [28]. Images were processed using Axiovision software with Image 4D (Versions 4.2 and 4.6; Zeiss), and Photoshop (Version 8; Adobe).
Measurements and Statistics
The surface area of the mCP (as defined by EtMn16 fluorescence) was measured using the outline measurement module (Axiovision 4.2 and 4.6). Values were entered into graphing software, Prism (Version 4; GraphPad) for histograms and normality statistics. Normality was determined for each of the distributions utilizing the D'Agostino & Pearson omnibus normality test (alpha = 0.05) and Shapiro-Wilk normality test (alpha = 0.05) (data not shown) for each of the treatments to verify that the Student T-test was appropriate (Version 4; GraphPad). Student T-tests were performed to determine statistical significance (two tailed with unequal distribution) in Excel (version 11.4.1; Microsoft).
Supporting Information
Variation of the DLV-PCeV junction. The DLV (arrows) bifurcated to join the PCeV and PCeV′ (open arrowheads) and the TCB (small arrowheads) connects the two junctions. This structure is plastic. The most common structure includes a third branch that transits between the DLV and the TCB (A–C), but in some instances the third DLV-TCB branch does not form by 5 dpf (D). The most rare occurrence is that the DLV does not develop (E). The PCeV and PCeV′ still meet in the location of the pCP, but lack blood flow (E, open arrowheads). All images are shown with anterior to the left. Scale bar is 50 µm.
(1.03 MB TIF)
(0.05 MB RTF)
(0.05 MB DOC)
Acknowledgments
Thanks to University of Minnesota Zebrafish Core Facility and to Aubrey Nielsen, Daniel Wolbrink, Daniel Carlson, and Rachel Bowers for excellent zebrafish care and maintenance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by National Institute of Health T32 EY007133-12-P.I. McLoon, University of Minnesota Department of Genetics, Cell, and Development, and Department of Pediatrics (BRB), Undergraduate Research Opportunities Program (JAM & EDY), Agency for Science, Technology and Research (A-STAR) of Singapore (VK). National Institute of Health R01 [GM63904 and DA14546 to SCE], Mayo Foundation (SCE), University of Minnesota, Department of Pediatrics (LAS), Minnesota Medical Foundation (LAS) and The Graduate Division of the University of Minnesota (LAS), and The University of Minnesota Medical School (Zebrafish Core Facility) (LAS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Netsky MG, Shuangshoti S, Tennyson VM, Brightman MW, Becker NH, et al. The Choroid Plexus in Health and Disease. Charlottesville, VA, USA: University Press of Virginia; 1975. [Google Scholar]
- 2.Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59:561–574. doi: 10.1093/jnen/59.7.561. [DOI] [PubMed] [Google Scholar]
- 3.Tennyson VM, Appas GD. The fine structure of the choroid plexus adult and developmental stages. Prog Brain Res. 1968;29:63–85. doi: 10.1016/s0079-6123(08)64149-7. [DOI] [PubMed] [Google Scholar]
- 4.Sturrock RR. A morphological study of the development of the mouse choroid plexus. J Anat. 1979;129:777–793. [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen M, Clausen PP, Jacobsen GK, Saunders NR, Mollgard K. Intracellular plasma proteins in human fetal choroid plexus during development. I. Developmental stages in relation to the number of epithelial cells which contain albumin in telencephalic, diencephalic and myelencephalic choroid plexus. Brain Res. 1982;255:239–250. doi: 10.1016/0165-3806(82)90024-4. [DOI] [PubMed] [Google Scholar]
- 6.Balciunas D, Davidson AE, Sivasubbu S, Hermanson SB, Welle Z, et al. Enhancer trapping in zebrafish using the Sleeping Beauty transposon. BMC Genomics. 2004;5:62. doi: 10.1186/1471-2164-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreto AS, Vassallo J, Queiroz Lde S. Papillomas and carcinomas of the choroid plexus: histological and immunohistochemical studies and comparison with normal fetal choroid plexus. Arq Neuropsiquiatr. 2004;62:600–607. doi: 10.1590/s0004-282x2004000400007. [DOI] [PubMed] [Google Scholar]
- 8.Marcus RC, Easter SS., Jr Expression of glial fibrillary acidic protein and its relation to tract formation in embryonic zebrafish (Danio rerio). J Comp Neurol. 1995;359:365–381. doi: 10.1002/cne.903590302. [DOI] [PubMed] [Google Scholar]
- 9.Strong LH. Early development of the ependyma and vascular pattern of the fourth ventricular choroid plexus in the rabbit. Am J Anat. 1956;99:249–290. doi: 10.1002/aja.1000990204. [DOI] [PubMed] [Google Scholar]
- 10.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 11.Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- 12.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 13.Irvin DK, Nakano I, Paucar A, Kornblum HI. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J Neurosci Res. 2004;75:330–343. doi: 10.1002/jnr.10843. [DOI] [PubMed] [Google Scholar]
- 14.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo SY, Kim M, Kim HS, Huh TL, Chitnis AB. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, et al. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 17.Holley SA, Julich D, Rauch GJ, Geisler R, Nusslein-Volhard C. her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development. 2002;129:1175–1183. doi: 10.1242/dev.129.5.1175. [DOI] [PubMed] [Google Scholar]
- 18.Amoyel M, Cheng YC, Jiang YJ, Wilkinson DG. Wnt1 regulates neurogenesis and mediates lateral inhibition of boundary cell specification in the zebrafish hindbrain. Development. 2005;132:775–785. doi: 10.1242/dev.01616. [DOI] [PubMed] [Google Scholar]
- 19.Wright GJ, Leslie JD, Ariza-McNaughton L, Lewis J. Delta proteins and MAGI proteins: an interaction of Notch ligands with intracellular scaffolding molecules and its significance for zebrafish development. Development. 2004;131:5659–5669. doi: 10.1242/dev.01417. [DOI] [PubMed] [Google Scholar]
- 20.Candal E, Anadon R, Bourrat F, Rodriguez-Moldes I. Cell proliferation in the developing and adult hindbrain and midbrain of trout and medaka (teleosts): a segmental approach. Brain Res Dev Brain Res. 2005;160:157–175. doi: 10.1016/j.devbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Lecea M, Kondrychyn I, Ye Z, Korzh V. In vivo Analysis of Choroid Plexus Morphogenesis in Transgenic Zebrafish. PLOS Biology. In Press;08-PLBI-RA-1885 doi: 10.1371/journal.pone.0003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter NL, Dymecki SM. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development. 2007;134:3449–3460. doi: 10.1242/dev.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- 24.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 25.Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- 26.Liem KF, Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Kokubo M, Marunouchi T. Asymmetric localization of Notch2 on the microvillous surface in choroid plexus epithelial cells. Histochem Cell Biol. 2007;127:449–456. doi: 10.1007/s00418-006-0260-8. [DOI] [PubMed] [Google Scholar]
- 28.Westerfield M. The Zebrafish Book. A Guide for the laboratory use of zebrafish (Danio rerio) Eugene: University of Oregon Press; 2000. [Google Scholar]
- 29.Davidson AE, Balciunas D, Mohn D, Shaffer J, Hermanson S, et al. Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev Biol. 2003;263:191–202. doi: 10.1016/j.ydbio.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Hermanson S, Davidson AE, Sivasubbu S, Balciunas D, Ekker SC. Sleeping Beauty transposon for efficient gene delivery. Methods Cell Biol. 2004;77:349–362. doi: 10.1016/s0091-679x(04)77019-3. [DOI] [PubMed] [Google Scholar]
- 31.Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38:1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 32.Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- 33.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 34.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, et al. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variation of the DLV-PCeV junction. The DLV (arrows) bifurcated to join the PCeV and PCeV′ (open arrowheads) and the TCB (small arrowheads) connects the two junctions. This structure is plastic. The most common structure includes a third branch that transits between the DLV and the TCB (A–C), but in some instances the third DLV-TCB branch does not form by 5 dpf (D). The most rare occurrence is that the DLV does not develop (E). The PCeV and PCeV′ still meet in the location of the pCP, but lack blood flow (E, open arrowheads). All images are shown with anterior to the left. Scale bar is 50 µm.
(1.03 MB TIF)
(0.05 MB RTF)
(0.05 MB DOC)