Abstract
In xanthorhodopsin, a retinal protein/carotenoid complex of Salinibacter ruber, the carotenoid salinixanthin functions as a light-harvesting antenna to supply additional excitation energy for retinal isomerization and proton transport. Another retinal protein, archaerhodopsin, has been shown to contain a carotenoid, bacterioruberin, but without an antenna function. We report here that the binding site confers a chiral geometry on salinixanthin in xanthorhodopsin, and confirm that the same is true for bacterioruberin in archaerhodopsin. Cell membranes containing these rhodopsins exhibit CD spectra with sharp positive bands in the visible region where the carotenoids absorb, and in the case of xanthorhodopsin a negative band at 536 nm, as well as bands in the UV. The carotenoid in ethanol has very weak optical activity in the visible. Denaturation of the opsin upon deprotonation of the Schiff base at pH 12.5 eliminates the induced CD bands in both proteins. In one of these proteins, but not in the other, the carotenoid binding site depends entirely on the retinal. Hydrolysis of the retinal Schiff base of xanthorhodopsin with hydroxylamine eliminates the induced CD bands of salinixanthin. In contrast, hydrolysis of the Schiff base in archaerhodopsin does not abolish the CD bands of bacterioruberin. Thus, consistent with its antenna function, the carotenoid binding site interacts closely with the retinal only in xanthorhodopsin and this interaction is the major source of the CD bands. In this protein, protonation of the counter-ion upon decreasing the pH from 8 to 5 causes significant changes in the CD spectrum. The observed spectral features suggest that binding of salinixanthin in xanthorhodopsin involves the cyclohexen-one ring of the carotenoid and its conformational heterogeneity is restricted.
Salinibacter ruber, an extremely halophilic eubacterium in hypersaline brines (1, 2) contains the carotenoid, salinixanthin (Fig. 1), responsible for the red color of the cell culture (3), and a retinal protein, xanthorhodopsin (4). The latter is a light-driven transmembrane proton pump like bacteriorhodopsin (5) and archaerhodopsin (6) of the archaea, proteorhodopsin of marine bacteria (7), and leptosphaerium rhodopsin in fungi (8), and exhibits sequence homology to these proteins (4, 9). An unusual feature of xanthorhodopsin is that besides retinal it contains bound salinixanthin, with a carotenoid/retinal stoichiometry near 1:1. Action spectra indicate that salinixanthin serves as a light-harvesting antenna, with about 40% of quanta absorbed by salinixanthin transferred to the retinal chromophore (4). Xanthorhodopsin therefore collects light like chlorophyll-based photosynthetic complexes, but instead of many antenna molecules that collectively funnel energy to the reaction center it utilizes only a single antenna in the complex. This system provides a new perspective on how antennae function, and raised the question (4) of how the carotenoid and the retinal could interact intimately in a heptahelical membrane protein.
Figure 1.
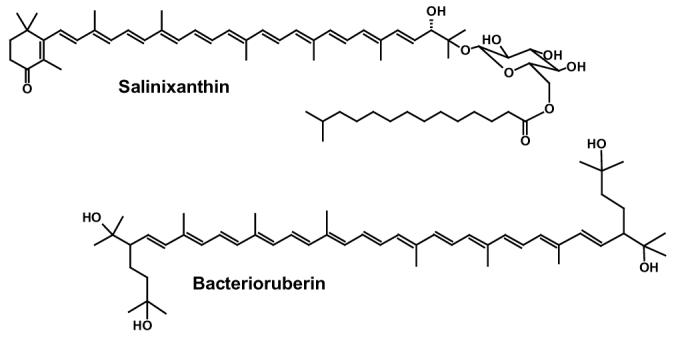
Chemical structures of: salinixanthin (3) and bacterioruberin (28).
The sharp, well-resolved bands at around 521 and 486 nm of bound salinixanthin appear in both the absorption spectrum of the native complex and the action spectrum for proton transport (4). Hydrolysis of the retinal Schiff base with hydroxylamine results not only in the expected disappearance of the absorption band of the retinal chromophore near 560 nm, but also in substantial broadening, decrease of extinction, and a small red shift of the carotenoid bands. Upon such bleaching, the spectrum of the carotenoid becomes almost identical to that of unbound carotenoid (4). These changes are reversed by reconstitution of the bleached complex with retinal, indicating close and specific interaction of the retinal and the carotenoid chromophores. This conclusion is supported by the observation of light-induced changes of carotenoid bands during the photocycle (4). Bacteriorhodopsin does not bind carotenoids, but another related retinal protein, archaerhodopsin, interacts with carotenoid bacterioruberin (Fig. 1) as follows from the sensitivity of the CD spectrum of cell membranes to the retinal Schiff base cleavage in archaerhodopsin (10). Bacterioruberin however does not have an antenna function as the action spectrum of proton pumping indicates (6). Xanthorhodopsin has not yet been crystallized, and the recent x-ray diffraction map of archaerhodopsin contains no clear density feature that could be modeled as a conjugated carotenoid chain (11).
Are the carotenoids bound to specific sites in xanthorhodopsin and archaerhodopsin, both small heptahelical membrane proteins that contain a single retinal, and if so does the binding site determine that there will be energy migration to the retinal in one rhodopsin but not the other? Circular dichroism has been used as a sensitive tool for studying chromophore-chromophore and chromophore-protein interactions in various proteins, including bacteriorhodopsin (12), halorhodopsin (13, 14), archaerhodopsin (10), and carotenoid/chlorophyll-protein complexes of photosynthetic membranes (15-19). It offers the possibility to test whether the carotenoid binds to a specific site where it can closely interact with the retinal, as implied by the spectroscopic results with xanthorhodopsin (4) but not by the crystallographic structure of archaerhodopsin (11).
In this study we examined the CD1 spectra of Salinibacter ruber cell membranes and solubilized xanthorhodopsin, and compared the spectra with those of Halorubrum membranes containing archaerhodopsin. We show that both rhodopsins exhibit substantial CD bands in the visible wavelength region, corresponding mostly to the structured spectra of the carotenoids, salinixanthin and bacterioruberin. Thus, both carotenoids acquire strong optical activity in their respective retinal protein/carotenoid complexes. However, only in xanthorhodopsin does the chirality of the bound carotenoid depend critically on the presence of retinal, indicating a tight interaction of the two bound polyenes. We show further that in xanthorhodopsin the CD spectrum is sensitive to the protonation state of the counter-ion to the retinal Schiff base.
MATERIALS AND METHODS
Growth of Salinibacter ruber, isolation of cell membranes, and partial purification of xanthorhodopsin upon solubilization in dodecylmaltoside were performed as described earlier (4). Briefly, cell membranes were obtained by breaking the cells with dialysis in the presence of DNAase vs. distilled water, and subsequently washing away other cell components and culture medium in at least 7 cycles of centrifugation in water. The membranes were finally resuspended in 100 mM NaCl, pH 8.5. Solubilization was performed in two steps. Membranes were first washed at low concentration of dodecylmaltoside (0.01%). This partially removed unbound carotenoid and some proteins, but did not solubilize the membranes. After that, xanthorhodopsin was solubilized in 0.15% dodecylmaltoside in 100 mM NaCl. The solubilized pigment was separated from the rest of the membrane by sedimentation at 250,000 g. This method yielded samples with highly purified xanthorhodopsin. Salinixanthin was extracted from cell membranes using an acetone/methanol (7:3) mixture, and purified by precipitating phospholipids with cold acetone and removing them by centrifugation as described earlier (3). Cell membranes containing archaerhodopsin-1 were isolated from Halorubrum sp. by dialysis and subsequent centrifugation in a way similar to that of Salinibacter ruber membranes. Protein identity was confirmed by sequence determination of the archaerhodopsin gene in this organism.
Absorption and circular dichroism spectra were measured on a Shimadzu UV-1601 spectrophotometer and a Jasco J-720 spectropolarimeter, respectively. The CD spectra are given in degree of ellipticity, θ, which is proportional to the difference in absorbance of left and right circularly polarized light: θ = 3300° (AL-AR) (15, 20). Quartz 1 mm and 4 mm pathlength cuvettes (from Helma and Starna Cells, respectively) were used. The CD spectra were recorded with 1 nm bandwidth resolution, and in 0.5 nm steps at 20°C. Typically 25 scans were averaged. Absorbance of the samples at the most intense carotenoid 487 nm band was 0.5-0.6. The CD spectra were corrected for the baseline distortion by subtracting reference spectra of the corresponding buffers or solvent. Absorption spectra were corrected for light scattering.
RESULTS
Complex CD spectra of xanthorhodopsin and archaerhodopsin, dominated by carotenoid bands
Fig. 2A (spectrum 1) shows the absorption spectrum of cell membranes of Salinibacter ruber with a high xanthorhodopsin content. The characteristic spectral signature of this protein is a shoulder at 560 nm from the broad retinal band and well-resolved sharp maxima from the bound salinixanthin at 519 and 487 nm. At shorter wavelengths an additional, smaller salinixanthin band at 320 nm is present. The CD spectrum of this sample (Fig. 2B, spectrum 1) exhibits a number of sharp positive and negative bands in the visible range, at 535 (−), 513 (+), 480 (+) and 455 nm (+). The large UV features are likely to originate mostly from the retinal chromophore and aromatic residues in the protein.
Figure 2.
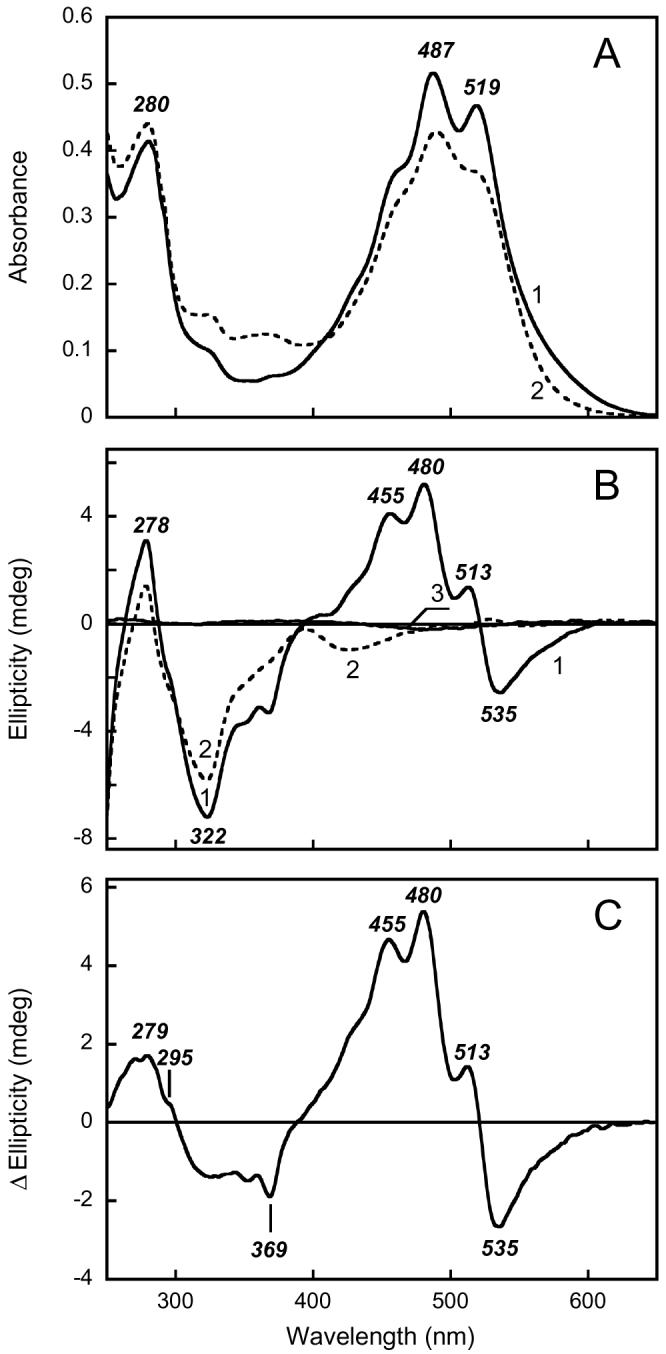
Absorption (A) and CD (B) spectra of xanthorhodopsin. Spectrum 1, suspension of Salinibacter ruber cell membranes containing xanthorhodopsin in 100 mM NaCl, pH 8.5; spectrum 2, after illumination at >550 nm for 3 hours in the presence of 200 mM hydroxylamine and 10 mg/ml of bovine serum albumin. Spectrum 3, salinixanthin in ethanol. C. Difference CD spectrum between unbleached and bleached cell membranes containing xanthorhodopsin: spectrum 1 minus spectrum 2 in Figure 1 B.
Cell membranes prepared from a Salinibacter culture which produced 3-fold less xanthorhodopsin exhibited an absorption spectrum with much less resolved bands from the excess, not bound, carotenoid. The CD spectrum was similar but had correspondingly smaller amplitude (not shown). This, as well as the positions of the band maxima, implies that the source of the CD bands is the retinal protein/carotenoid complex, xanthorhodopsin. The CD spectrum of solubilized Salinibacter membranes contains essentially the same bands for xanthorhodopsin as the native membranes (e.g., see Fig 5 below), without any obvious changes caused by the detergent except some reduction in the amplitude.
Figure 5.
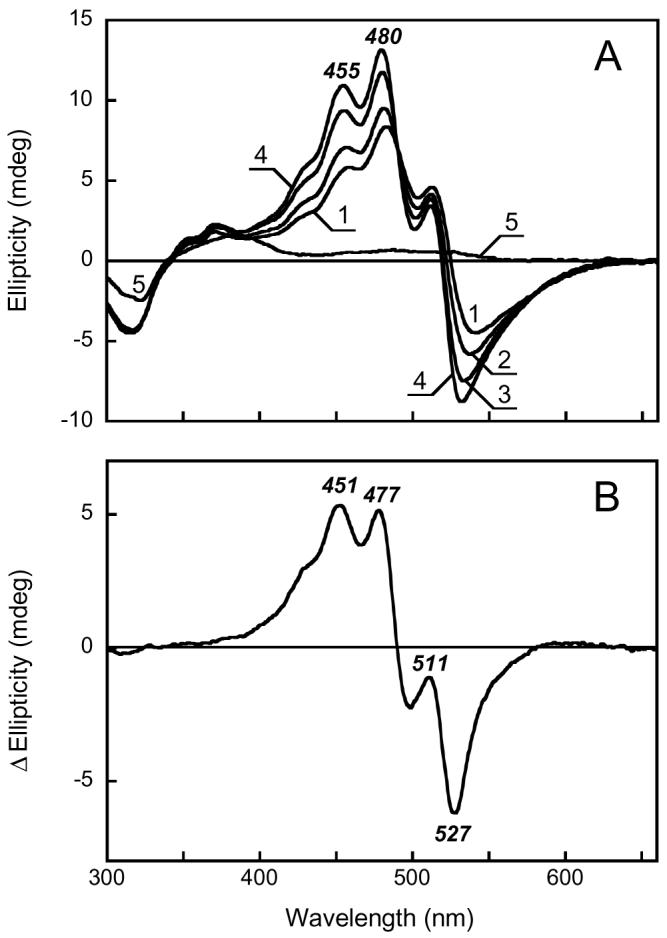
pH dependence of the CD spectrum of xanthorhodopsin. A. Purified xanthorhodopsin solubilized in 0.15% dodecylmaltoside at pH 4.5, 5.9, 6.7, 8.5, and 12.8, in spectra 1 through 5, respectively. B. Difference spectrum “pH 8.5 minus pH 4.5” (spectrum 4 minus spectrum 1 in Fig. 5A).
Hydrolysis of the xanthorhodopsin retinal Schiff base with hydroxylamine results in the disappearance of the 560 nm retinal band, the appearance of absorption at 320-380 nm from the retinal oxime, and the apparent dissociation (or loosening of binding) of salinixanthin in the complex (4), as indicated by the broadening of its bands (Fig. 2A, spectrum 2). Under these conditions the CD bands in the visible region disappear (Fig. 2B, spectrum 2), and two bands with much smaller amplitude appear at 490 nm (+) and 528 nm (−). The bands in the UV change also, but less. From these observations the sharp CD bands at 513, 480 and 455 nm can be assigned to salinixanthin bound to xanthorhodopsin. Extracted salinixanthin in ethanol shows virtually no optical activity (Fig. 2B, spectrum 3 and (3)). The retinal chromophore contributes mostly to the negative CD band at > 550 nm. In addition to the CD bands of the retinal and the carotenoid, the difference CD spectrum produced by reaction with hydroxylamine exhibits a band in the UV with a maximum at 279 nm and a shoulder at 295 nm (Fig. 2C), indicating that aromatic amino acids undergo chirality changes upon hydrolysis of the retinal Schiff base and the subsequent loss of specific salinixanthin binding.
Figs. 3A and B show absorption and CD spectra for membranes containing archaerhodopsin. As in xanthorhodopsin, the sharp absorption bands of the bacterioruberin at 543, 506 and 477 nm are superimposed on the band of the retinal chromophore near 570-580 nm (Fig. 3A, spectrum 1). Also as in xanthorhodopsin, there are strong positive CD bands at 550, 516, and 485 nm, that correlate but do not coincide with, the carotenoid absorption bands, with a contribution of a positive band from the retinal between 550 and 600 nm (Fig. 3B, spectrum 1). As for xanthorhodopsin, there are characteristic features in the UV as well. The main difference between the CD spectra of the two proteins is that, when calculated for equivalent concentrations of the proteins, the amplitudes of the bands are about 2 fold greater for archaerhodopsin, and the negative feature at > 500 nm is absent.
Figure 3.
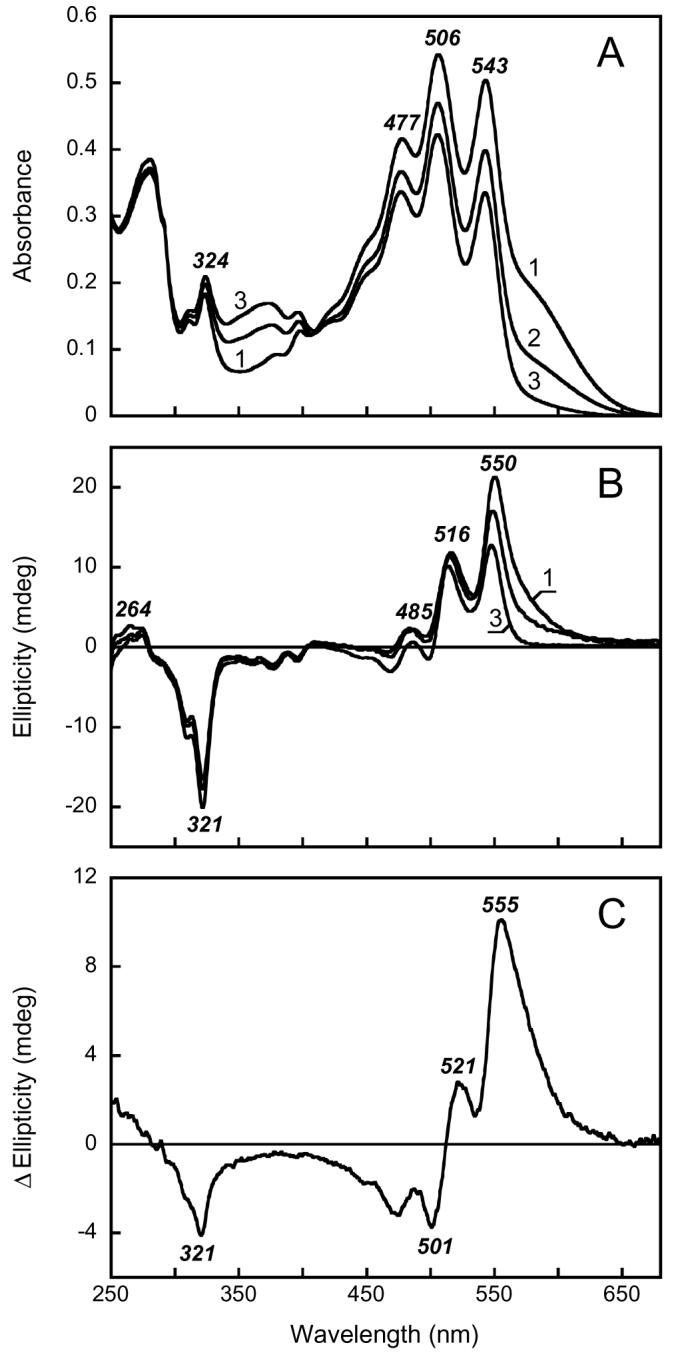
Absorption (A) and CD (B) spectra of archaerhodopsin. Spectra 1 through 3, initial spectrum of Halorubrum sp. membranes at pH 7.5 and after illumination for 2 and 9 hrs at >550 nm in the presence of 200 mM hydroxylamine and 10 mg/ml of bovine serum albumin, respectively. After 9 hrs of illumination (spectrum 3) the absorption band of the retinal chromophore decreased to less than 10% of initial value, as indicated by lowered absorption at 600 nm and laser flash induced absorption changes (not shown). C. Difference CD spectrum: spectrum 1 minus spectrum 3 in Fig. 2B.
The spectral changes in archaerhodopsin upon hydroxylamine bleaching of its retinal band are in sharp contrast with those described for the xanthorhodopsin. Fig. 3A (spectra 2 and 3) shows the progressive removal of the retinal band during the hydroxylamine treatment. Unlike the salinixanthin bands, the well-resolved absorption bands of bacterioruberin do not broaden and do not shift, when the Schiff base linkage with retinal is hydrolyzed. As reported earlier (10), and confirmed here (Fig. 3B, spectrum 3), in this protein the induced carotenoid CD bands of archaerhodopsin are somewhat affected, but largely preserved, after hydroxylamine treatment. The difference CD spectrum between the unbleached and bleached sample in Fig. 3C contains a large contribution from retinal at > 550 nm, as a positive rather than a negative band, but much smaller contributions from changes in the carotenoid.
Restoration of the CD bands upon reconstitution of hydroxylamine-bleached membranes with all-trans retinal
Addition of all-trans retinal to the Salinibacter ruber membranes, bleached with hydroxylamine and subsequently washed from it, induces reconstitution of both the retinal chromophore band and sharp bands of the bound salinixanthin (Fig. 4A). This is accompanied by the appearance of the CD bands in the visible which are practically identical to those observed in the membranes before bleaching (See Fig. 4B, curve 1). An interesting question is whether the shape of the CD spectrum depends substantially on the fraction of reconstitution, as was observed for bacteriorhodopsin in purple membrane. In the latter case, apparently interaction of pigment molecules within the trimers affected the CD spectrum by adding a bilobe component to a broad positive band (21). This feature was observed only at high occupancy of trimers and was eliminated upon complete solubilization of the membrane in Triton (22). It was proposed that bilobe feature might originate from exciton interaction of molecules in trimers (21), although this explanation was later questioned for lack of evidence for efficient energy transfer between molecules in trimers and other observations (23-25).
Figure 4.
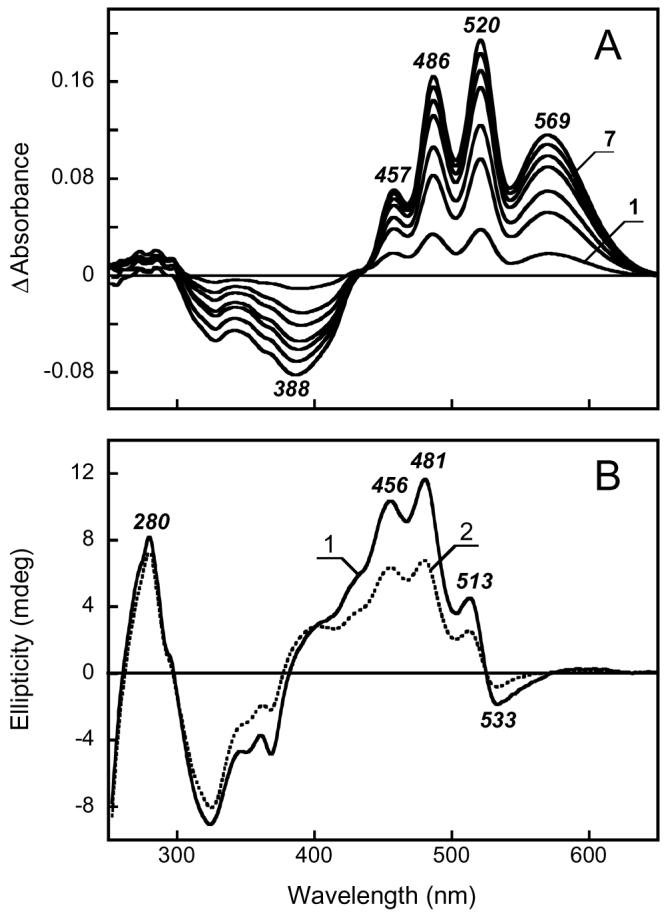
A. Absorption changes observed upon reconstitution with all-trans retinal the Salinibacter ruber membranes bleached with hydroxylamine. Curve 1 through 7, spectra taken 2, 6, 10, 20, 30, 50, 90 min after addition of retinal. B. CD spectra of reconstituted membranes: 1, 100% reconstitution; 2, 50% reconstitution.
In analogy with bacteriorhodopsin, if xanthorhodopsin is arranged in dimers or trimers, the shape of its CD spectrum should depend on the fraction of reconstitution. We produced partial reconstitution, by adding 50% of the retinal needed for complete occupancy. The resulting spectrum (curve 2 in Figure 4B) shows all the features present in the spectrum of the fully reconstituted sample, and thus does not provide evidence for a significant contribution from a possible supramolecular arrangement of the chromophores to the CD spectrum.
Probing the interaction between the retinal Schiff base and the carotenoid with the pH dependence of the CD spectrum
Lowering the pH from 8.5 to 4.5 causes minor changes in the absorption spectrum of xanthorhodopsin, which include a ca 3-5 nm red shift of the retinal chromophore band and a slight (ca 0.5 nm) blue shift of the carotenoid antenna absorption bands (26). These changes are accompanied by a change of the photocycle from one that includes M (with deprotonated retinal Schiff base) to another that does not, as in bacteriorhodopsin at low pH (27), and were attributed (26) to protonation of the proton acceptor and counter-ion to the Schiff base (presumably Asp83, analogous to Asp85 in bacteriorhodopsin). In the CD spectrum raising the pH causes much more extensive changes: overall increase of the amplitude of the carotenoid antenna bands (Fig. 5A spectra 1 to 4), with an apparent pKa 6.4, near that of the pKa of the counter-ion (ca 6.0 in (26)), and alterations in their relative amplitude and peak positions, as seen in the difference CD spectrum between pH 8.5 and 4.5 (Fig. 5B). The observed changes of the peak ratio and their exact position indicates that CD spectrum is likely to be composed of several components, which vary differently with the environment in the protein.
Raising the pH to > 12.5 causes not only a large blue-shift of the retinal absorption band from ca. 560 nm to 372 nm from titration of the Schiff base (26), but also decrease in the extinction and resolution of the carotenoid absorption bands analogous to those observed upon hydrolysis of the Schiff base with hydroxylamine (26). Raising the pH to 13 results in the complete loss of the intensity of the CD bands (Fig. 5A, spectrum 5). The same was observed for archaerhodopsin (not shown), confirming that, like salinixanthin, bacterioruberin does not have intrinsic optical activity in the visible. Abolition of the induced CD bands will have been caused by either deprotonation of the retinal Schiff base or the subsequent denaturation of the opsin. In archaerhodopsin it was possible to decide between these alternatives. Because removing the retinal Schiff base did not abolish the optical activity of the carotenoid (Fig. 3B), but raising the pH of a hydroxylamine-bleached archaerhodopsin sample eliminated all induced CD bands, we can conclude that the loss of bacterioruberin chirality at high pH is from denaturation of the opsin.
DISCUSSION
Origin of the complex CD spectra of xanthorhodopsin and archaerhodopsin
The sharp positive maxima in the CD spectrum of xanthorhodopsin resemble the absorption maxima of salinixanthin but do not coincide with them. The same can be said about archaerhodopsin. The intense absorption bands of carotenoids with a long chain, such as salinixanthin (3) (11 conjugated bonds in the chain plus two in the ring) and bacterioruberin (28) (13 conjugated bonds in the chain), in the visible range arise from transition to vibrational sublevels of the second excited state, S2, since transition to the first singlet excited state is prohibited for symmetry reasons (reviewed in ref. (29)). The sum of vibronic transitions to the 0, 1, 2, and 3 sublevels of the C=C and C-C stretching vibrations of the conjugated chain is almost entirely responsible for the typical four-band carotenoid spectra of rhodopin glucoside in the light-harvesting complex of Rhodopseudomonas acidophila (17). A similar origin for the absorption maxima should be expected for salinixanthin and bacterioruberin. Each of these vibrational levels will contribute to the CD spectrum.
According to a previous (3) and the present study, salinixanthin has very weak optical activity in ethanol solution or in the membrane when not bound to a retinal protein. As we report here, large CD bands arise only when the carotenoid is in the native rhodopsin complex, i.e., when perturbed by the constraints of a rigid binding site as for other carotenoids in photosynthetic membranes (30). For example, optical activity was observed for the otherwise nonchiral lycopene and rhodopin of light-harvesting complexes of photosynthetic bacteria (17). The CD bands in such cases will originate from interaction of the chromophore with the protein in an asymmetric environment, or from an enforced asymmetric conformation of the carotenoid chain. In some cases, the CD spectrum is “conservative,” and contains negative and positive lobes resembling the first derivative of the absorption spectrum. The maxima of the xanthorhodopsin CD bands are 5-8 nm blue-shifted relative to the absorption maxima, and very nearly coincident with the maxima of the first derivative of the absorption spectrum (compare Fig. 6A, spectra 1 and 2). When this contribution is subtracted, the remaining spectrum (Fig. 6A, spectrum 3) has an overall bilobe character, with a broad positive band near 450-500 nm (from the carotenoid) and a negative band at 550 nm (from the retinal). This bilobe might originate from dipole interaction between salinixanthin and the retinal in xanthorhodopsin. Such interaction is expected because the two chromophores must be very near each other in order energy migration with quantum efficiency of ca. 40% from salinixanthin to retinal could occur (4). This becomes clear if one takes into account that the energy transfer should occur from the very short-lived S2 state (lifetime typically ca. 250 fs) because S1 state is expected to be below the lowest retinal singlet excited state. The requirement for rapid transfer from S2 is relevant also to the spectral changes which salinixanthin undergoes upon formation of the native xanthorhodopsin complex. The narrowing of the absorption bands indicates restriction of out of plane motions within the conjugated chain. The prime candidate for a site, which undergoes such immobilization, is the ring. Its angular movements around the C6-C7 bond are most likely primarily responsible for the broadening of the spectrum of unbound salinixanthin as was found for retinal (31, 32) and other carotenoids (33). If this is so, the ring and part of the chain close to the C6-C7 bond is the site of interaction of salinixanthin with the retinal protein. Immobilization of this part will serve two purposes: improve alignment of the two chromophores, and increase the life time of the S2 state by lowering the rate of internal conversion. The result is an increase in the rate and the quantum efficiency of energy transfer. The ring is fixed not in plane of the conjugated chain but apparently at some angle to it because a slight blue shift is observed in the absorption maximum upon formation of the complex. This asymmetric conformation is probably one of the sources of chirality and optical activity of bound salinixanthin. It might not be the only source (others, like an asymmetric environment) and exciton coupling might also contribute.
Figure 6.
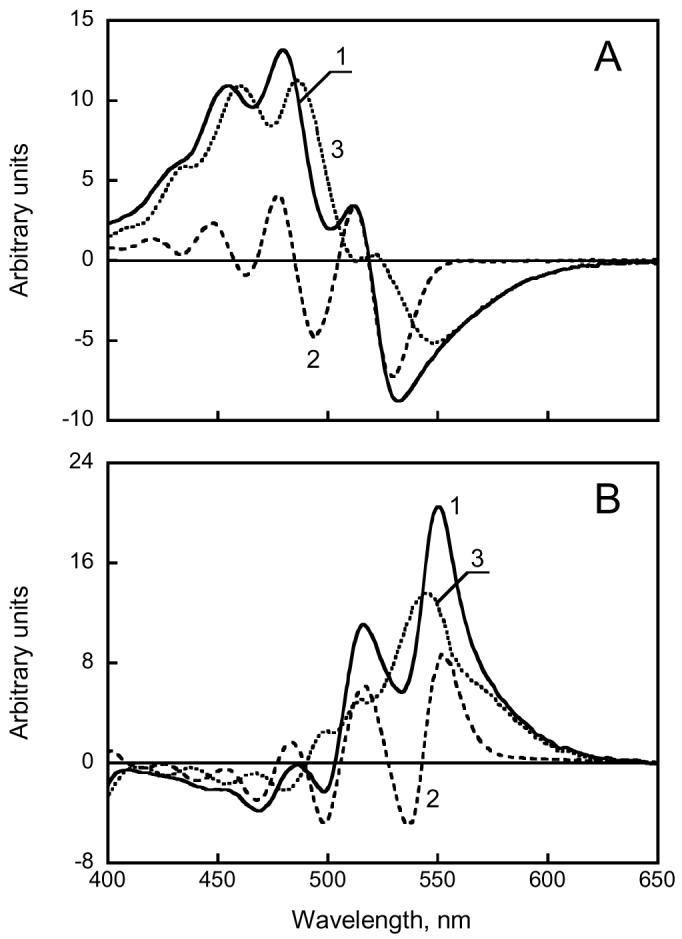
Decomposition of the CD spectra of xanthorhodopsin (A) and archaerhodopsin (B) to conservative and non-conservative components. The former was approximated by the first derivative of the absorption spectrum. A. Spectrum 1, CD spectrum of xanthorhodopsin solubilized in 0.15% dodecylmaltoside at pH 8.5; spectrum 2, first derivative of the absorption spectrum dA/dλ, multiplied by λ2 (λ2dA/dλ = − dA/dν) and scaled to fit the amplitude of main peaks in the CD spectrum; spectrum 3, the difference “spectrum 1 minus spectrum 2”. B. Spectrum 1, CD spectrum of cell membranes containing archaerhodopsin; spectrum 2, first derivative multiplied by −λ2; spectrum 3, “spectrum 1 minus spectrum 2”.
Bacterioruberin does not contain a ring but exhibits a strong CD signal when it interacts with archaerhodopsin. The CD maxima of archaerhodopsin are 7-10 nm red-shifted from the absorption maxima, and they agree with the first derivative of the absorption spectrum as in xanthorhodopsin but taken with a minus sign (compare the CD spectrum to the first derivative of the absorption spectrum in Fig. 6B, spectra 1 and 2). However, when this conservative contribution is subtracted from the CD spectrum, the spectrum that remains (Fig. 6B, spectrum 3) does not have a bilobe character. Instead, the contributions of the carotenoid and the retinal seem to be both positive. Such dissection of the CD spectra into components raises an interesting question: do the differences in the complex CD spectra provide evidence for exciton exchange between carotenoid and retinal in xanthorhodopsin but not in archaerhodopsin, consistent with the antenna function of the carotenoid in this protein only? The bilobe feature of the CD spectrum in xanthorhodopsin is consistent with excitonic interaction, but experiments with retinal and carotenoid analogues that affect their interaction, and calculations when the geometry of the donor and acceptor pair become available, will be necessary to draw a firmer conclusion.
The carotenoid binding sites appear to be specific enough in both rhodopsins to confer chirality on the chromophores. If the carotenoids are bound inside the helical bundles their geometry will be fully dependent on the binding site, but if they are bound to the hydrophobic surfaces of these integral membrane proteins (11) the sites must have distinct shapes also to ensure a fixed geometry. In the crystal structure of bacteriorhodopsin (34) the lipid chains at the protein periphery follow the grooves formed by side-chains of the protein. Importantly, however, the sites differ in the two rhodopsins we examine here. The abolition of the CD bands upon removal of the retinal Schiff base by hydroxylamine bleaching indicates close retinal-carotenoid interaction in xanthorhodopsin, but this is not so in archaerhodopsin. The results are thus consistent with the earlier finding of antenna function in the former (4), but not the latter, complex as earlier (10) and our unpublished data indicate.
Electrostatic effects of the retinal Schiff base region on bound salinixanthin
The spatial relationship of retinal and salinixanthin in xanthorhodopsin is not yet clear, but an obvious question is if the retinal Schiff base – counter-ion dipole is near enough to the carotenoid to affect it. Indeed, we find that, protonation of the Schiff base counter-ion decreases considerably the overall amplitude of the CD spectrum (Fig. 5A). This indicates that the charge state of the counter-ion is a major factor in determining the interaction of the retinal protein with the carotenoid antenna. In spite of the large decrease in the amplitude of the CD spectrum at low pH, there are only minor effects on the absorption spectrum, with the carotenoid maxima undergoing very small blue-shifts without substantial change of the resolution of the vibronic bands (26). The latter is mostly determined by steric factors restricting movements of the chain and particularly the ring. Thus the effect of the protonation of the counter-ion on the CD spectrum must be for electrostatic rather than steric reasons.
In conclusion, the CD spectrum of xanthorhodopsin and its changes upon retinal chromophore hydrolysis and protonation of counter-ion provides evidence for tight interaction of the carotenoid antenna with the retinal. This kind of interaction is not present in archaerhodopsin in which no energy transfer from carotenoid to the retinal takes place.
ACKNOWLEDGEMENT
The authors thank Prof. Josefa Antón for providing the strain of Halorubrum sp. containing archaerhodopsin and Jennifer Wang for assistance.
Footnotes
This work was supported by grants from NIH (GM29498 to J. K. L.), DOE (DEFG03-86ER13525 to J.K.L.) and ARO (W911NF-06-01-0020 to S.P.B. and J.K.L.)
Abbreviations: CD, circular dichroism; dodecylmaltoside, n-dodecyl-α-D-maltopyranoside.
REFERENCES
- 1.Antón J, Rosselló-Mora R, Rodríguez-Valera F, Amann R. Extremely halophilic Bacteria in crystallizer ponds from solar salterns. Appl. Environ. Microbiol. 2000;66:3052–3057. doi: 10.1128/aem.66.7.3052-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antón J, Oren A, Benlloch S, Rodríguez-Valera F, Amann R, Rosselló-Mora R. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Micrbiol. 2002;52:485–491. doi: 10.1099/00207713-52-2-485. [DOI] [PubMed] [Google Scholar]
- 3.Lutnaes BF, Oren A, Liaaen-Jensen S. New C-40-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J. Nat. Prod. 2002;65:1340–1343. doi: 10.1021/np020125c. [DOI] [PubMed] [Google Scholar]
- 4.Balashov SP, Imasheva ES, Boichenko VA, Antón J, Wang JM, Lanyi JK. Xanthorhodopsin: A proton pump with a light-harvesting carotenoid antenna. Science. 2005;309:2061–2064. doi: 10.1126/science.1118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oesterhelt D, Stoeckenius W. Functions of a new photoreceptor membrane. Proc. Natl. Acad. Sci. U.S.A. 1973;70:2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukohata Y, Sugiyama Y, Ihara K, Yoshida M. An australian halobacterium contains a novel proton pump retinal protein: archaerhodopsin. Biochem. Biophys. Res. Commun. 1988;151:1339–1345. doi: 10.1016/s0006-291x(88)80509-6. [DOI] [PubMed] [Google Scholar]
- 7.Béjà O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich SB, Gates CM, Feldman RA, Spudich JL, Spudich EN, DeLong EF. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science. 2000;289:1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 8.Waschuk SA, Bezerra J,AG, Shi L, Brown LS. Leptosphaeria rhodopsin: Bacteriorhodopsin-like proton pump from a eukaryote. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6879–6883. doi: 10.1073/pnas.0409659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mongodin EF, Nelson KE, Daugherty S, DeBoy RT, Wister J, Khouri H, Weidman J, Walsh DA, Papke RT, Sanchez Perez G, Sharma AK, Nesbø CL, MacLeod D, Bapteste E, Doolittle WF, Charlebois RL, Legault B, Rodriguez-Valera F. The genome of Salinibacter ruber: Convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18147–18152. doi: 10.1073/pnas.0509073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukohata Y, Ihara K, Uegaki K, Miyashita Y, Sugiyama Y. Australian Halobacteria and their retinal-protein ion pumps. Photochem. Photobiol. 1991;54:1039–1045. doi: 10.1111/j.1751-1097.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 11.Enami N, Yoshimura K, Murakami M, Okumura H, Ihara K, Kouyama T. Crystal structures of archaerhodopsin-1 and -2: Common structural motif in archaeal light-driven proton pumps. J. Mol. Biol. 2006;358:675–685. doi: 10.1016/j.jmb.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Cassim JY. Unique biphasic band shape of the visible circular-dichroism of bacteriorhodopsin in purple membrane - excitons, multiple transitions of protein heterogeneity. Biophys. J. 1992;63:1432–1442. doi: 10.1016/S0006-3495(92)81701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duschl A, McCloskey MA, Lanyi JK. Functional reconstitution of halorhodopsin. J. Biol. Chem. 1988;263:17016–17022. [PubMed] [Google Scholar]
- 14.Hasselbacher CA, Spudich JL, Dewey TG. Circular dichroism of halorhodopsin: comparison with bacteriorhodopsin and sensory rhodopsin I. Biochemistry. 1988;27:2540–2546. doi: 10.1021/bi00407a041. [DOI] [PubMed] [Google Scholar]
- 15.Parson WW, Nagarajan V. Optical spectroscopy in photosynthetic antennas. In: Green BR, Parson WW, editors. Light-Harvesting Antennas in Photosynthesis. Kluwer Academic Publishers; Dordrecht / Boston / London: 2003. pp. 83–127. [Google Scholar]
- 16.Davis CM, Bustamante PL, Loach PA. Reconstitution of bacterial core light-harvesting complexes of Rhodobacter sphaeroides and Rhodospirillum rubrum with isolated α- and β -polypeptides, bacteriochlorophyll a and carotenoid. J. Biol. Chem. 1995;270:5793–5804. doi: 10.1074/jbc.270.11.5793. [DOI] [PubMed] [Google Scholar]
- 17.Georgakopoulou S, van Grondele R, van der Zwan G. Circular dichroism of carotenoids in bacterial light-harvesting complexes: experiments and modeling. Biophys. J. 2004;87:3010–3022. doi: 10.1529/biophysj.104.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgakopoulou S, Frese RN, Johnson E, Koolhaas C, Cogdell RJ, van Grondele R, van der Zwan G. Absorption and CD spectroscopy and modeling of various LH2 complexes from purple bacteria. Biophys. J. 2002;82:2184–2197. doi: 10.1016/S0006-3495(02)75565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogdell RJ, Scheer H. Circular dichroism of light-harvesting complexes from purple photosynthetic bacteria. Photochem. Photobiol. 1985;42:669–678. [Google Scholar]
- 20.Buchecker R, Noack K. Circular dichroism. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Birkhäuser Verlag; Basel, Boston, Berlin: 1995. pp. 63–116. [Google Scholar]
- 21.Heyn MP, Bauer P-J, Dencher NA. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem. Biophys. Res. Commun. 1975;67:897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- 22.Becher B, Ebrey TG. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacteriumhalobium. Biochem. Biophys. Res. Commun. 1976;69:1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed MA, Lin CT, Mason WR. Is there an excitonic interaction or antenna system in bacteriorhodopsin? Proc. Natl. Acad. Sci. U.S.A. 1989;86:5376–5379. doi: 10.1073/pnas.86.14.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SG, El-Sayed MA. CD spectrum of bacteriorhodopsin - best evidence against exciton model. Biophys. J. 1991;60:190–197. doi: 10.1016/S0006-3495(91)82042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnaukhova E, Vasileiou C, Wang A, Berova N, Nakanishi K, Borhan B. Circular dichroism of heterochromic and partially regenerated purple membrane: Search for exciton coupling. Chirality. 2006;18:72–83. doi: 10.1002/chir.20222. [DOI] [PubMed] [Google Scholar]
- 26.Imasheva ES, Balashov SP, Wang JM, Lanyi JK. pH dependent transitions in xanthorhodopsin. Photochem. Photobiol. 2006 doi: 10.1562/2006-01-15-RA-776. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SY. Light-induced currents from oriented purple membrane. I. Correlation of the microsecond component (B2) with the L-M photocycle transition. Biophys. J. 1990;57:943–950. doi: 10.1016/S0006-3495(90)82614-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Handbook. Birkhauser Verlag; Basel, Boston, Berlin: 2004. [Google Scholar]
- 29.Polivka T, Sundström V. Ultrafast dynamics of carotenoid excited states -from solution to natural and artificial systems. Chem. Rev. 2004;104:2021–2071. doi: 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]
- 30.Cogdell RJ, Crofts AR. Analysis of the pigment content of an antenna pigment-protein complex from three strains of Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta. 1978;502:409–416. doi: 10.1016/0005-2728(78)90074-9. [DOI] [PubMed] [Google Scholar]
- 31.Honig B, Ebrey TG. The structure and spectra of the chromophore of the visual pigments. Annu. Rev. Biophys. Bioengin. 1974;1974:151–177. doi: 10.1146/annurev.bb.03.060174.001055. [DOI] [PubMed] [Google Scholar]
- 32.Christensen RL, Kohler BE. Low resolution optical spectroscopy of retinyl polyenes: low lying electronic levels and spectral broadness. Photochem. Photobiol. 1973;1973:293–301. [Google Scholar]
- 33.Britton G. UV/Visible Spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Birkhäuser Verlag; Basel, Boston, Berlin: 1995. pp. 13–62. [Google Scholar]
- 34.Luecke H, Schobert B, Richter H-T, Cartailler J-P, Lanyi JK. Structure of bacteriorhodopsin at 1.55 Å resolution. J. Mol. Biol. 1999;291:899–911. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]


