Abstract
Lens transparency and high refractive index presumably depend on the appropriate arrangement and distribution of lens proteins among lens fiber cells. Intercellular gap junction channels formed by α3 and α8 connexins are known to transport small molecules, ions and water, but not proteins, in the lens. Mosaic expression of green fluorescent protein (GFP) in the lens is a useful marker for monitoring macromolecule distribution between fiber cells and for constructing 3-dimensional images of living lens cells. In α3(−/−) α8(−/−) double knockout (DKO) lenses, three-dimensional images of GFP-positive cells demonstrate the changes of epithelial cell surfaces and insufficient elongation of inner fiber cells. Uniform distribution of GFP between inner lens fiber cells is observed in both wild-type and α3(−/−) lenses. In contrast, uniform GFP distribution is slightly delayed in α8(−/−) lenses and is abolished in DKO lenses. Without endogenous wild-type α3 and α8 connexins, knock-in α3 connexin (expressed under the α8 gene promoter) restores the uniform distribution of GFP protein in the lens. Thus, the presence of either α3 or α8 connexins seems sufficient to support the uniform distribution of GFP between differentiated lens fiber cells. Although the mechanism that drives GFP transport between fiber cells remains unknown, this work reveals that gap junction communication plays a novel role in the regulation of intercellular protein distribution in the lens.
Keywords: Connexin, Gap junction, Cell-cell communication
1. Introduction
The ocular lens is a syncytial unit consisting of a single monolayer of epithelial cells at the anterior surface and a bulk mass of elongated lens fibers that extend from the anterior to posterior poles (Mathias et al., 1997). During lens formation, the embryonic lens vesicle is a spherical monolayer of epithelial cells. The posterior cells of the lens vesicle differentiate and elongate to form primary lens fiber cells that fill the lumen of the lens vesicle. The anterior cells remain in a monolayer to become lens epithelial cells that cover the anterior hemisphere of the lens. Lifelong lens growth relies on epithelial cell proliferation, elongation and differentiation into secondary lens fibers, which surround previous generations of fibers, at the lens equator (McAvoy et al., 1999; Piatigorsky, 1981). During the late stages of fiber cell differentiation, inner differentiated fiber cells break down cytoplasmic organelles and nuclei to eliminate light scattering structures and become mature fiber cells in the lens core (Bassnett, 2002).
The lens must maintain transparency and develop appropriate high refractive index in order to transmit and focus light images onto the retina. High concentrations of lens proteins, metabolites, ions and water presumably need to be arranged and distributed among lens fiber cells to achieve transparency with appropriate high refractive index (Delaye and Tardieu, 1983; Jones et al., 2005). The mechanisms that control lens refractive index remain largely unknown. A recent study reports that the loss of Lim2 (or MP20) in mouse lenses impairs the internal refractive quality (Shiels et al., 2007). Lim2 belongs to the Pmp22/claudin protein family, and members of this protein family mediate cell adhesion and junction formation (Arneson and Louis, 1998; Chen et al., 2003; Van Itallie and Anderson, 2006). However, the molecular basis for the abnormal refraction in the Lim2 knockout lenses and the function(s) of Lim2 in the lens are unknown. Presumably, the regulation of lens protein concentration is important for the development of appropriate reflective index in the lens.
Gap junction channels, formed by connexins, allow the passage of small (<1200 Da) molecules, including ions and metabolites, directly from cell to cell (Gilula et al., 1972; Simpson et al., 1977; Yeager et al., 2000). The extensive network of gap junctions facilitates electrical and metabolic coupling between lens epithelial-epithelial, epithelial-fiber and fiber-fiber cells (Goodenough, 1992). The lens expresses at least three connexin subunits, α1 (Cx43), α3 (Cx46) and α8 (Cx50) (Beyer et al., 1989; Paul et al., 1991; White et al., 1992). In mammalian lenses, connexin α1 is expressed at low levels in epithelial cells and is absent in lens fibers (Beyer et al., 1989). Connexin α3 is highly expressed during fiber differentiation (Gong et al., 1997), while connexin α8 is expressed in lens epithelial cells and has increased expression in differentiated fibers (Dahm et al., 1999; Rong et al., 2002).
Connexin-mediated intercellular gap junction communication is crucial for lens growth and transparency (Gong et al., 2007; Mathias et al., 2007). Connexin α3(−/−) homozygous knockout mice develop nuclear cataracts (Gong et al., 1997), and connexin α8(−/−) homozygous knockout mice have significantly smaller lenses with zonular pulverulent nuclear cataracts (Rong et al., 2002; White et al., 1998). The heterozygous knockout mice of α3 and/or α8 develop normal and transparent lenses while the α3 and α8 double homozygous knockout (DKO) mice have severe nuclear cataracts and microphthalmia (Xia et al., 2006a). Furthermore, knock-in α3 connexin, genetic replacement of endogenous α8 connexin with wild-type α3 connexin by homologous recombination (α3 connexin expressed from the α8 locus, hereafter referred toas KIα3 mice), prevents lens opacification but does not rescue the small lens size caused by the absence of α8 (White, 2002). Knock-in α3 alleles alone are also sufficient to maintain lens transparency and partially restore fiber cell coupling (Martinez-Wittinghan et al., 2003; Martinez-Wittinghan et al., 2004; Xia et al., 2006b).
Green fluorescent protein (GFP) is a useful marker for observing the properties of living lens cells during development. At least two GFP transgenic mouse lines develop mosaic expression patterns of GFP in lens epithelial cells (Shestopalov and Bassnett, 2003; Xia et al., 2006c). Previous studies have uncovered a macromolecular exchange pathway that mediates the uniform distribution of GFP in the lens, and it has been hypothesized that the plasma member fusion of inner fiber cells leads to the uniform GFP distribution (Shestopalov and Bassnett, 2000;2003). However, this hypothesis remains to be tested, and the molecular nature of this macromolecular exchange pathway needs to be elucidated. By using compound mutant mice that contain disrupted α3 and/or α8 connexin genes with a GFP-transgene, we have found that gap junction communication influences intercellular GFP protein distribution in differentiated lens fiber cell before undergoing cell maturation. A loss of α3 and/or α8 connexins also affects other properties of lens epithelial and fiber cells.
2. Methods
2.1 Mice
This study followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and an ACUC approved animal protocol (UC Berkeley). The generation and genotype of α3(−/−), α8(−/−), DKO and α3(−/−) α8(KIα3/−) mice have been previously described (Gong et al., 1997; Okabe et al., 1997; Rong et al., 2002; White, 2002). GFP-positive (GFP+) connexin knockout or knock-in mice were generated by intercrossing connexin knockout or knock-in mice with GFP transgenic mice (Okabe et al., 1997). GFP+ mice were screened using a UV lamp. Heterozygous knockout mice were then mated to generate homozygous knockout mice that were genotyped by previously described PCR methods (Gong et al., 1997; Rong et al., 2002; White, 2002). Homozygous knockout or knock-in GFP+ mice (with one copy of the GFP transgene) were maintained for this study.
2.2 Microscopy
Confocal microscopy was used to evaluate the distribution of GFP in living lenses. GFP+ mice were euthanized at postnatal day 7 (P7), P14, P21 and P90. Fresh intact lenses were dissected out of the whole eye immediately before imaging. The confocal microscope (Leica) was equipped with an argon laser, and a 40× oil lens was used. Lenses were maintained in DMEM at 37°C on the stage of the confocal microscope.
2.3 Epithelial and Fiber Cell Evaluation
Z-stacks for GFP lenses were collected with 1 μm z-steps, and AxioVision 4.5 was used to analyze epithelial cell and fiber cell shape, length and surface characteristics. Bright GFP+ epithelial cells surrounded by GFP-negative cells from the center region of the monolayer of epithelial cells were used for 3D reconstruction.
2.4 Vibratome Sections
Lens vibratome sections were prepared using a previously described method (Gilliland et al., 2001). Briefly, freshly dissected lenses from P10 mice were fixed for 15 hours in 3% paraformaldehyde at room temperature. Fixed lenses were then mounted anterior side up with super glue onto an aluminum sectioning stand. Lenses were covered with warm 2.5% agar noble, then submerged in 1X phosphate buffered saline at 6–10°C and sectioned using a vibrating knife microtome with an amplitude setting of 1 and a speed of approximately 0.2 mm/s. Lens cross sections between 250–300 μm in thickness from similar depths (approximately 500–600 μm from the anterior surface of the lens) in different lens samples were collected onto glass slides and fixed for 5 minutes with 3% paraformaldehyde before mounting with mounting medium for fluorescence (Vector Laboratories, Inc., Burlingame, CA). Fluorescent images were collected using a Leica confocal microscope.
The radial distance of the mosaic GFP fibers was quantified. For each genotype, vibratome section images of 6 lenses from 3 mice were analyzed with ImageJ. The average and standard error were plotted in Excel. Student t-test was used to determine statistical significance.
2.5 Immunocytochemistry
A previously described method was used for preparing lens cryosections for immunohistochemical staining (Gong et al., 1997). Briefly, mouse eyes were fixed with fresh 4% formaldehyde in PBS for 30 minutes, then washed with cold 1X PBS twice and soaked overnight in 30% sucrose in PBS at 4°C. Cryosections between 8–10 μm in thickness from P1 and P7 lenses were collected onto glass slides. Sections were gently washed two times with 1X PBS and one time with ddH2O and mounted with mounting medium for fluorescence with DAPI (Vector Laboratories, Inc. Burlingame, CA). Images were collected by a fluorescence microscope (Axiovert 200; Zeiss).
3. Results
3.1 The lateral surface structure of lens epithelial cells was influenced by α8 connexin while the apical surface structure was affected by a combination of α3 and α8 connexins
We evaluated the epithelial cells and lens suture formation by directly taking high-resolution images of GFP+ living lenses. In order to examine living epithelial and fiber cells, lenses were dissected out of enucleated eyeballs of GFP+ mice at P7, P14, P21 and P90 immediately before imaging. Epithelial cells exhibited a mosaic GFP expression pattern in all lenses at P7 (Figure 1) and at all other ages examined (data not shown). The size and shape of epithelial cells in connexin single knockout lenses and the wild-type lens were similar even though both α8(−/−) and DKO lenses are smaller than the wild-type and α3(−/−) lenses (Rong et al., 2002; Xia et al., 2006a). There were some small irregularities in epithelial cells, especially cells at the anterior pole, of the DKO lens (arrowheads, Figure 1).
Fig. 1.
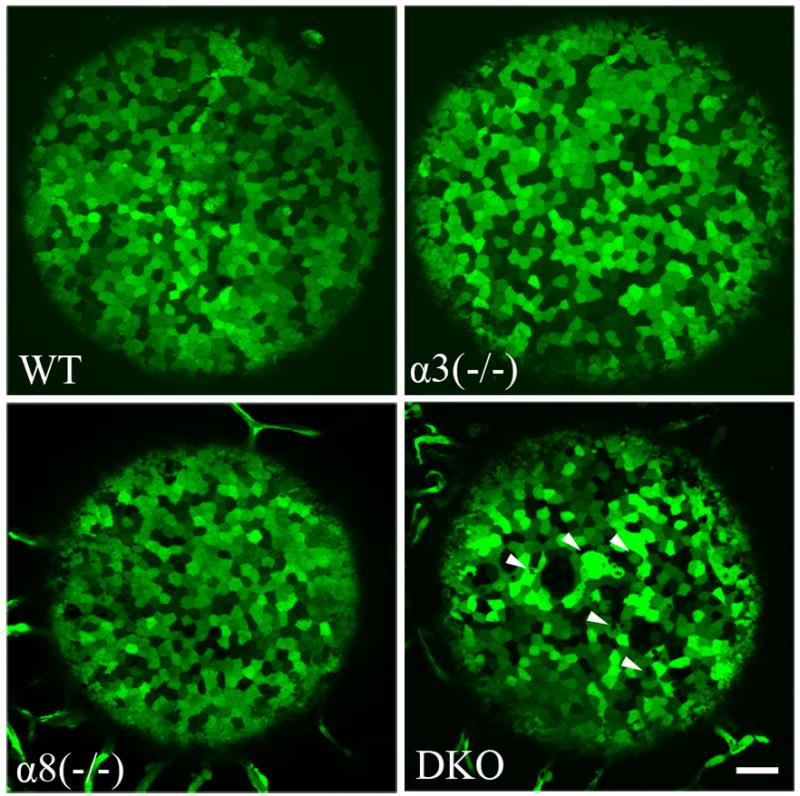
Wild-type (WT), α3(−/−), α8(−/−) and double knockout (DKO) mouse lenses were dissected from fresh eyes and imaged at postnatal day 7 (P7). The epithelial cells exhibited a mosaic expression pattern in all lenses with different genotypes. The size and shape of epithelial cells appeared to be similar in all lenses. Note that α8(−/−) and DKO lenses are smaller than WT and α3(−/−) lenses. Some small irregularities were present in the DKO epithelial cells (white arrowhead). Scale bar, 50 μm.
Using z-stacks collected from GFP+ lenses, we reconstructed and rendered 3D images of bright GFP+ epithelial cells from P7 wild-type, α3(−/−), α8(−/−) and DKO lenses (Figure 2). Lens epithelial cells in the center region were chosen for these reconstructions and comparisons. The basal surfaces (left column of Figure 2) of the epithelial cells were similar between wild-type and different mutant mice. However, the apical surface (middle column of Figure 2) of DKO epithelial cells was more smooth compared to wild-type and single knockout epithelial cells. Moreover, the lateral surfaces of both α8(−/−) and DKO cells were much more smooth (right columns of Figure 2) compared to wild-type and α3(−/−) cells. In contrast, surface structures of α3(−/−) lens epithelial cells were similar to those of wild-type. These data suggest that α8 gap junctions are crucial for the formation of lateral surface structures of epithelial cells while both α3 and α8 are essential for the normal formation of apical structures of epithelial cells.
Fig. 2.

Three-dimensional epithelial cell reconstruction using AxioVision. All samples were P7, and cells from the center of the lens anterior were used for these reconstructions. The left column shows the basal surface of epithelial cells. The middle column shows the apical surface of epithelial cells. The right columns show the lateral surface of epithelial cells. Scale bar, 20 μm. A magnified view of each cell’s lateral surface is shown to the right. Scale bar, 5 μm. DKO and α8(−/−) epithelial cells had more smooth lateral cell surfaces, and DKO cells had no processes on the apical surface.
3.2 A wide open anterior suture was observed only in DKO lenses
We further evaluated fiber cells of GFP+ living lenses from different mutant mice. A typical Y-shaped anterior suture, where the ends of opposing lens fiber cells are in contact, was observed in wild-type, α3(−/−) and α8(−/−) lenses at P7 (white dashed lines mark the center of the suture in Figure 3), and GFP fluorescent signals were uniformly distributed in these lens fibers (Figure 3). However, the ends of opposing fiber cells in DKO lenses of neonatal mice failed to contact each other, resulting in a “wide open anterior suture” (right bottom panel in Figure 3). GFP+ globules were also present in this open anterior suture. Our previous work showed that 2-week old DKO lenses appeared to have degenerating fibers in the lens core while peripheral fiber cells differentiated normally and formed end-to-end contacts at the anterior pole (Xia et al., 2006a). Thus, these GFP+ globules could result from degenerating inner fiber cells and/or abnormal epithelial cells. Similar to wild-type lenses, the suture structure at the posterior pole is normal in α3(−/−), α8(−/−) and DKO lenses, and the ends of the secondary fiber cells of DKO lenses eventually contacted each other at the posterior pole (data not shown).
Fig. 3.
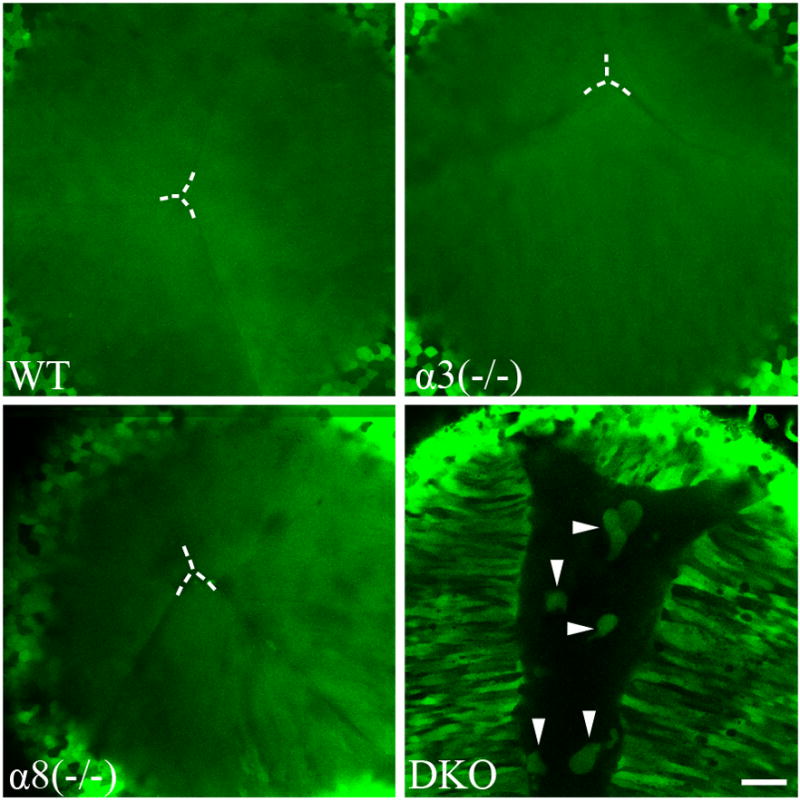
Lens fibers from WT, α3(−/−), α8(−/−) and DKO were imaged in P7 GFP+ lenses. WT and single knockout lenses had proper suture formation and uniform GFP distribution in lens fiber cells. The centers of the Y-suture in these lenses are indicated by dashed lines. The loss of both connexins in the DKO lens caused incomplete suture formation, and bright GFP+ cellular structures appeared in the center of the DKO lens (white arrowheads). DKO lens fibers did not show uniform GFP distribution. Scale bar, 50 μm.
Surprisingly, DKO lens fibers displayed a mosaic pattern of GFP expression, which differed from a uniform profile of GFP signal seen in wild-type and single knockout lens fibers. Z-stacks of lens fibers and 3D reconstruction revealed that the ends of opposing fiber cells never came in contact with each other, causing a large GFP-negative (black) chamber in the anterior lens core (Figure 4A). Thus, this chamber with an open suture is correlated to a triangle-shaped cataract seen in DKO lenses (Xia et al., 2006a). Detailed 3D reconstruction of fibers at the edge of the open Y-suture showed that fiber cells adjacent to the lumen appeared to be intact and had mosaic GFP signal and that some inner fiber cells were not fully elongated (Figure 4B). These imaging results of DKO living lenses indicate that the secondary lens fiber cells do not elongate properly to form the lens suture on time at neonatal stage, and primary lens fiber cells probably undergo degeneration in the lens core.
Fig. 4.
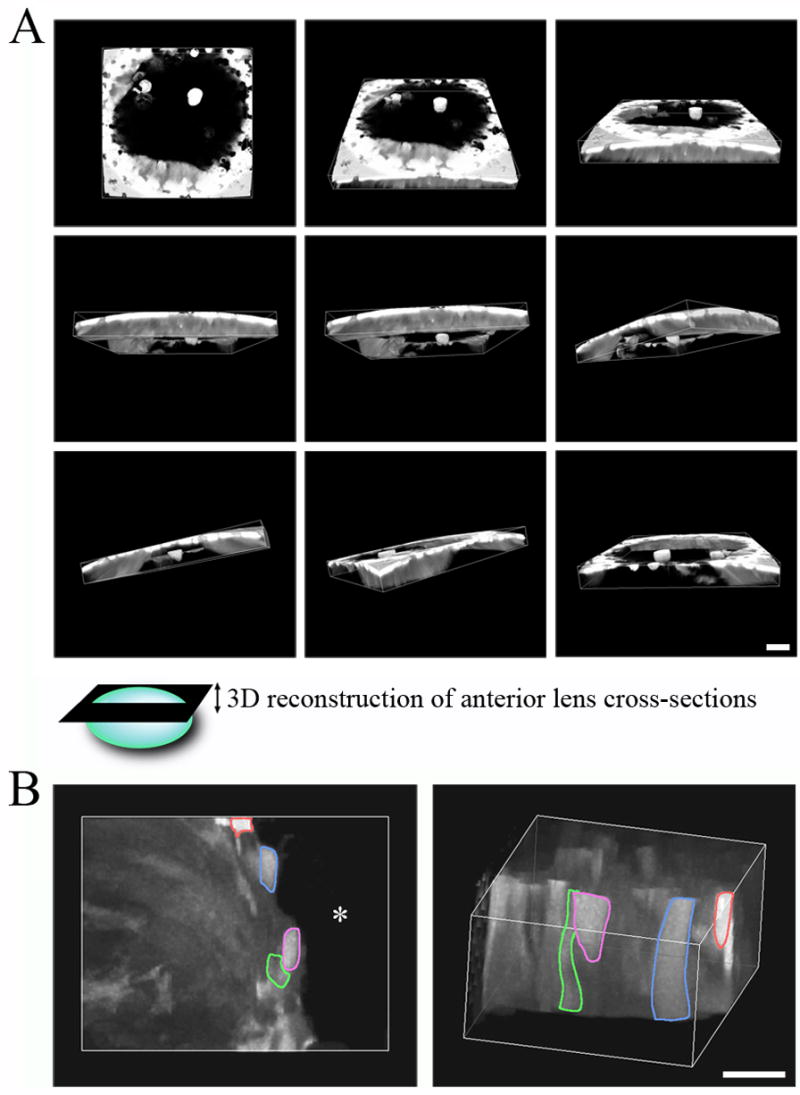
Three-dimensional reconstruction of GFP+ P7 DKO fiber cells using AxioVision. A) Each panel shows a different view of the fiber cells, demonstrating that the cells remained intact and had mosaic GFP signal. It is evident that the suture was not properly closed in the DKO lens. The epithelial cells were removed from this reconstruction. Scale bar, 50 μm. B) Detailed view of secondary fibers at the leading edge of the Y-suture. The image on the left shows the fibers in a top-down view. Four separate fibers are highlighted by different colors, and the asterisk denotes the lumen in the center of the DKO lens. The image on the right shows a lateral view, showing that fibers remained intact and had different levels of GFP signal. Loss of α3 and α8 in fibers caused incomplete fiber elongation as shown by the short fibers highlighted in red and purple. Scale bar, 20 μm.
3.3 Absence of both α3 and α8 connexin abolished the uniform distribution of GFP signal in the lens core
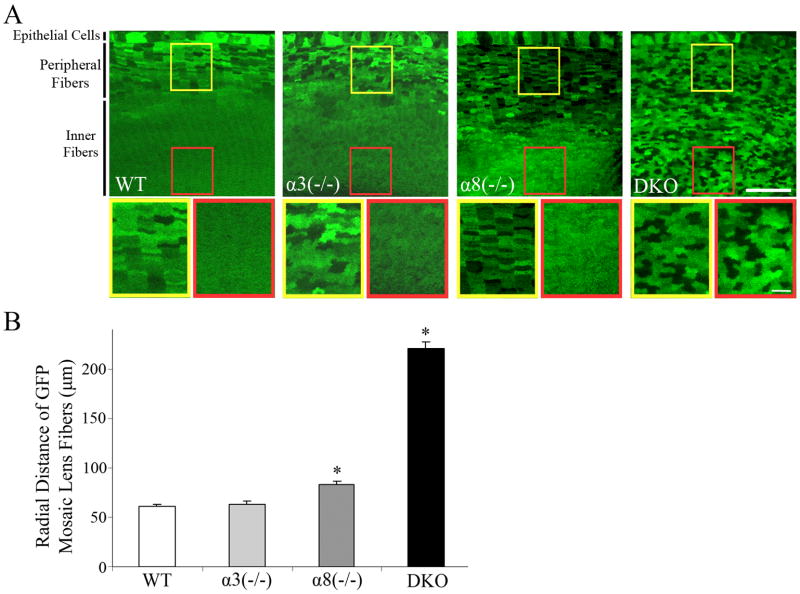
In order to confirm the mosaic GFP expression pattern in inner fibers of DKO lenses, we collected images of vibratome cross sections from the lens equatorial region. Wild-type, α3(−/−) and α8(−/−) lenses showed mosaic GFP signals in epithelial cells and peripheral fiber cells while their inner fiber cells displayed uniform GFP signals (Figure 5A). In contrast, DKO lens sections revealed that the mosaic GFP pattern remained in all lens fiber cells (Figure 5A). We further quantified the radial distance of mosaic GFP fibers, from the boundary of uniform GFP distribution to the epithelial-fiber interface, by measuring 6 lens sections of 3 mice for each genotype (Figure 5B). The mosaic GFP fiber distance is about 60 μm for wild-type and α3(−/−) lenses and about 80 μm for α8(−/−) lenses (Figure 5B). However, the mosaic GFP fiber cells were observed at a depth of 250 μm in DKO lenses, which was far deeper than the depths of 60-80 μm in wild-type, α3(−/− ) or α8(−/−) lenses. Due to the open anterior-suture and fiber cell degeneration, we were unable to collect reliable GFP signal in the DKO lens core. There is also a statistically significant difference between wild-type and α8(−/−) lenses. Therefore, these data suggest that the loss of α8 connexin slightly delays the uniform distribution of GFP in inner lens fiber cells while the loss of both α3 and α8 abolishes the uniform distribution of GFP proteins in the lens.
Fig. 5.
A) Vibratome cross sections of the equatorial region of WT, α3(−/−), α8(−/−) and DKO P10 GFP+ lenses were imaged. Scale bar, 50 μm. Magnified cortical (left) and inner fiber regions (right) are shown below each vibratome section image. Scale bar, 10 μm. The fibers cells of WT, α3(−/−) and α8(−/−) lenses had mosaic GFP expression in the periphery of the lens and uniform GFP expression in the center of the lens. In contrast, DKO lens fibers showed mosaic GFP expression throughout the lens. B) The radial distance of the GFP mosaic lens fibers was quantified from vibratome sections of 6 lenses from 3 mice of each genotype. The average and standard error were plotted for comparison. The change in radial distance of GFP mosaic fibers for α8(−/−) and DKO lenses was statistically different from WT lenses. (P> 0.001). Due to the open anterior Y-suture, we were unable to evaluate the fiber cells of DKO lenses past ~250 μm, but all fibers present in DKO vibratome sections had mosaic GFP expression.
3.4 Inner DKO lens fibers underwent maturation with delayed denucleation similar to inner α8(−/−) lens fibers
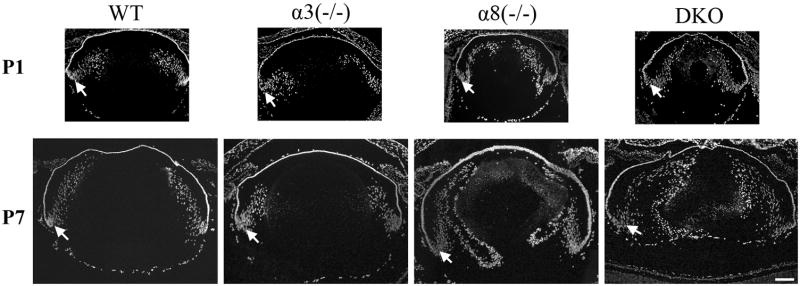
Fiber cell denucleation is a hallmark of fiber cell differentiation and maturation. As shown in Figure 5 and in a previous report (Shestopalov and Bassnett, 2003), GFP exchange between fiber cells occurs in the early stages of fiber cell differentiation before cell denucleation. In order to understand whether the mosaic GFP distribution in DKO lens fibers was associated with delayed fiber cell differentiation and maturation, we evaluated fiber cell denucleation in different mutant lenses by DAPI staining. Wild-type and α3(−/−) lenses displayed normal fiber cell denucleation that occurred at about 400 μm from the lens capsule (Figure 6). Although DKO and α8(−/−) mutant lenses showed abnormal distribution of fiber cell nuclei and a delayed denucleation process, fiber cells in the lens core had no remaining nuclei. The DKO mutant lenses had some DAPI staining in the center indicating that some nuclear remnants remained after nuclei were degraded. This result suggests gap junction communication plays a novel role in the regulation of intercellular GFP distribution besides its important role in fiber cell denucleation. Therefore, we tested whether restored gap junction communication via a knock-in α3 allele could rescue abnormalities observed in DKO lenses.
Fig. 6.
DAPI-stained cell nuclei of lens frozen sections from WT, α3(−/−), α8(−/−) and DKO mice at P1 and P7. Both WT and α3(−/−) lenses displayed normal denucleation of inner fibers while α8(−/−) and DKO lenses showed delayed denucleation of inner fibers. White arrows indicate lens bow region. Scale bar, 200 μm.
3.5 Knock-in α3 connexin restored the uniform GFP distribution in inner fiber cells
To test whether knock-in α3 connexin could restore uniform GFP signal in inner fiber cells, we generated GFP+ α3(−/−) α8(KIα3/−) mutant mice, which contained only one allele of knock-in α3 connexin without endogenous wild-type α3 or α8 connexins, and evaluated the epithelial and fiber cells of living lenses. The GFP+ α3(−/−) α8(KIα3/−) lens was clear and displayed mosaic GFP signal in epithelial cells that had normal shape and size (Figure 7A and 7B). Imaging of the lens equatorial region demonstrated that cortical fiber cells maintained a mosaic GFP expression pattern while inner fiber cells had uniform GFP distribution (Figure 7C). These results confirmed that gap junction communication facilitated by knock-in α3 connexin was sufficient to restore the uniform distribution of GFP in inner fiber cells.
Fig. 7.
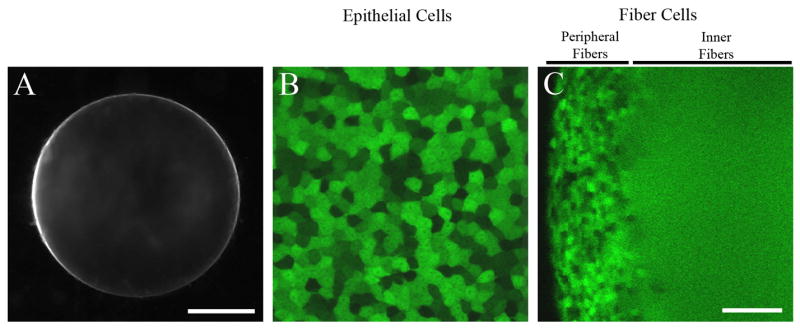
A) Lens picture of a freshly dissected α3(−/−) α8(KIα3/−) lens at the age of P16. The lens is clear, but small due to the lack of α8 connexin. Scale bar, 500 μm. B and C) GFP fluorescent images of lens epithelial cells and fiber cells in a freshly dissected P7 α3(−/−) α8(KIα3/−) lens. Both epithelial cells and peripheral fiber cells displayed a mosaic expression pattern of GFP while the inner fibers had uniform GFP signal. Scale bar, 50 μm.
4. Discussion
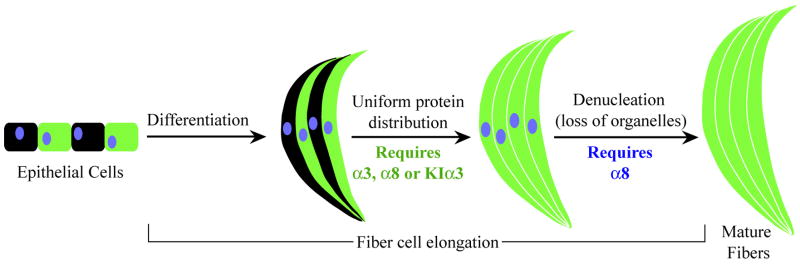
This is the first time that gap junctions (or connexins) have been demonstrated to influence the intercellular protein distribution in the lens in addition to their unique roles in fiber cell elongation and maturation (Figure 8). It is well known that gap junction channels directly transport small molecules between cells of different organs. In the lens, the regulation of protein distribution is presumably necessary for generating appropriate refractive index in the lens core (Delaye and Tardieu, 1983; Jones et al., 2005). Our previous work suggests that gap junction communication is important for fiber cell maturation processes, such as denucleation, and the maintenance of lens mature fiber cells (Gong et al., 1997; Rong et al., 2002; Xia et al., 2006a). This work suggests a novel role of intercellular gap junction communication in the regulation of intercellular distribution of macromolecules, such as GFP, in differentiated lens fiber cells. Endogenous wild-type α8 connexin alone in α3(−/−) lenses seems sufficient to support the uniform distribution of GFP and fiber cell denucleation while endogenous wild-type α3 connexin alone in α8(−/−) lenses is less sufficient, which leads to delayed GFP exchange and cell denucleation. The absence of both α3 and α8 connexins in DKO lenses abolishes GFP protein exchange in inner differentiated fiber cells, inhibits complete fiber cell elongation, disrupts denucleation and causes degeneration in the lens core. Interestingly, the knock-in α3 connexin alone is also sufficient to restore the uniform distribution of GFP. The mechanism for how gap junction communication regulates GFP protein transport in differentiated fiber cells remains unknown. The formation of macromolecular exchange pathway is one of the events occurred during the progression of fiber cell differentiation (Figure 8). We hypothesize that gap junction communication may directly influence the formation of the macromolecular exchange pathway in differentiated fiber cells or that altered gap junction communication perturbs the properties of differentiated fiber cells or the progression of fiber cell differentiation which in turn influences the formation of the macromolecular exchange pathway.
Fig. 8.
A summary of the roles of connexins during fiber cell differentiation, elongation and maturation. The diagram illustrates distinct stages during the transition of lens epithelial cells to mature fiber cells. Neither α3 nor α8 connexins are essential for the differentiation of epithelial cells to differentiating fiber cells. However, uniform distribution of GFP in inner differentiated fiber cells requires the presence of endogenous α3, endogenous α8 or knock-in α3 connexin. Normal fiber cell denucleation requires the presence of endogenous α8, but α3 connexin.
The physical nature of the macromolecule transport pathway between lens fiber cells is unclear. With about 10–15 Angstroms pore size (Fleishman et al., 2004; Oshima et al., 2007; Unger et al., 1999), gap junction channels are unlikely to directly transport the native form of GFP between differentiated fiber cells. Therefore, a different transport pathway is likely to be responsible for the uniform GFP distribution between lens inner fiber cells. Previous studies have suggested that the uniform GFP distribution in inner lens fibers is mediated by a macromolecular diffusion pathway, probably resulting from plasma membrane fusion of neighboring fibers (Shestopalov and Bassnett, 2000;2003). The presence of cell fusion has been reported in lenses of different species, such as rat and chick (Kuszak et al., 1985; Shestopalov and Bassnett, 2000). However, no reported evidence demonstrate that fiber cell fusion occurs in cortical fiber cells, which transport GFP, in mouse lenses. Substantial published evidence also contradict the fiber cell fusion model. Electrical impedance studies provide quantitative data of fiber-to-fiber coupling conductance via intercellular gap junction communication but do not detect the presence of other intercellular diffusion pathways in the lens (Baldo et al., 2001; Baldo and Mathias, 1992; Duncan et al., 1981; Martinez-Wittinghan et al., 2004). Moreover, it has been difficult to observe fiber cell fusion in wild-type, α3(−/−) or α8(−/−) mouse lenses by thin-section microscopic analysis (Dunia et al., 2006) or in wild-type mouse lenses by scanning electron microscopy (Blankenship et al., 2007) or freeze-fracture electron microscopy (Lo and Reese, 1993). In addition, α3(−/−) lenses display no electrical coupling conductance between inner fiber cells at a distance of ~200 μm and greater from the lens capsule (Gong et al., 1998). Unexpectedly, the uniform distribution of GFP in α3(−/−) lens fibers is sustained. Thus, the presence of endogenous α8 connexin in the lens cortex seems sufficient to support a uniform distribution of GFP proteins (Figure 5). Our work and a previous study clearly show that a uniform GFP distribution occurs in inner differentiated fiber cells before undergoing denucleation (Shestopalov and Bassnett, 2003). Therefore, the hypothesis that fiber cell fusion provides the macromolecular diffusion pathway remains to be evaluated. A recent study reports that the adhesion of gap junctions is necessary for their function in the neocortex (Elias et al., 2007). Future studies will be needed to clarify the nature of the pathway that mediates intercellular distribution of GFP and to elucidate how gap junction communication influences this macromolecular exchange pathway in the lens.
Using the GFP signal, we are also able to study the 3D morphology of living cells without fixation and processing artifacts associated with traditional histology techniques. Three-dimensional reconstruction of epithelial cells shows key differences between wild-type and knockout cells. DKO and α8(−/−) epithelial cells have more smooth lateral surfaces, indicating that gap junctions formed by α8 connexin are important for communication and adhesion contacts between epithelial cells. Expression of α8 connexin in epithelial cells has been reported previously, and dye transfer experiments further confirm the importance of α8 connexin in lens epithelial cell coupling (Dahm et al., 1999; White et al., 2001). However, the apical surface structures, which contact the anterior tips of fiber cells, changed only in DKO lenses. Thus, both α3 and α8 connexins probably contribute to gap junctions between epithelial and fiber cells. GFP-transgenic mutant mice are a useful system for studying cellular morphology and for investigating protein transport in the lens.
Different mechanisms are suggested to be associated with their cataract formation between α3(−/−) and DKO lenses (Gong et al., 1997; Xia et al., 2006a). We are puzzled by the fact that substantial reduction of γ-crystallin proteins, without obvious degradation, occurs in DKO lenses while degradation and aggregation of crystallin proteins are detected in α3(−/−) lenses (Xia et al., 2006a). This work reveals that the delayed elongation of the anterior ends of the secondary fiber cells in DKO lenses results in an anterior Y-shaped gap correlating to the shape of the cataract (Xia et al., 2006a). However, Inner fiber cells remain intact in DKO lenses, even though some of them fail to fully elongate. Only a limited number of degenerated cells are observed in the gap of the open anterior suture. This new morphological information provides additional evidence to support different mechanisms lead to nuclear cataract in DKO and α3(−/−) lenses.
Acknowledgments
We thank Dr. Woo-Kuen Lo for his helpful insights and discussion regarding this manuscript. We also thank other members of the Gong laboratory for the critical reading of this manuscript. This work was supported by grant EY013849 (XG) from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arneson ML, Louis CF. Structural arrangement of lens fiber cell plasma membrane protein MP20. Experimental eye research. 1998;66:495–509. doi: 10.1006/exer.1998.0477. [DOI] [PubMed] [Google Scholar]
- Baldo GJ, Gong X, Martinez-Wittinghan FJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J Gen Physiol. 2001;118:447–456. doi: 10.1085/jgp.118.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo GJ, Mathias RT. Spatial variations in membrane properties in the intact rat lens. Biophys J. 1992;63:518–529. doi: 10.1016/S0006-3495(92)81624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Experimental eye research. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. The Journal of cell biology. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship T, Bradshaw L, Shibata B, Fitzgerald P. Structural specializations emerging late in mouse lens fiber cell differentiation. Investigative ophthalmology & visual science. 2007;48:3269–3276. doi: 10.1167/iovs.07-0109. [DOI] [PubMed] [Google Scholar]
- Chen T, Li X, Yang Y, Erdene AG, Church RL. Does lens intrinsic membrane protein MP19 contain a membrane-targeting signal? Molecular vision. 2003;9:735–746. [PubMed] [Google Scholar]
- Dahm R, van Marle J, Prescott AR, Quinlan RA. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a reevaluation of its role in the lens. Experimental eye research. 1999;69:45–56. doi: 10.1006/exer.1999.0670. [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Duncan G, Patmore L, Pynsent PB. Impedance of the amphibian lens. J Physiol. 1981;312:17–27. doi: 10.1113/jphysiol.1981.sp013613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunia I, Cibert C, Gong X, Xia CH, Recouvreur M, Levy E, Kumar N, Bloemendal H, Benedetti EL. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur J Cell Biol. 2006;85:729–752. doi: 10.1016/j.ejcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Fleishman SJ, Unger VM, Yeager M, Ben-Tal N. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Molecular cell. 2004;15:879–888. doi: 10.1016/j.molcel.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Gilliland KO, Freel CD, Lane CW, Fowler WC, Costello MJ. Multilamellar bodies as potential scattering particles in human age-related nuclear cataracts. Molecular vision. 2001;7:120–130. [PubMed] [Google Scholar]
- Gilula NB, Reeves OR, Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Gong X, Baldo GJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses lacking alpha3 connexin. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15303–15308. doi: 10.1073/pnas.95.26.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Cheng C, Xia CH. Connexins in Lens Development and Cataractogenesis. J Membr Biol. 2007 doi: 10.1007/s00232-007-9033-0. [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Seminars in cell biology. 1992;3:49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- Jones CE, Atchison DA, Meder R, Pope JM. Refractive index distribution and optical properties of the isolated human lens measured using magnetic resonance imaging (MRI) Vision Res. 2005;45:2352–2366. doi: 10.1016/j.visres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Macsai MS, Bloom KJ, Rae JL, Weinstein RS. Cell-to-cell fusion of lens fiber cells in situ: correlative light, scanning electron microscopic, and freeze-fracture studies. J Ultrastruct Res. 1985;93:144–160. doi: 10.1016/0889-1605(85)90094-1. [DOI] [PubMed] [Google Scholar]
- Lo WK, Reese TS. Multiple structural types of gap junctions in mouse lens. Journal of cell science. 1993;106(Pt 1):227–235. doi: 10.1242/jcs.106.1.227. [DOI] [PubMed] [Google Scholar]
- Martinez-Wittinghan FJ, Sellitto C, Li L, Gong X, Brink PR, Mathias RT, White TW. Dominant cataracts result from incongruous mixing of wild-type lens connexins. The Journal of cell biology. 2003;161:969–978. doi: 10.1083/jcb.200303068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Wittinghan FJ, Sellitto C, White TW, Mathias RT, Paul D, Goodenough DA. Lens gap junctional coupling is modulated by connexin identity and the locus of gene expression. Investigative ophthalmology & visual science. 2004;45:3629–3637. doi: 10.1167/iovs.04-0445. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The Lens Circulation. J Membr Biol. 2007 doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiological reviews. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye (London, England) 1999;13(Pt 3b):425–437. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. 'Green mice' as a source of ubiquitous green cells. FEBS letters. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10034–10039. doi: 10.1073/pnas.0703704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. The Journal of cell biology. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation; research in biological diversity. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development (Cambridge, England) 2002;129:167–174. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. Journal of cell science. 2000;113(Pt 11):1913–1921. doi: 10.1242/jcs.113.11.1913. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Development of a macromolecular diffusion pathway in the lens. Journal of cell science. 2003;116:4191–4199. doi: 10.1242/jcs.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, King JM, Mackay DS, Bassnett S. Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2. Investigative ophthalmology & visual science. 2007;48:500–508. doi: 10.1167/iovs.06-0947. [DOI] [PubMed] [Google Scholar]
- Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeating the junctional membrane channels. Science (New York, NY) 1977;195:294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science (New York, NY) 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- White TW. Unique and redundant connexin contributions to lens development. Science (New York, NY) 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Molecular biology of the cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. The Journal of cell biology. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Sellitto C, Paul DL, Goodenough DA. Prenatal lens development in connexin43 and connexin50 double knockout mice. Investigative ophthalmology & visual science. 2001;42:2916–2923. [PubMed] [Google Scholar]
- Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Experimental eye research. 2006a;83:688–696. doi: 10.1016/j.exer.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Xia CH, Cheung D, DeRosa AM, Chang B, Lo WK, White TW, Gong X. Knock-in of alpha3 connexin prevents severe cataracts caused by an alpha8 point mutation. Journal of cell science. 2006b;119:2138–2144. doi: 10.1242/jcs.02940. [DOI] [PubMed] [Google Scholar]
- Xia CH, Liu H, Chang B, Cheng C, Cheung D, Wang M, Huang Q, Horwitz J, Gong X. Arginine 54 and Tyrosine 118 residues of {alpha}A-crystallin are crucial for lens formation and transparency. Investigative ophthalmology & visual science. 2006c;47:3004–3010. doi: 10.1167/iovs.06-0178. [DOI] [PubMed] [Google Scholar]
- Yeager M, Nicholson BJ, Elliot LH. Advances in Molecular and Cell Biology. Elsevier; 2000. Structure and biochemistry of gap junctions; pp. 31–98. [Google Scholar]