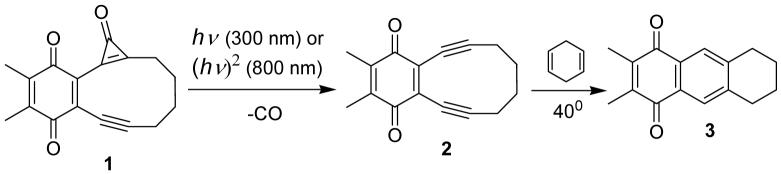
Abstract
p-Quinoid cyclopropenone-containing enediyne precursor (1) has been synthesized by mono-cyclopropanation of one of the triple bonds in p-dimethoxy substituted 3,4-benzocyclodeca-1,5-diyne followed by oxidative demethylation. Cyclopropenone 1 is stable up to 90°C but readily produces reactive enediyne 2 upon single-photon (Φ300nm = 0.46) or two-photon (σ800 nm = 0.5 GM) photolysis. The photo-product 2 undergoes Bergman cyclization at 40°C with the life time of 88 h.
Introduction
The cytotoxicity of enediyne antitumor antibiotics is attributed to the ability of the (Z)-3-ene-1,5-diyne fragment to undergo Bergman1 cyclization. The p-benzyne diradical produced in this reaction is believed to abstract hydrogen atoms from both strands of DNA, ultimately causing double-strand DNA scission.2 These natural products are highly potent antineoplastic agents, but their clinical use is hampered by inadequate anti-tumor selectivity. The cycloaromatization of enediynes is also employed in the development of novel potent nucleases3 and p-phenylene polymers for microelectronic fabrication.4 The photochemical triggering of enediyne cycloaromatization is a very attractive idea as it allows for the spatial and temporal control of the Bergman cyclization. The direct irradiation of acyclic5,6 and cyclic7 enediynes, as well as of natural antibiotic Dynemicin A,8 is known to cause light-induced cycloaromatization. However, quantum and chemical yields of this process are usually low. The efficiency of the photochemical Bergman cyclization can be substantially improved by adjusting the electronic properties of substituents9 and/or using different modes of excitation energy transfer, for example MLCT. In addition, several caged enediynes have been prepared, which undergo conventional chemical activation after the photochemical uncaging step.10
Our group explores the alternative strategy: the in situ photochemical generation of reactive enediynes from thermally stable precursors. The photo-generated enediyne then undergoes facile thermal Bergman reaction. Thus, replacement of one of the triple bonds in an enediyne structure with a cyclopropenone group produces thermally stable precursors.11 UV photolysis of these compounds results in the decarbonylation of cyclopropenone moiety12 and the formation of reactive enediynes. UV irradiation, however, is not compatible with many biomedical applications, which require the use of light in a so-called “phototherapeutic window”, a region of relative tissue transparency between 650 and 950 nm. The energy of red or NIR photons, on the other hand, is not sufficient to trigger most photochemical reactions. One of the approaches allowing for the alleviation of this problem is to employ nonresonant two-photon excitation (2PE). At high light fluxes chromophores might simultaneously absorb two red/NIR photons producing excited states same as or similar to ones accessible by excitation with UV light of twice the frequency.13 In addition, 2PE also allows for the 3-D spatial control of photo-induced processes.14 While many efficient two-photon fluorophores have been reported,15 the field of two-photon photochemistry remains relatively unexplored.16 Even fewer examples of two-photon induced activation or release of bilogicaly-relevant structures are known.17
This report describes the first two-photon induced generation of reactive enediyne, as well as the Bergman cyclization of the photoproduct (Scheme 1). We also report direct determination of the two-photon absorption cross-section of the precursor 1.
Scheme 1.
Results and Discussion
Synthesis of Cyclopropenone 1
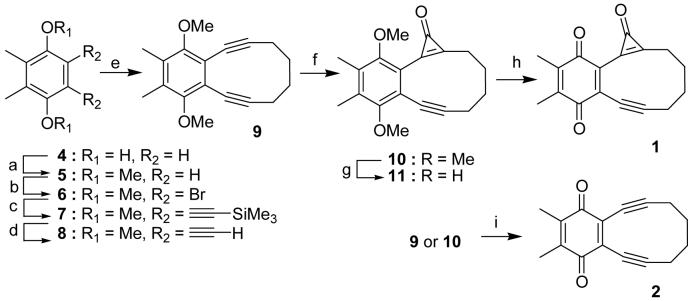
Cyclopropenone 1 was prepared in eight steps starting from 2,3-dimethyl-1,4-hydroquinone (4). The metylation of hydroquinone 4 followed by the bromination of the product 5 provided 1,2-dibromo-3,6-dimethoxy-4,5-dimethylbenzene (6) in a good yield. The Pd(0)/Cu(I) mediated coupling of dibromide 6 with trimethylsilyl acetylene in piperidine and the subsequent cleavage of trimethylsilyl protection in methanol under basic conditions afforded diacetylene 8 in 71% yield. The benzannulated enediyne 9 has been prepared by the reaction of dianion of 8 with 1,4-diiodobutane in THF-HMPA solvent. The crucial mono-cyclopropanation step was achieved by the addition of dichlorocarbene, generated in situ from chloroform and n-BuLi, followed by the hydrolysis in concentrated hydrochloric acid at -78°C to form cyclopropenone 10 in excellent yield (Scheme 2).
Scheme 2.
Reagents and conditions: (a) Me2SO4, K2CO3, acetone; (b) Br2, CHCl3, 77% (two steps); (c) HC≡CSiMe3, Pd(PPh3)2Cl2, Cul, PPh3, piperidine; (d) K2CO3, MeOH, 71% (two steps); (e) n-BuLi, I(CH2)4I, THF, HMPA, - 78°→r.t., 42%; (f) CHCl3, n-BuLi, THF, -78°C, 86%; (g) BBr3, CH2Cl2, - 78°C→r.t.; (h) FeCl3, THF, 23% (two steps); (i) CAN in aq. acetonitrile, 81% (10) or 89% (9)
The oxidative demethylation of hydroqiunone moiety of 10 proved to be challenging. Complex mixtures of ring-open products were formed under various conditions (e.g., AgO/HNO318 or H2SO4/HNO319). Treatment of 10 with CAN in aqueous acetonitrile20 resulted in clean formation enediyne 2. Recognizing that the cyclopropenone group might be extremely sensitive to strong oxidants we turned our attention to stepwise demethylation and oxidation protocols. Reaction of cyclopropenone 10 with boron tribromide gave rise to hydorquinone 11, which in turn was oxidized by FeCl3 to produce the cyclopropenone-containing enediyne precursor 1 in 23% yield. Enediyne 2 was prepared by CAN oxidation of 9 (Scheme 2).
Single-Photon Photochemistry of 1
The UV spectrum of the cyclopropenone (1) in methanol shows a strong absorption at 298 nm (logε = 3.99) and a somewhat weaker band at 393 nm (logε = 3.06, Fig 1). The irradiation of 1 in methanol using 300 nm broad-band lamps, as well as monochromatic 355 nm light from the frequency tripled Nd:YAG laser, results in the rapid decarbonylation of the substrate and the formation of enediyne 2. The quantum yield of this reaction at room temperature is Φ = 0.46 ± 0.04 at 300 nm in methanol. Incomplete photolysis (up to 40% conversion) of the precursor 1 produces only the target enediyne 2. However, further irradiation results in the formation of substantial amounts of by-products and reduces the isolated yield of 2 to 76%. The UV spectrum of the product overlaps substantially with the starting material (Fig.1), which makes us believe that the lower yield of the complete conversion photolysis is due to a secondary photochemical reaction.
Figure 1.
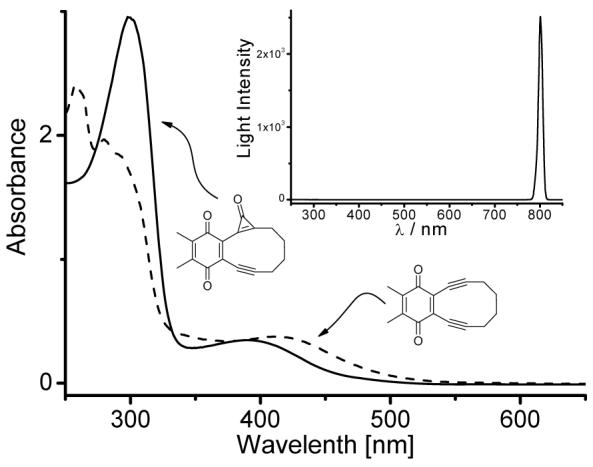
UV spectra of ca. 3*10-4 M methanol solutions of cyclopropenone 1 (solid line) and enediyne 2 (dotted line). The insert shows the spectral width of the Ti:Sapphire laser pulse.
Two-Photon induced generation of enediyne 2
The irradiation of 1 in methanol with 800 nm pulses from a Ti:Sapphire laser results in the same process as the UV photolysis, i.e. decarbonylation of the cyclopropenone group and the formation of enediyne 2. The progress of this reaction was monitored by HPLC following the disappearance of starting material 1, as well as the formation of 2 (Figure 2, Table S121). It is interesting to note that the two-photon induced decarbonylation of cyclopropenone 1 is much cleaner than the single-photon photolysis. The HPLC analysis of reaction mixtures was unable to detect any by-products in the former case.
Figure 2.
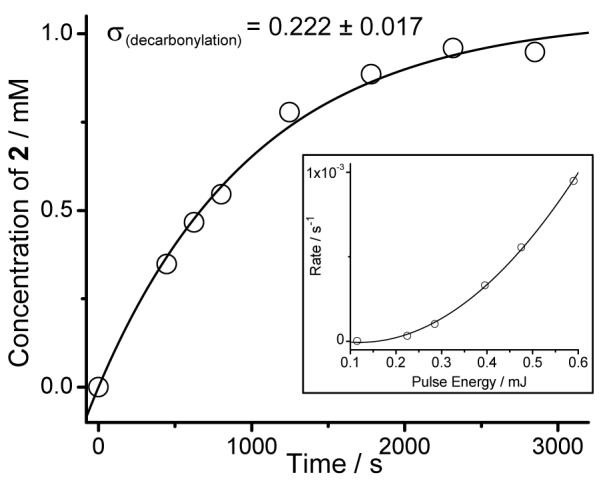
Formation of enediyne 2 in the two-photon induced decarbonylation of 1 mM methanol solutions of cyclopropenone 1. Line shown was drawn using parameters obtained by the least-squares fitting of the experimental data to eq. 1. The insert illustrates the dependence of the rate of the photochemical reaction on the pulse energy. The line shows the fit of the data to a second order polynomial equation.
The conversion of the starting material (in terms of molar concentration, C) by the two-photon induced photoreaction can be described by the Eq. (1), which has been derived from the differential form of Beer’s law for the two-photon absorption.22 In Eq. 1 I2 is a squared light flux (photons2 cm-4 s-2), which is integrated for the duration of the laser pulse, ν represents the repetition rate, and σR is a two-photon cross-section for the induction of photodecarbonylation reaction. The latter term can be further defined as σR
| (1) |
= Φ2PE*σ, where Φ2PE is a fraction of two-photon excited molecules that undergo chemical transformation, and σ is the 2PE cross-section of the substrate.
Least-squares fitting of the experimental data to the equation (Eq. 1) gave us the σR= 0.222 ± 0.017 GM.23 To convert experimentally determined two-photon cross-section for the induction of the photodecarbonylation reaction, σR, into the two-photon absorption cross-sections of enediyne precursor 1, we need to know the fraction of two photon excited molecules that undergo decarbonylation, Φ2PE. The excited state initially populated upon two-photon excitation might be different from that initially populated upon single-photon excitation. However, according to Kasha’s rule, photochemical reactions generally occur from the lowest singlet or triplet excited states regardless of the excitation method and the initial exited state.24 Thus, we can assume that the quantum yield of two-photon initiated process is equal to its single-photon counterpart, Φ2PE = ΦSPE. The two-photon absorption cross-sections of cyclopropenone 1, is, therefore, equal to σ2PE(800) = σR(800) / ΦSPE = 0.483 ± 0.058 GM.
Bergman cyclization of 2,3(octa-1,7-diyne-1,8-diyl)-5,6-dimethylhydroqiunone 1,4-dimethyl ether (2)
The enediyne 2 undergoes efficient Bergman cyclization upon heating in the degassed benzene at 75°C in the presence of 1,4-cyclohexadiene. The starting material is completely consumed within 4 h producing 2,3-dimethyl-5,6,7,8-tetrahydroanthracene-1,4-dione (3) in 87% yield (Scheme 1). The rate of Bergman cyclization of 2 was measured in a 2-propanol solution at 40°C. The progress of the reaction was followed by HPLC (Fig. 3).
Figure 3.
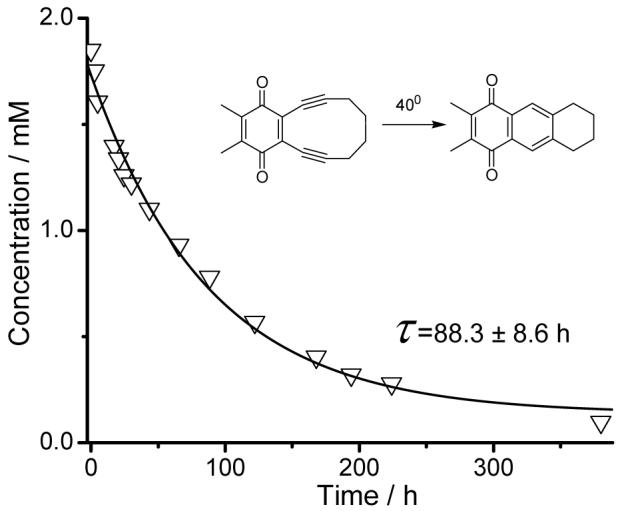
Decay of enediyne 2 in 2-propanol at 40°C. Curve represents the calculated fit to a single exponential equation.
The observed rate of the Bergman cyclization of 2, k40°C = (3.14 ± 0.31)*10-6 s-1, is much faster than that of the parent 3,4-benzocyclodeca-1,5-diyne. The latter is stable below 50°C and cyclize at 84°C with k = 8 *10-6 s-1.25 Direct comparison of these rates, however, should be done with caution because the rate of enediyne cyclization is known to depend on the solvent, as well as on the concentration and nature of hydrogen donor.
It is important to note that the starting cyclopropenone 1 is perfectly stable at 40°C and shows no signs of decomposition up to 90°C.
Conclusions
We have shown the feasibility of the in situ two-photon induced generation of reactive enediynes using light within the “phototherapeutic window”. The cyclopropenone-containing enediyne precursor 1 is stable in the dark even at elevated temperatures but undergoes efficient photo-decarbonylation producing reactive enediyne 2. The latter undergoes Bergman cyclization at biologically relevant temperatures. The two-photon induced photochemical reaction of 1 is much cleaner because it is not accompanied by the secondary photochemistry.
Experimental Section
Single-Photon Photochemistry
The preparative and analytical photolyses of 1 were conducted in methanol solutions using Rayonet photoreactor. Preparative 300 nm irradiation of 1 in methanol allowed us to isolate 2,3-(octa-1,7-diyne-1,8-diyl)-5,6-dimethyl-1,4-benzoquinone (2), which was found to be identical to the sample prepared independently. The quantum yield of the photo-decarbonylation reaction of 1 was measured in methanol solutions using ferrioxalate actinometry.26
Two-photon Induced Enediyne Generation
TPE experiments were conducted using 800 nm pulses generated by an amplified Ti:Sapphire laser operating at 1 kHz. The laser beam was attenuated by a diaphragm with a 6.15 mm opening. The power output of the laser after the diaphragm was 0.61 W, which was reduced to 0.55 W after passing through the sample. At the concentration of the substrate used in these experiments the loss of energy after the sample is mostly due to the losses on the phase boundaries, which allows us to evaluate the laser power within the sample as 0.58 W or 580 μJ per pulse-1. The shape of the laser pulse was determined to be close to Gaussian with the half-height width of 94 fs. Using these parameters we have calculated the distribution of light intensity and squared light intensity within the pulse assuming ideal Gaussian shape of the pulse. For the integration of the squared light intensity we have selected the integration limits of ± 100 fs from the center of the pulse, as the value of I2 at these extremes drops to less than 0.2% of the maximum.
1,2-dibromo-3,6-dimethoxy-4,5-dimethylbenzene (6).27
A suspension of 2,3-dimethyl-1,4-hydroquinone (4) (5 g, 36.23 mmol), Me2SO4 (10.4 g, 72.46 mmol), and K2CO3 (25 g) in acetone (300 mL) was refluxed for 24 hours under argon. The reaction mixture was cooled to room temperature, filtered, and concentrated in vacuum. The oily residue was dissolved in ethyl acetate - hexanes (1:12) mixture, passed through a short silica gel column, and concentrated under vacuum to give 5.2 g of crude 2,3-dimethyl-1,4-dimethylbenzene (5).
A solution of bromine (11 g, 68.3 mmol) in 50 mL of chloroform was added dropwise to a solution of crude 5 in chloroform (100 mL), the resulting mixture was protected from light and stirred for 60 min at room temperature. The reaction mixture was washed with aqueous solution of Na2S2O5, water, dried over anhydrous MgSO4, and concentrated. The residue was purified by chromatography on silica gel (ethyl acetate - hexanes 1:20) to give 9 g (27.8 mmol, 77%) of 1,2-dibroro-3,6-dimethoxy-4,5-dimethylbenzane (6). Rf=0.55 (ethyl acetate - hexanes 1:5); Mp 117-118°C, lit 117-119°C.
1,2-Diethynyl-3,6-dimethoxy-4,5-dimethylbenzene (8).28
Pd(PPh3)2Cl2 (1 g, 1.43 mmol), Cul (0.36 g, 1.88 mmol), and trimethylsilylacetylene (17.0 g, 173 mmol) were added to a degassed solution of dibromide 6 (8 g, 24.70 mmol) in piperidine (ca 120 mL) at room temperature. The reaction vessel was sealed and the mixture was stirred for 24 hours at 85°C. After cooling to room temperature, the reaction mixture was filtered and concentrated in vacuum. The residue was dissolved in ethyl acetate - hexanes (1:30) mixture, passed through a short silica gel column, and concentrated in vacuum to give crude 1,4-dimethoxy-2,3-dimethyl-5,6-bis-trimethylsilanylethynylbenzene (7). Rf=0.36 (ethyl acetate - hexanes 1:20); 1H NMR (300 MHz, CDCl3) δ 3.82 (s, 6 H), 2.16 (s, 6 H), 0.27 (s, 18 H); 13C NMR (75 MHz, CDCl3) δ 155.9, 132.5, 117.8, 102.3, 99.6, 60.5, 12.8, 0.0; MS calc for C20H30 O2Si2 (M+) 358, found 358.
A methanol solution of crude diacetylene 7 was added to a stirred suspension of K2CO3 (14 g, 100 mmol) in methanol (120 mL), and the resulting mixture was stirred for 1 h at room temperature. The reaction was quenched by saturated aqueous NH4Cl, most of solvent was removed in vacuum. Ethyl acetate was added to the mixture, organic layer separated, washed with water, brine, dried over anhydrous MgSO4, and concentrated in vacuum. The residue was purified by chromatography on silica gel (ethyl acetate - hexanes 1:25) to give 3.76 g of 8 (17.57 mmol, 71% over two steps) as a white powder. Rf=0.38 (ethyl acetate - hexanes 1:5); 1H NMR (300 MHz, CDCl3) δ 3.81 (s, 6 H), 3.49 (s, 2 H), 2.17 (s, 6 H); 13C NMR (75 MHz, CDCl3) δ 156.2, 133.1, 117.2, 84.5, 78.3, 60.8, 12.9; MS calc for C14H14 O2 (M+) 214, found 214.
2,3-(octa-1,7-diyne-1,8-diyl)-5,6-dimethyl-1,4-hydroquinone dimethyl ether (9)
n-BuLi (2.5M solution in hexanes, 7.85 mL, 19.6 mmol) was added to a stirred solution of 1,2-diethynyl-3,6-dimethoxy-4,5-dimethylbenzene (8) (2.0 g, 9.35 mmol) in THF (400 mL) and HMPA (20 mL) at -78°C under argon. After two hours at this temperature, 1,4-diiobutane (2.91 g, 9.40 mmol) was added dropwise, the reaction mixture was allowed to reach room temperature, and stirred for a 24h. The reaction was quenched by addition of phosphate buffer, partially concentrated, diluted with hexanes passed through a short silica gel column, and concentrated in vacuum. The residue was purified by chromatography on silica gel (ethyl acetate - hexanes 1:30→1:25) to give 1.05 g (3.91 mmol, 42%) of 2,3-(octa-1,7-diyne-1,8-diyl)-5,6-dimethylhydroquinone 1,4-dimethyl ether (9) as colorless crystals, which decompose upon heating. Rf=0.40 (ethyl acetate - hexanes 1:5); 1H NMR (300 MHz, CDCl3) δ 3.85 (s, 6 H), 2.49 (m, 4 H), 2.16 (s, 6 H), 1.96 (m, 4 H); 13C NMR (75 MHz, CDCl3) δ 153.3, 130.8, 120.8, 102.4, 79.0, 60.8, 28.6, 21.9, 12.7; HRMS calc for C18H20 O2 (M+) 268.1463, found 268.1462.
Cyclopropenone 10
A solution of n-BuLi (2.5M solution in hexanes, 2.34 mL, 5.87 mmol) was added dropwise over ca 1.5 hours to a stirred solution of enediyne 9 and CHCl3 (0.8 g 6.67 mmol) in THF at -78°C. The resulting solution was stirred for 30 min, quenched by 3 mL of concentrated HCl and slowly warmed to a room temperature. Most THF was removed in vacuum. The reaction mixture was diluted with ether, washed with saturated solution of NaHCO3, water, brine, dried over anhydrous MgSO4, and concentrated. The residue was purified by chromatography on silica gel (CH2Cl2 - hexanes 1:5 → CH2Cl2 → ethyl acetate) to give 0.353 g (1.19 mmol, 86% calculated on recovered enediyne) of cyclopropenone 10 as dark orange oil, and 0.347 g (1.29 mmol) of enediyne 9. Rf=0.42 (ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 3.91, 3.89 (s, 6 H), 3.04 (t, J=6.6 Hz, 2 H), 2.52 (t, J=5.4 Hz, 2 H), 2.26 (s, 6 H), 2.09-2.05 (m, 2 H), 1.8-1.72 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ 157.7, 156.6, 155.2, 154.3, 153.2, 136.4, 132.3, 120.8, 114.9, 102.0, 96.1, 78.9, 62.5, 61.0, 26.39, 26.41, 26.0, 18.8, 13.2, 12.8; IR (CCl4) 2934 (m), 2858 (w), 1840 (s), 1627 (s), 1461 (m), 1397 (m); HRMS calc for C19H20 O3 (M+) 296.1412, found 296.1414.
Cyclopropenone 1
Solution of BBr3 (1 M in CH2Cl2, 7 mL, 7 mmol) was added dropwise to a solution of dimethyl ether 10 (0.45 g, 1.52 mmol) in 80 mL of CH2Cl2 at - 78°C. The resulting mixture was stirred for 4 h at -78°C, slowly warmed to room temperature, and stirred for another 4 h. Reaction was quenched by water (50 mL), organic layer separated, and the aqueous layer was extracted with CH2Cl2. Combined organic layers were dried over anhydrous sodium sulfate, and concentrated in vacuum to give 0.2 g of crude hydroquinone 11. 1H NMR (300 MHz, CDCl3) δ 2.91 (t, J=6.6 Hz, 2 H), 2.52 (t, J=6.0 Hz, 2 H), 2.24, 2.23 (s, 6 H), 2.05-1.95 (m, 2 H), 1.83-1.75 (m, 2 H); MS calc for C17H16 O3 (M+) 268, found 268.
Anhydrous FeCl3 was added to the solution of crude hydroquinone 11 in 30 mL of THF at room temperature. The reaction mixture was stirred for 30 min, and quenched with water and ethyl acetate. The organic layer was separated, washed with water, brine, dried over anhydrous sodium sulfate, and concentrated in vacuum. The residue was purified by chromatography on silica gel (ethyl acetate - hexanes 5:1) to give 92 mg (0.346 mmol, 23% over two steps) of quinoid cyclopropenone 1 as deep orange oil, which crystallizes upon standing in the refridgerator. Rf=0.29 (ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 2.96 (t, J=6.6 Hz, 2 H), 2.55 (t, J=5.4 Hz, 2 H), 2.058, 2.045 (s, 6 H), 1.73-1.64 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ 182.9, 182.2, 163.1, 157.2, 152.0, 141.9, 141.3, 133.7, 130.3, 117.2, 79.1, 27.1, 26.3, 26.05, 19.6, 12.62, 12.58; HRMS calc for C17H14 O3 (M+) 266.0943, found 266.0931.
2,3-(Octa-1,7-diyne-1,8-diyl)-5,6-dimethyl quinone (2)
A solution of 9 (0.1 g, 0.373 mmol) in 5 mL of acetonitrile was added to a stirred solution of CAN (1.63 g, 2.98 mmol) in 10 mL of acetonitrile - water mixture (1:1). Reaction mixture was stirred for 45 min, diluted with water and CH2Cl2. The organic layer was separated, the aqueous layer was extracted with 2X 5 mL of CH2Cl2. Combined organic layers were washed with water and dried over anhydrous sodium sulfate. The solvent was removed in vacuum to give 79 mg (0.332 mmol, 89%) of quinone 2 as yellow crystalline material. Mp 118 - 120°C with decomposition. Rf=0.25 (EtOAc:Hex 1:5); 1H NMR (300 MHz, CDCl3) δ 2.51 (s, b, 4 H), 2.00 (s, 6 H), 1.96-1.90 (m, 4 H); 13C NMR (75 MHz, CDCl3) δ 182.6, 140.8, 137.3, 114.9, 79.6, 28.2, 22.4, 12.5; HRMS calc for C16H14 O2 (M+) 238.0994, found 238.0997.
2,3-dimethyl-5,6,7,8-tetrahydroanthracene-1,4-dione (3)
A solution of 2 (0.087 g, 0.37 mmol) in benzene:1,4-cyclohaxadiene (4:1, 15 mL) was degassed, the reaction vessel was sealed and the mixture was stirred for ∼5 hours at 75°C. After cooling down and removing solvent the residue was by chromatography on silica gel (ethyl acetate - hexanes 1:1) to give to give 78 mg (0.33 mmol, 87%) of 3 as a yellow crystals. Mp 159 - 161°C. Rf=0.25 (EtOAc:Hexanes 1:5); H NMR (300 MHz, CDCl3) δ 7.73 (s, 2 H), 2.87 (m, 4 H), 2.15 (s, 6 H), 1.83 (m. 4 H); 13C NMR (75 MHz, CDCl3) δ 185.1, 143.5, 143.2, 129.7, 127.0, 29.7, 22.6, 12.8; HRMS calc for C16H16 O2 (M+) 240.1150, found 240.1143.
Supplementary Material
Acknowledgment
Authors thank the National Institutes of Health (grant CA91856-01A1) and the National Science Foundation (grant CHE-0449478) for the support of this project, as well as the Ohio Laboratory for Kinetic Spectroscopy for the use of instrumentation and technical assistance. A.P. thanks the McMaster Endowment for a research fellowship.
References
- 1.Bergman RG. Acc.Chem.Res. 1973;6:25. [Google Scholar]
- 2.For recent reviews see:Borders DB, Doyle TW, editors. Enediyne Antibiotics as Antitumor Agents. Marcel Dekker; New York: 1995. Jones GB, Fouad FS. Curr.Pharm.Design. 2002;8:2415. doi: 10.2174/1381612023392810.Nicolaou KC, Dai W-M. Angew.Chem., Int.Ed.Engl. 1991;30:1387.Nicolaou KC, Smith AL. Acc.Chem.Res. 1992;25:497.Danishefsky SJ, Shair MD. J. Org. Chem. 1996;61:16.
- 3.Dai W-M, Lai KW, Wu A, Hamaguchi W, Le MYH, Zhou L, Ishii A, Nishimoto S. J.Med.Chem. 2002;45:758. doi: 10.1021/jm015588e. [DOI] [PubMed] [Google Scholar]; Tuntiwechapikul W, David WM, Kumar D, Salazar M, Kerwin SM. Biochemistry. 2002;41:5283. doi: 10.1021/bi025535e. [DOI] [PubMed] [Google Scholar]; Rawat DS, Zaleski JM. J.Am.Chem.Soc. 2001;123:9675. doi: 10.1021/ja011215r. [DOI] [PubMed] [Google Scholar]; Jones GB, Hynd G, Wright JM, Purohit A, Plourde G, II, Huber RS, Mathews JE, Li A, Kilgore MW, Bubley GJ, Yancisin M, Brown MA. J.Org.Chem. 2001;65:3688. doi: 10.1021/jo0055842. [DOI] [PubMed] [Google Scholar]; Takahashi T, Tanaka H, Yamada H, Matsumoto T, Sugiura Y. Angew. Chem., Int. Ed. Engl. 1977;36:1524. [Google Scholar]
- 4.Smith DW, Jr., Babb DA, Snelgrove RV, Townsend PH, III, Martin SJ. J. Am. Chem. Soc. 1998;120:9078. [Google Scholar]; John JA, Tour JM. Tetrahedron. 1997;53:15515. [Google Scholar]; Chen X, Tolbert LM, Hess DW, Henderson C. Macromolecules. 2001;34:4104. [Google Scholar]; Shah HV, Babb DA, Smith DW., Jr. Polymer. 2000;41:4415. [Google Scholar]
- 5.Kagan J, Wang X, Chen X, Lau KY, Batac IV, Tuveson RW, Hudson JB. J.Photochem.Photobiol. B. 1993;21:135. doi: 10.1016/1011-1344(93)80175-9. [DOI] [PubMed] [Google Scholar]; Turro NJ, Evenzahav A, Nicolaou KC. Tetrahedron Lett. 1994;35:8089. [Google Scholar]; Kaneko T, Takahashi M, Hirama M. Angew.Chem.Int.Ed. 1999;38:1267. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1267::AID-ANIE1267>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]; Plourde G, II, El-Shafey A, Fouad F, Purohit A, Jones G. Bioorg.Med.Chem.Lett. 2002;12:2985. doi: 10.1016/s0960-894x(02)00618-2. [DOI] [PubMed] [Google Scholar]
- 6.Benites PJ, Holmberg RC, Rawat DS, Kraft BJ, Klein LJ, Peters DG, Thorp HH, Zaleski JM. J.Am.Chem.Soc. 2003;125:6434. doi: 10.1021/ja020939f. [DOI] [PubMed] [Google Scholar]; Kraft B,J, Coalter NL, Nath M, Clark AE, Siedle AR, Huffman JC, Zaleski JM. Inorg.Chem. 2003;42:1663. doi: 10.1021/ic0207045. [DOI] [PubMed] [Google Scholar]
- 7.Choy N, Blanco B, Wen J, Krishan A, Russell KC. Org. Lett. 2000;2:3761. doi: 10.1021/ol006061j. [DOI] [PubMed] [Google Scholar]; Funk RL, Young ERR, Williams RM, Flanagan MF, Cecil TL. J.Am.Chem.Soc. 1996;118:3291. [Google Scholar]
- 8.Shiraki T, Sugiura Y. Biochemistry. 1990;29:9795. doi: 10.1021/bi00494a006. [DOI] [PubMed] [Google Scholar]
- 9.Alabugin IV, Manoharan M. J.Phys.Chem.A. 2003;107:3363. [Google Scholar]; Alabugin IV, Manoharan M, Kovalenko SV. Org.Lett. 2002;4:1119. doi: 10.1021/ol0255054. [DOI] [PubMed] [Google Scholar]; Clark AE, Davidson ER, Zaleski JM. J.Am.Chem.Soc. 2001;123:2650. doi: 10.1021/ja0039987. [DOI] [PubMed] [Google Scholar]
- 10.Nicolaou KC, Dai W-M, Wendeborn SV, Smith AL, Torisawa Y, Maligres P, Hwang C-K. Angew.Chem.Int.Ed.Engl. 1991;30:1032. [Google Scholar]; Wender PA, Zercher CK, Beckham S, Haubold E-M. J.Org.Chem. 1993;58:5867. [Google Scholar]; Basak A, Bdour HM, Shain JC, Mandal S, Rudra KR, Nag S. Bioorg.Med.Chem.Lett. 2000;10:1321. doi: 10.1016/s0960-894x(00)00237-7. [DOI] [PubMed] [Google Scholar]
- 11.Poloukhtine A, Popik VV. Chem.Commun. 2005:617. doi: 10.1039/b414951c. [DOI] [PubMed] [Google Scholar]; Poloukhtine A, Popik VV. J.Org.Chem. 2005;70:1297. doi: 10.1021/jo048065y. [DOI] [PubMed] [Google Scholar]
- 12.Poloukhtine A, Popik VV. J.Org.Chem. 2003;68:7833. doi: 10.1021/jo034869m. [DOI] [PubMed] [Google Scholar]
- 13.Göppert-Mayer M. Ann.Phys.(Leipzig) 1931;9:273. [Google Scholar]; Lakowicz JR. Two-Photon And Non-Linear Induced Fluorescence. Plenum Press; New York: 1991. [Google Scholar]; Callis PR. Ann.Phys.Rev.Chem. 1997;48:271. doi: 10.1146/annurev.physchem.48.1.271. [DOI] [PubMed] [Google Scholar]; Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grötzinger C. Nature Biotech. 2001;19:327. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]; Rubat M. Circ.Res. 2004;95:1154. doi: 10.1161/01.RES.0000150593.30324.42. [DOI] [PubMed] [Google Scholar]; Kauffman JF, Turner JM, Alabugin IV, Breiner B, Kovalenko SV, Badaeva EA, Masunov A, Tretiak S. J.Phys.Chem. A. 2006;110:241. doi: 10.1021/jp056127y. [DOI] [PubMed] [Google Scholar]
- 14.König K. J.Microsc. 2000;200:2. doi: 10.1046/j.1365-2818.2000.00738.x. [DOI] [PubMed] [Google Scholar]; Weissleder R. Nature Biotech. 2001;19:316. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]; Brown EB, Shear JB, Adams SR, Tsien RY, Webb WW. Biophys.J. 1999;76:489. doi: 10.1016/S0006-3495(99)77217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao S, Belfield KD. J.Org.Chem. 2005;70:5156. doi: 10.1021/jo0503512. [DOI] [PubMed] [Google Scholar]; Yang WJ, Kim DY, Jeong M-Y, Kim HM, Lee YK, Fang X, Jeon S-J. Chemistry. 2005;11:4198. doi: 10.1002/chem.200401134. [DOI] [PubMed] [Google Scholar]; Drobizhev M, Stepanenko Y, Dzenis Y, Karotki A, Rebane A, Taylor PN, Anderson HL. J.Am.Chem.Soc. 2004;126:15352. doi: 10.1021/ja0445847. [DOI] [PubMed] [Google Scholar]; Pond SJK, Tsutsumi O, Rumi M, Kwon O, Zojer E, Bredas J-L, Marder SR, Perry JW. J.Am.Chem.Soc. 2004;126:9291. doi: 10.1021/ja049013t. [DOI] [PubMed] [Google Scholar]; Beverina L, Fu J, Leclercq A, Zojer E, Pacher P, Barlow S, Van Stryland EW, Hagan DJ, Bredas J-L, Marder SR. J.Am.Chem.Soc. 2005;127:7282. doi: 10.1021/ja050688l. [DOI] [PubMed] [Google Scholar]
- 16(a).Belfield KD. Spectrum. 2001;14:1. [Google Scholar]; b) Marder SR, et al. Nature. 1999;398:51. [Google Scholar]; c) Fedoryak OD, Dore TM. Org.Let. 2002;4:3419. doi: 10.1021/ol026524g. [DOI] [PubMed] [Google Scholar]; d) Kim H-C, Kreiling S, Greiner A, Hampp N. Chem.Phys.Let. 2003;372:899. [Google Scholar]; e) Dyer J, Jockusch S, Balsanek V, Sames D, Turro NJ. J.Org.Chem. 2005;70:2143. doi: 10.1021/jo048053c. [DOI] [PubMed] [Google Scholar]; f) Goodwin AP, Mynar JL, Ma Y, Fleming GR, Frechet JMJ. J.Am.Chem.Soc. 2005;127:9952. doi: 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]; g) Urdabayev NK, Popik VV. J.Am.Chem.Soc. 2004;126:4058. doi: 10.1021/ja0497328. [DOI] [PubMed] [Google Scholar]
- 17.Nikolenko V, Yuste R, Zayat L, Baraldo LM, Etchenique R. Chem.Commun. 2005:1752. doi: 10.1039/b418572b. [DOI] [PubMed] [Google Scholar]; Fedoryak OD, Sul J-Y, Haydon PG, Ellis-Davies GCR. Chem.Commun. 2005:3664. doi: 10.1039/b504922a. [DOI] [PubMed] [Google Scholar]; Zhao YR, Zheng Q, Dankin K, Xu K, Martinez ML, Li W-H. J.Am.Chem.Soc. 2004;126:4653. doi: 10.1021/ja036958m. [DOI] [PubMed] [Google Scholar]; Lu M, Fedoryak OD, Moister BR, Dore TM. Org.Lett. 2003;5:2119. doi: 10.1021/ol034536b. [DOI] [PubMed] [Google Scholar]; Kiskin NI, Chillingworth R, McCray JA, Piston D, Ogden D. Eur.Biophys.J. 2002;30:588. doi: 10.1007/s00249-001-0187-x. [DOI] [PubMed] [Google Scholar]; Furuta T, Wang SSH, Dantzker JL, Dore TM, Bybee WJ, Callaway EM, Denk W, Tsien RY. Proc.Natl.Acad.Sci.USA. 1999;96:1193. doi: 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adams SR, Lev-Ram V, Tsien RY. Chem.Biol. 1997;4:867. doi: 10.1016/s1074-5521(97)90119-8. [DOI] [PubMed] [Google Scholar]
- 18.Parker KA, Spero DM, Koziski KA. J.Org.Chem. 1987;52:183. [Google Scholar]
- 19.Waterlot C, Haskiak B, Couturier DJ. Chem. Res. Synop. 2000;3:106. [Google Scholar]
- 20.Jacob P, Callery PS, Shulgin AT, Castagnoli N. J.Org.Chem. 1976;41:3627. doi: 10.1021/jo00884a035. [DOI] [PubMed] [Google Scholar]
- 21.Supporting information
- 22.Urdabaev NK, Poloukhtine A, Popik VV. Chem.Comm. 2006:454. doi: 10.1039/b513248g. [DOI] [PubMed] [Google Scholar]
- 23.1 GM = 10-50 cm4 s photon-1 molecule-1
- 24.Turro NJ. Modern Molecular Photochemistry. University Science Books; Sausalito: 1991. [Google Scholar]
- 25.Semmelhack MF, Neu T, Foubelo F. J.Org.Chem. 1994;59:5038. [Google Scholar]; Semmelhack MF, Sarpong R. J.Phys.Org.Chem. 2004;17:807. [Google Scholar]
- 26.Murov SL, Carmichael I, Hug GL. Handbook of Photochemistry. Marcel Dekker; New York: 1993. p. 299. [Google Scholar]
- 27.Lawson D, McOmie J, West D. J.Chem.Soc.C. 1968:2414. [Google Scholar]
- 28.Nishinaga T, Miyata Y, Nodera N, Komatsu K. Tetrahedron. 2004;60:3375. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.