Abstract
The Pfizer Healthcare Informatics team conducted a series of guided interviews with 35 Pfizer senior leaders to elicit their understanding, desires, and expectations of how Electronic Health Records (EHR) might be used in the pharmaceutical industry today and/or in the future. The interviews yielded fourteen use case categories comprising 42 specific use cases. The highest priority use cases were “Drug Safety & Surveillance,” “Clinical Trial Recruitment,” and “Support Regulatory Approval.” Fifteen EHR companies were surveyed to assess their functionality against the specified use cases. Self-reported responses from the EHR companies were highest for “Virtual Phase IV Trials” and “Document Management for Clinical Trials.” This research identifies preliminary opportunities for EHR products to provide aggregate, blinded data to address the interests of the pharmaceutical industry. However, further collaboration between the stakeholders will be necessary to ensure the full realization of the opportunities for data re-use.
Introduction
The pharmaceutical industry is an information intense business, relying on the proper utilization of information in order to effectively develop and deliver its medicines to patient populations. The information can be created from within organizations through ongoing clinical trials (Phase I, II, III, and/or IV), or through real-world observational data after a product receives regulatory approval from the FDA. Real-world situations may consist of patients on multiple medications, co-morbidities, wider patient demographic, and provide a different type of insight as compared to traditional randomized clinical trials. Until recently, real-world observational data generally consisted of claims data providing limited perspective. But as the nation continues towards increasing utilization of electronic health records (EHRs), the potential value of ancillary activities such as monitoring quality, 1 assessing population health, 2 and clinical research, 3 is becoming possible.
Opportunities for EHR-generated patient data to support data re-use, and clinical research in particular, were highlighted during the First National Health Information Network (NHIN) Forum, 4 and testimony to the National Committee for Vital and Health Statistics (NCVHS). 5 In addition, the American Medical Informatics Association (AMIA) developed a national framework for data re-use, 6 regarding issues of identification, data stewardship, control, and access for the use cases of Quality, Public Health, Research, and Commercial Uses, 7 with subsequent testimony to NCVHS. 8 Despite this growing awareness, there is little specific research assessing the capabilities of EHRs for clinical research or other areas that are of interest to the commercial practices of the pharmaceutical industry. This case study attempts to identify the areas of value for EHRs to support data re-use to address the needs of pharmaceutical manufacturers.
Case Description
Pfizer is the world's largest research-based biomedical and pharmaceutical company, with over $7.6B invested in research and development in 2006, including over 240 novel compounds in development, 9 and as of November 2005, had 324 clinical trials registered on ClinicalTrials.gov. 10 To manage all the active clinical trials, Pfizer has enabled electronic data capture (EDC) for many clinical trials and for new clinical trial startups. In an effort to access information more efficiently, Pfizer had started to investigate potential opportunities around how electronic health records (EHRs) might be used to augment, supplement, or replace EDC based systems. In addition, other departments (e.g., Outcomes Research, Safety) within Pfizer traditionally relied on claims data for various analyses; they too are attempting to evaluate the value of real-world clinical data available from EHRs.
The Pfizer Healthcare Informatics (PHI) group conducted an initial assessment and identified several ongoing evaluations of EHR data. In an effort to identify the potential opportunities of EHR and how it could impact the different business areas of Pfizer, PHI led this research project to interview senior leaders within the research, development, outcomes research, safety and regulatory departments to identify how opportunities with EHR data could address the changing business needs. Pfizer then contacted a number of EHR vendors to validate the opportunities identified during the interviews with Pfizer senior leadership.
Methods
Our research team identified a preliminary list of department heads, vice presidents and executive directors at Pfizer Inc. A guided interview (Please see Supplemental Appendix, Table 3, available as a JAMIA online data supplement at www.jamia.org) was conducted by at least two members of the research team. The summaries of the interviews were collated, tabularized, and analyzed for trends and similarities to develop distinct use cases. The project team examined the list of EHR companies who received certification by CCHIT in the Fall of 2006 and identified a subset of companies based on analyst recommendations, years in business, and public statements about their intent on supporting clinical research (Please see Supplemental Appendix, Table 4, available as a JAMIA online data supplement at www.jamia.org). We invited a subset of companies to respond to a survey evaluating their products' functionalities across the identified clinical research use case categories. Companies providing EHR self-reported whether their products met, partially met, or did not meet the functionality defined in the use cases, and if not, if there were plans for future development or not. We collated, tabularized, and analyzed the EHR vendor survey results.
Results
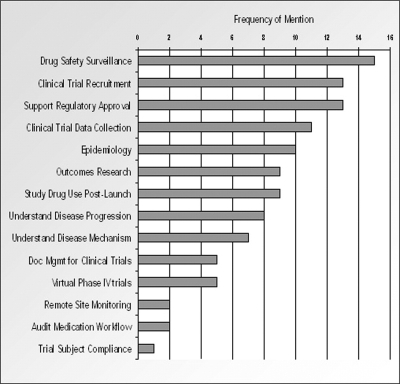
Thirty-five Pfizer senior executives across Research, Development, Operations, Outcomes Research, Safety & Risk Management, and Regulatory, provided input for this project. Interviewees were not specifically asked to identify use cases and requirements. The project team distilled the interview notes into 42 distinct use cases (▶, details are available in the Supplemental Appendix, Table 5, available as a JAMIA online data supplement at www.jamia.org) across 14 categories.
Table 1.
Table 1 Use Case Details by Category
| Use Case Categories | Use Cases | |
|---|---|---|
| 1) Audit Medication Workflow | a) Audit medication workflow | |
| 2) Clinical Trial Data Collection |
|
|
| 3) Clinical Trial Recruitment |
|
|
| 4) Document Management for Clinical Trials | a) Collect & document patient consent | b) Retain trial subject records |
| 5) Drug Safety Surveillance |
|
|
| 6) Epidemiology | a) Study disease prevalence in populations | |
| 7) Outcomes Research |
|
|
| 8) Remote Site Monitoring | a) Monitor sites remotely - Phase IIa | b) Monitor sites remotely - Phase IIb/III |
| 9) Study Drug Use Post-Launch | a) Evaluate optimal dose | b) Capture post-approval drug usage data |
| 10) Support Regulatory Approval |
|
|
| 11) Trial Subject Compliance | a) Track patient compliance with protocol | |
| 12) Understand Disease Progression | a) Understand Disease Progression | |
| 13) Understanding Disease Mechanism |
|
b) Genetic association & linkage analysis |
| 14) Virtual Phase IV trials | a) Allow health plans earlier access to new medications | b) Virtual Phase IV trials |
EHR = electronic health record.
“Drug Safety & Surveillance,” “Clinical Trial Recruitment,” and “Support Regulatory Approval” were the most oft-mentioned scenarios during the interviews (▶), in which the senior executives believed that EHR data would prove valuable.
Table 2.
 |
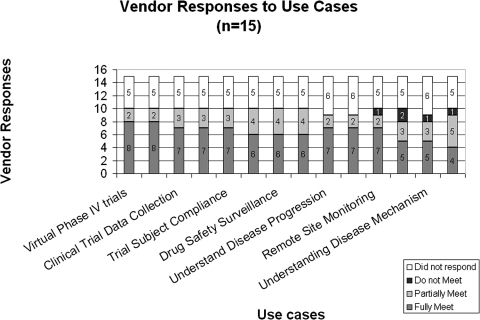
Of the 15 companies identified and invited to respond to the survey, nine companies provided a full response, five companies chose not to respond, and one company provided a partial response regarding their products' ability to support the use cases identified. ▶ shows the self reported survey results of how the EHR companies' products support the functionalities identified from the Pfizer senior leadership interviews.
Figure 1.
Vendor responses to use case categories.
Discussion and Lessons Learned
The pharmaceutical industry is a consumer of healthcare data throughout the different functions needed for the research, development, and safety monitoring of medicines. Clinical data sets are being made available through commercial initiatives such as GE's Medical Quality Improvement Consortium (MQIC), or the Anceta subsidiary of the American Medical Group Association (AMGA), and other companies are considering such commercial efforts. The pharmaceutical industry could more effectively research, develop, and monitor safety of medicines by utilizing such clinical databases that are made possible by the growing adoption of EHRs in healthcare institutions.
In responding to the research team's survey, several companies indicated their ability to support some of the 14 use cases for pharmaceutical research. At present, most of the surveyed EHR companies seem to have the capability to capture the data necessary for “Clinical Trial Recruitment,” “Drug Safety Surveillance,” and some of the use cases for retrospective analyses or observational studies. But it is also important to note that the use case to support regulatory approval was one of the lowest rated; with 33% indicating they fully meet and 13% having no intent of developing such functionality. Although in theory the needed data can be captured by EHRs, unless it is actually acquired with the quality and completeness necessary for regulatory bodies, there is limited value for pharmaceutical companies who must adhere strictly to regulatory requirements; e.g., 21CFR Part 11, which pertains to electronic signatures and audit trails.
Organizations such as the Holston Medical Group and Cleveland Clinic are already conducting patient recruitment for clinical trials through their EHR systems, 5,6 highlighting the feasibility of utilizing EHR to support the research and development of new medicines. In addition, the MQIC database and General Practice Research Database (GPRD) frequently enable research and drug safety analyses. Several of the remaining 14 use case categories that are identified, could be made operational to support research and development today within current EHR implementations.
While EHRs can clearly provide some support to the pharmaceutical industry for data re-use, an ongoing dialogue must continue among EHR companies, research based organizations, and the pharmaceutical industry to ensure that the data being captured, aggregated, and analyzed can produce the value necessary for all stakeholders. The pharmaceutical industry is interested in population health, and EHR vendors can help to better capture and analyze health information. Particularly in the areas of drug safety surveillance, clinical trial recruitment, and in observational studies, the EHR vendors can partner with the pharmaceutical industry to help satisfy regulatory requirements in order to bring life-saving drugs to patients faster. In addition, it is important that the analytical integrity of the data be maintained and that patient privacy and confidentiality is ensured in any such data re-use. Further research is necessary to identify the requirements for EHRs to support each data re-use use case for the pharmaceutical industry, and an environment is needed in which such functionality can be tested and demonstrated.
Acknowledgments
The authors thank the numerous Pfizer executives who participated in this research project, both as interviewees and on the steering committee, the EHR companies who participated in the surveys, John M. Apathy and Lawrence Hanrahan from Accenture for their work in interviewing and synthesizing data, and William A. Yasnoff, MD, PhD (NHII Advisors) for his guidance and advice in writing this paper.
Footnotes
Funding for this research was provided by Pfizer Inc.
References
- 1.American Health Information Community—Quality WorkgroupU.S. Department of Health & Human Serviceshttp://www.hhs.gov/healthit/ahic/quality/Accessed July 15, 2007.
- 2.American Health Information Community—Population Health WorkgroupU.S. Department of Health & Human Serviceshttp://www.hhs.gov/healthit/ahic/population/Accessed July 15, 2007.
- 3.“(Draft) Agenda 3rd Nationwide Health Information Network Forum: Prototypes and Business Models”, Business Model Plenary Discussion, Jan 26, 2007http://www.hhs.gov/healthit/documents/DraftAgenda070125.pdfAccessed July 23, 2007.
- 4.1st Nationwide Health Information Network Forum: Functional Requirements, June 28-29, 2006http://www.hhs.gov/healthit/nhin/forum_june2006.htmlAccessed Aug 15, 2007.
- 5.Apathy J. “Testimony to NCVHS representing Clinical Research Community”, Hearing on the Functional Requirements for the Nationwide Health Information Network, Department of Health & Human Services, National Committee on Vital and Health Statistics, July 27, 2006http://www.ncvhs.hhs.gov/060727tr.htmAccessed July 23, 2007.
- 6.Safran C, Bloomrosen ML, Hammond E, et al. “Toward a National Framework for the Secondary Use of Health Data: An American Medical Informatics Association White Paper” JAMIA 2007;14(1):1-9Jan–Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safran C, Detmer D. “2007 Invitational Conference on Secondary Use of Health Data”http://www.amia.org/inside/initiatives/healthdata/2007/index.asp 2007. Accessed Aug 7, 2007.
- 8.Labkoff S, Bloomrosen M, Safran C. “Toward a National Framework on Secondary Use of Health Data”, Testimony to N.C.V.H.S., July 18, 2007http://www.amia.org/inside/initiatives/healthdata/2007/labkoffbloomrosenncvhssecondarydata.pdf 2007. Accessed Aug 8, 2007.
- 9. Pfizer Inc. 2006 Financial Reporthttp://www.pfizer.com/files/annualreport/2006/financial/financial2006.pdf 2007. Accessed Feb 28, 2008.
- 10.Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005 N Engl J Med 2005;353:2779-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]