Abstract
This article describes the motivation, development, and implementation of a software tool, www.vaccineselection.com, introduced to assist health care professionals and public health administrators in managing pediatric vaccine purchase decisions and making economically sound formulary choices. The tool integrates general operations research methodologies with specific local practice choices to solve for the lowest overall cost set of vaccines required to immunize a child according to the Recommended Childhood Immunization Schedule. A description of the tool's capabilities is provided. Results on the use of the software tool are reported and discussed.
Introduction
The objective of this article is to chronicle the history, development, and use of www.vaccineselection.com, a pediatric vaccine formulary selection algorithm website, designed to assist health care professionals and public health decision makers responsible for purchasing pediatric vaccines.
Background
The United States first introduced a harmonized childhood immunization schedule in 1995. 1 Since that time, the Recommended Childhood Immunization Schedule has grown in scope and complexity, requiring numerous clinic visits over several years, with several injections administered during each such visit. 2 Per Food and Drug Administration (FDA) guidelines and requirements, these clinic visits must be scheduled based on the specifications and requirements of each vaccine.
Pharmaceutical companies are actively working to develop new vaccines to protect children from existing and emerging infectious diseases. However, there may be negative implications with adding more vaccines into the Recommended Childhood Immunization Schedule. 3 In particular, a growing number of physicians and health care providers have expressed concerns about the increased number of injections required to take advantage of new vaccine products. 4–6 Indeed, the trend of continuing to expand the Recommended Childhood Immunization Schedule shows no signs of abating. To satisfy the 2007 Recommended Childhood Immunization Schedule, as many as 18 injections in total may be required over the first 3 recommended immunization visits (2, 4, and 6 months). Looking into the future, it is possible to envision vaccination crowding as a discouraging factor for the Advisory Committee on Immunization Practices (ACIP) to add new vaccines to the Recommended Childhood Immunization Schedule, even when worthwhile vaccine candidates become available.
As additional pediatric vaccines are developed and added to the Recommended Childhood Immunization Schedule, upward pressure is being placed on both the volume and frequency of immunization visits. To overcome this challenge, vaccine manufacturers are becoming more adept in combining various vaccines to reduce the number of injections and clinic visits. 7,8 However, such combination vaccines create their own unique set of problems, challenges, and questions. 9 For example, which vaccines should be combined, and are such combinations biologically compatible? How should such combination vaccines be sequenced and timed to ensure that immunity is achieved without compromising safety and/or efficacy? Given that combination vaccines may lead to extravaccination, which is more likely to occur if immunization documentation is incomplete or unavailable, to what extent should this be tolerated, with the objective of minimizing the risk of harmful side effects and the cost of administering an antigen that is not medically required? Moreover, how does one determine an economical package of vaccine types and brands that should be procured for a particular clinic environment to ensure that the immunization schedule can be satisfied? Note that such a package of vaccines is referred to as the lowest overall cost vaccine formulary, 10 which is the set of vaccines to stock, based on a given set of economic criteria, including vaccine price, vaccine preparation, vaccine administration, and clinic visits. Health care professionals and public health administrators require answers to such questions to effectively distribute pediatric vaccines to where they are needed and to safeguard the nation's population against the threat of both existing and emerging infectious diseases.
Because several vaccines are available from more than one vaccine manufacturer and the number of available combination vaccines with partially overlapping components is growing, the potential exists for a combinatorial explosion of choices in stocking the minimal number of vaccine products to satisfactorily vaccinate children as recommended and required. The resulting combinatorial chaos is a direct consequence of the growing number of feasible formularies that exist. This looming situation, coupled with changes in public health policy, such as immunization requirements for all children entering public schools in the United States, makes it exceedingly challenging for even the most skilled and knowledgeable health care providers and public health administrators to determine the ideal formulary of vaccine types and brands that should be procured for their particular health care immunization environment.
In spite of all these problems and challenges, combining antigens protective against multiple diseases into a single injection clearly has numerous advantages and is the direction in which vaccine manufacturers and immunization practice is moving. 11,12 In fact, combination vaccines are officially preferred over their individual component vaccines in their ability to simplify the immunization process 13 and reduce both the number of injections required during each clinic visit and the total number of injections required to satisfy the Recommended Childhood Immunization Schedule. 14–16
As of January 2008, there were 4 combination vaccines under federal contract for pediatric immunization: diphtheria-tetanus-acellular pertussis–hepatitis B–inactivated polio vaccine (DTaP-HepB-IPV); diphtheria-tetanus-acellular pertussis–Haemophilus influenzae b (DTaP-Hib); Hepatitis B–Haemophilus influenzae b (HepB-Hib); and measles-mumps-rubella–varicella (MMR-Var), with a fifth and sixth vaccine (DTaP-Hib-IPV, DTaP-IPV) gaining FDA approval in June 2008; see http://www.cdc.gov/nip/vfc/cdc_vac_price_list.htm for a complete list of all vaccines under federal contract, including packaging, federal contract and private sector prices, contract end dates, and manufacturers. Despite the early optimism that combination vaccines would solve the problem of excessive injections, their actual development has been more difficult, lengthy, and costly than initially anticipated. 16 This has been primarily because of immunologic interference within a specific vaccine. Therefore, when a new combination vaccine becomes available, the inevitable challenge arises of determining its economic worth and value within the public health community. Two closely related questions can be posed by the 2 primary groups with the greatest interest in such vaccines. First, vaccine manufacturers ask, “What price should be set for this vaccine,” so as to maximize profit and secure market share for the product; whereas health care providers, public health administrators, and insurance companies ask, “Should this vaccine be purchased, and, if so, at what price?” These questions become even more challenging when 2 or more partially overlapping combination vaccines are available; only 1 such vaccine should be stocked in a single formulary, given the immunologic importance of brand matching, which ensures immunological effectiveness in a vaccine recipient, as well as the fact that the cost of managing and administering 2 distinct formularies would be prohibitive. 17
Once vaccine manufacturers have set the price for their products, tools are needed to assist public health administrators and health care providers to make vaccine procurement decisions based on sound economic criteria. For example, a state public health administrator responsible for purchasing vaccines through the Vaccine for Children program may wish to determine which combination vaccine provides the best value for their particular state immunization environment. Similarly, a group of health care providers in a large health maintenance organization (HMO) may wish to determine whether a new combination vaccine provides good value for their practice, based on the price that it is being offered to them. Vaccine formulary decisions for a clinic or practice are often made on a case-by-case basis. Issues that are considered when stocking a vaccine formulary include availability through the Vaccine for Children program (to simplify vaccines that must be available), the ease of administering a vaccine, the number of injections that are required to achieve full immunity, 11 and reimbursement issues. 18 A quantitative tool designed to assist such vaccine formulary decision makers would provide significant practical value.
Design Objectives
Since 1996, personnel within the Centers for Disease Control and Prevention (CDC) have collaborated with operations researchers to explore how operations research modeling and analysis tools can be used to address pediatric immunization and vaccine formulary optimization issues, and to make such tools available and accessible to the pediatric health care and public health communities. A major research accomplishment and transition from this collaboration has been the adaptation of optimization models and algorithms into the web-based tool available at www.vaccineselection.com, for use by private-sector health care entities, such as HMOs, private practice pediatricians, and family practice clinics, and for public health agencies, such as state and county public health departments and administrators. The objective of the web-based tool is to provide such individuals with a mechanism to use economic factors beyond vaccine purchase price to design and maintain pediatric vaccine formularies for a defined pediatric immunization environment, including the value of existing and new combination vaccines. The objective of the user interface is to facilitate such a mechanism, using point-and-click tools, so that any such individuals can make vaccine formulary decisions based on such criteria.
Quantitative computer software tools such as www.vaccineselection.com could provide much-needed help for the pediatric health care community to address the combinatorial chaos that will occur as additional combination vaccines enter the pediatric immunization market. This combinatorial chaos is a direct result of the large number of feasible choices that exist when stocking a vaccine formulary. Therefore, the linkage between the underlying operations research models and a publicly accessible internet interface at www.vaccineselection.com has put this useful and convenient tool into the hands of immunization program policymakers and vaccine purchasers at the local and state levels, in both the public and private sectors. To our knowledge, no other tools, neither algorithms nor websites, have been created and disseminated to assist such groups with vaccine formulary selection decisions. Note that this tool only considers the full Recommended Childhood Immunization Schedule, and does not include the “catch-up” schedule, which is also published by the CDC. 2 However, because the methodology used and approach taken by the tool supports inventory management, and not the vaccination of individuals, and given that the vaccines used for “catch-up” patients are the same for those patients that are on schedule, 2,19 this limitation of our system should not impact the tool's usefulness or generalizability.
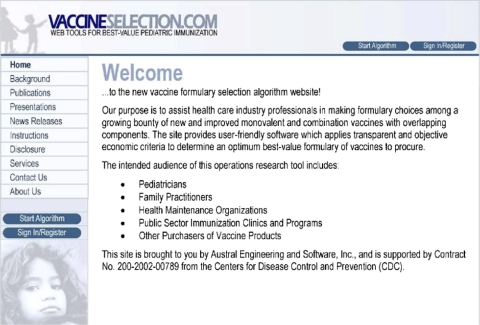
The computer software tool requires an objective function to be defined and constraints to be formulated. The objective function captures the costs associated with an immunization program, including the cost of the available vaccines, the cost of preparing each vaccine by medical staff, the cost of administering each vaccine by medical staff, the cost of each clinic visit, and if desired, the cost of vaccine wastage. Although other costs exist associated with immunization, such as the cost of medical care for children who contract the disease because full immunity was not achieved or the vaccine was not effective, or the cost of treating adverse side effects due to the vaccine itself or due to extravaccination, such costs were not included in the objective function, given the dearth of economic data needed to supports such analyses. 20 The constraints modeled in the tool were captured based on the design of the Recommended Childhood Immunization Schedule and the nature of the vaccines being administered, including biological contraindications. The goal of the tool is to capture the key cost drivers for health care professionals and public health administrators responsible for purchasing pediatric vaccines to stock formularies. As the front page of www.vaccineselection.com notes (▶), “The intended audience of this operations research tool includes: Pediatricians, Family Practitioners, Health Maintenance Organizations, Public Sector Immunization Clinics and Programs, Other Purchasers of Vaccine Products.”
Figure 1.
Homepage for www.vaccineselection.com.
System Description
The website, labeled as “Web Tools for Best-Value Pediatric Immunization,” funded through a Small Business Innovation Research contract with the CDC, allows health care providers, HMOs, health insurance companies, and government public health agencies to input specific prices for all the vaccines that are available for them to purchase for their vaccine formulary, as well as any combination vaccines that may become available in the future, to determine the lowest overall cost vaccine formulary for each child within their specific pediatric immunization environment. Given a particular set of possible vaccines and user inputs (e.g., vaccine prices, vaccine preparation costs, vaccine injection costs), the website executes an operations research algorithm to efficiently search through all possible sets of vaccine formularies to find the optimal such set for a local practice based on several health care economic factors and criteria. Note that no other website exists to achieve this objective. At present, given the number of pediatric vaccines available, the algorithm takes 1 s of computing time to solve for the largest possible problem that can be formulated. Overviews of the algorithm have been previously described, 10,21 with a general outline of the specifics applied to vaccination management found in the next section.
Note that computer-based systems exist to manage medical and health supplies. Miyata et al. 22 describe a chemotherapy registration system to manage chemotherapy for outpatient cancer treatment. Roberts et al. 23 describe a computer-based system for managing supplies in a clinical laboratory. Bramstedt and Young 24 describe how the internet can be used to make the patient selection process for heart transplantation more transparent. Wicks et al. 25 discuss how radiofrequency identification can be used to manage medical supplies. All of these medical management systems are descriptive, focusing on tracking supplies. The web tool www.vaccineselection.com is prescriptive in that it uses medical input data to provide an optimal vaccine selection solution.
Search Engine
The search engine used to solve for each solution generated on the website is customized, using a modified branch and remember (B&R) algorithm that exploits the structure of the problems created on the website. This customized search engine was created to avoid the use of commercial software packages like CPLEX, and hence, eliminate costly software license fees. More importantly though, the customized search engine allows one to exploit the requirements of the Recommended Childhood Immunization Schedule with specific algorithmic features unique to the model that is being solved, which results in faster real-time search engine processing times. For example, a preprocessing algorithm is applied before the B&R algorithm, which removes a monovalent vaccine if another monovalent vaccine of the same type is available, at a lower cost. This is because the higher-cost monovalent vaccine would never be included in an optimal formulary, hence there is no need to consider its use during the B&R algorithm. Such a situation can occur between 2 vaccines from different manufactures or between 2 vaccines with different packaging from the same manufacturer. Note that a practice that has need of individual vaccines because of specific clinical requirements could always buy them on the margin, on a case-by-case basis. The preprocessing algorithm substantially reduces the number of solutions that the B&R algorithm must evaluate for typical problem instances.
The B&R algorithm uses depth-first search to enumerate the feasible solutions of the problem. Suppose that the δ diseases are denoted as d 1 , d 2 , … ,d δ and the τ months are denoted as m 1 , m 2 , … ,m τ. A subproblem consists of a disease/month pair (d, m) and a partial solution S dm that specifies which vaccines have been given before month m and for diseases preceding disease d during month m. Additional subproblems are generated from this subproblem by specifying which vaccine, if any, will be given for disease d during month m. Note that the B&R algorithm does not use bounds to prune the search tree, but rather, it prunes whenever the current partial solution is infeasible or when it is dominated (by cost) by a previously generated partial solution; this is the “Remember” portion of the algorithm, which will be explained below. Such pruning significantly improves the computational speed of the search engine so that solutions can be obtained in just a few seconds for any reasonable set of inputs.
The requirements of the Recommended Childhood Immunization Schedule are modeled as objects in the C++ implementation of the B&R algorithm. This representation was chosen to handle the variation in the requirements that can be selected by the user (see the discussion later) and to facilitate modifying the code whenever the Recommended Immunization Schedule is changed by the ACIP. A Basic Requirement (BR) is a single linear equality or inequality that models a requirement on a set of vaccines V R for a given disease d during a set of months M R. The linear inequality for this BR is
where x vm = 1 (0) if vaccine v is (not) given during month m. For example, the 2008 Recommended Childhood Immunization Schedule requires that a vaccine be given for hepatitis B during month 4. The BR for this requirement would have V R equal to the set of all vaccines that contain an antigen for hepatitis B, M R = {4}, and r = 1.
Almost all of the requirements in the Recommended Childhood Immunization Schedule can be modeled as a BR. Examples include requiring at least 1 dose or exactly 1 dose for disease d during month m or during a time window of several months, or prohibiting a vaccine from being given during month m for disease d. However, some of the requirements are too complicated to be modeled in this manner. For example, the Recommended Childhood Immunization Schedule requires that a Hib vaccine be given during months 2, 4, and 6, unless Merck products are used in months 2 and 4, in which case the dose in month 6 may be skipped. To model this special case, the C++ code creates an object called an Or-Requirement, which is a set of BRs. For a schedule to be feasible, it must satisfy at least 1 BR in each Or-Requirement.
Given a subproblem consisting of a disease/month pair (d, m) and a partial solution S dm, the algorithm determines whether any of the BRs or Or-Requirements are violated, which means that S dm does not satisfy the requirement and that future doses cannot cause the requirement to be satisfied. Whenever this occurs, the subproblem can be pruned.
As mentioned above, the algorithm also prunes subproblems using memory. For example, suppose that 2 partial solutions, S 1 and S 2, both schedule vaccines through month m. If these partial solutions have made precisely the same progress toward satisfying the immunization requirements, although they may have used different vaccines to accomplish this, then only the lower-cost partial solution can lead to an optimal solution, and hence, the higher-cost partial solution can be pruned. Determining whether or not the 2 partial solutions have made the same progress toward satisfying the requirements can be done by comparing the left-hand side of every BR. If the left-hand side is the same for every BR, then the 2 partial solutions have made the same progress toward satisfying the requirements. At certain times when the B&R generates a new subproblem, the memory is checked to see whether a subproblem that dominates it has already been generated. If so, the new subproblem is pruned. The list of subproblems generated during the B&R algorithm is stored in a hash table to permit efficient look up.
The model and customized search engine were validated by randomly generating several thousand sets of actual (monovalent and combination) and hypothetical combination vaccines, and solving for the lowest-cost optimal formulary. Each of these problem instances was also formulated as an integer programming model, and the commercial software package, CPLEX, was used to solve it. In all instances generated, the solutions matched.
Web-based User Interface
The web-based user interface was developed and is being maintained by Austral Engineering and Software (AES), Inc, a software development firm based in Athens, Ohio (http://www.aes.ws). The decision-making capabilities built into the website were presented to the ACIP during their October 2001 meeting. This presentation highlighted the purpose of the website, its current and future role in supporting pediatric vaccine economic decision-making, and the ease of use of the website. The website provides health care administrators with a software tool to make vaccine purchasing decisions for a wide variety of actual and hypothetical health care environments. It allows such administrators to input any or all available vaccine products at federal contract, private-sector retail, or private-sector discounted prices, with specific vaccine-administration costs, including the costs of administering an injection, preparing vaccines, and clinic visits, to obtain the vaccine formulary with the lowest overall cost for their specific pediatric immunization environments. They can also use the website to study various vaccination scenarios and to play “what if” games across numerous immunization scenarios, such as to determine the economic impact of reductions in vaccine prices or the addition of new vaccines on the overall cost and design of their vaccine formulary.
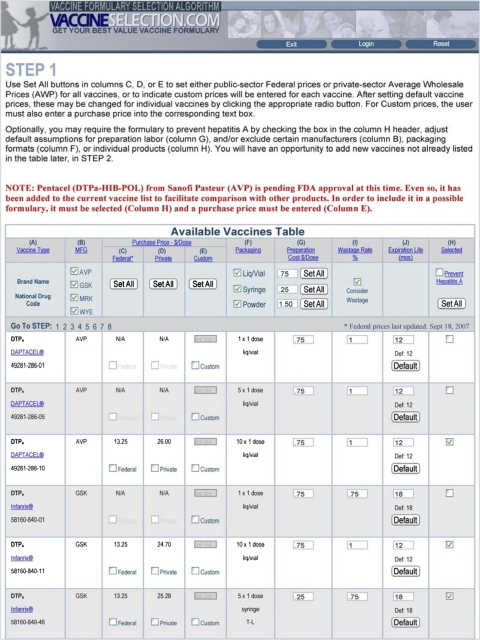
The website provides step-by-step guidelines that allow users to exploit the power of the operations research tools available; see http://www.vaccineselection.com/instructions.aspx for up-to-date details on these guidelines. The website uses a point-and-click data input format whenever possible to simplify the data input process from the default settings (▶). The website incorporates all of the vaccine features captured in the Recommended Childhood Immunization Schedule, as well as biological restrictions, as determined by the FDA and recommended by the ACIP, for all vaccines under federal contract. The user can choose to input public-sector federal contract prices, private-sector retail prices, or insert their own customized prices, which is particularly valuable when a vaccine purchaser wishes to negotiate a new price for an existing vaccine or determine an appropriate price for a new combination vaccine. As an initial starting point, the website default setting is to allow the list of vaccines currently under federal contract, in all packaging formats, with the flexibility to exclude any of these vaccines from consideration. The website also allows certain vaccine restrictions that are not universally required for all children, or applicable to all immunization environments. For example, the user may include or exclude all vaccines produced by a specific manufacturer or all vaccines with a particular packaging: prefilled syringes, liquid vaccines in vials, powdered/lyophilized vaccines. The user may also specify the preparation costs for each vaccine, which includes the estimated cost of labor to open and prepare a vaccine for administration. Default preparation costs are $0.25 for prefilled syringes, $0.75 for liquid vaccines in vials, and $1.50 for powdered/lyophilized vaccines, 10 although customized values for these costs can be inserted by the user. The website also allows the user to incorporate the impact of vaccine wastage on their optimal vaccine formulary; see Jacobson et al. 26 for a description of how this is computed, using data reported by Setia et al., 27 and to add new combination vaccines that are not under federal contract. The costs of a clinic visit, with default value $10, and administering an injection, with default value $5, can also be set by the user, based on the perspective from which the user wishes to determine their vaccine formulary. For example, the problem can be solved from the societal perspective, the health professional's perspective, or from the patient's perspective; see Weniger et al. 20 for a discussion of these issues. Lastly, 2 issues that the user can choose to include or exclude are acellular pertussis brand matching, which requires that all DTaP vaccines, including combinations, be administered from the same manufacturer, and perinatal HepB doses, assuming that a birth dose of hepatitis B is given to a child. The default is to enforce acellular pertussis brand matching, because it is highly encouraged, 28 whereas the perinatal HepB dose is handled by choosing across 3 defined scenarios: scenario 1 (the default setting) requires that 3 doses of HepB be scheduled, starting at age 2 months; scenario 2 schedules a perinatal outpatient visit (age 0-to-1 month) for the first HepB dose, with the vaccine, preparation, visit, and injection costs for this dose computed; scenario 3 assumes that only 2 remaining doses of the HepB vaccine are required, and ignores all costs of the first HepB dose. After entering all of the data, the user clicks a solve button, after which a table is generated that displays the lowest-cost vaccine formulary obtained by the search engine. As of January 15, 2008, version Beta 8.0 is being used to obtain this solution.
Figure 2.
Vaccine formulary selection algorithm user interface.
Status Report
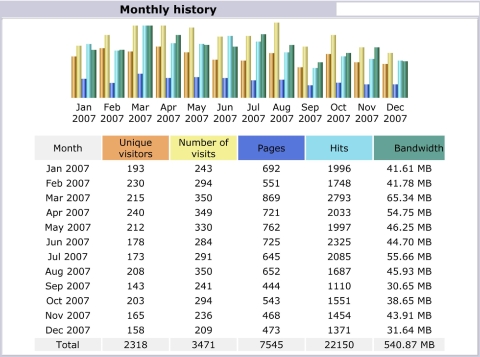
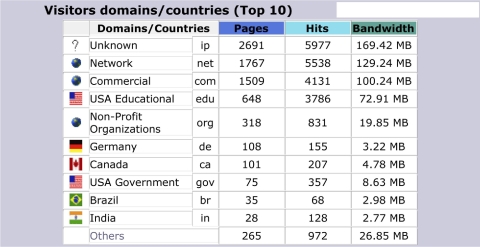
To date, numerous health care personnel and researchers have visited this website to learn more on how the capability offered by the site can be used to help address pediatric vaccine formulary design questions. Confidentiality agreements prevent the disclosure of information about specific users and applications, and their demographics. However, the number of users of the website has grown since it first became available in 2002, with a peak in 2005, and a drop-off since then of approximately 25%. The approximate number of unique visitors to the website was 295 in 2002 (the year it was first launched, while still under development), 337 in 2003, 1,201 in 2004, 3,185 in 2005, 2,952 in 2006, and 2,318 in 2007, with many of these individuals and organizations making multiple visits. ▶ provides a screenshot of the user statistics for www.vaccineselection.com in 2007, which includes the number of unique visitors (broken down by each month), the total number of visits (because users may return more than once), the number of pages viewed (which provides an indication of how much of the website was used by visitors), the number of hits (which indicates the number of requests from the web browser for data from the website), and the bandwidth (which indicates the amount of data transmitted to the web browser from the website, which in turn serves as a surrogate for the extent to which the optimization search algorithm was used to solve actual formulary design problems). ▶ provides a summary of the top 10 user domains of the website. Approximately 20% of the hits and page views were from United States commercial domains (.com), 6.7% of the visitors were from international domains, 4.2% were from not-for-profit domains (.net), and 8.5% were from United States educational domains (.edu). Although these domains give some indication of the sources of the users, they do not provide a precise snapshot of their demographics and interests. Note that these latter users (United States educational domain users) had a disproportionately high number of hits (17% of the total for the entire year). One possible explanation for this high usage level is that the website is being used as a research and educational tool. Of course, to determine the exact background of all the users would require a detailed survey of their motivation, interests, and reasons for visiting the website. ▶ provides a breakdown in the number of pages, hits, and bandwidth (as well as the percentage of the total number in each of these categories) used by commercial (.com), United States educational (.edu), and not-for-profit organizations (.org), from 2004 through 2007. The most interesting trend is that not-for-profit organizations seem to be growing in their use of the website, although commercial and United States educational organizations continue to be the 2 largest communities who visit and use the website.
Figure 3.
User Statistics for www.vaccineselection.com in 2007.
Figure 4.
Domains of user statistics for www.vaccineselection.com in 2007.
Table 1.
Table 1 Trends in Domains of Users for www.vaccineselection.com, 2004 to 2007 ∗
| Year | .com Pages | .com Hits | .com Bandwidth | .edu Pages | .edu Hits | .edu Bandwidth | .org Pages | .org Hits | .org Bandwidth |
|---|---|---|---|---|---|---|---|---|---|
| 2007 | 1,509 (20.0) | 4,131 (18.7) | 100.24 MB (18.5) | 648 (8.6) | 3,786 (17.1) | 72.91 MB (13.5) | 318 (4.2) | 831 (3.8) | 19.85 MB (3.7) |
| 2006 | 1,535 (18.1) | 4,416 (17.5) | 107.01 MB (15.2) | 1,469 (17.3) | 4,435 (17.6) | 170.79 MB (24.3) | 67 (0.8) | 335 (1.3) | 6.79 MB (1.0) |
| 2005 | 1,116 (17.2) | 2,705 (15.7) | 70.99 MB (16.3) | 267 (4.1) | 1,289 (7.5) | 37.03 MB (8.5) | 94 (1.5) | 425 (1.5) | 11.58 MB (2.7) |
| 2004 | 983 (23.3) | 3,342 (24.7) | 122.44 MB (28.7) | 172 (4.1) | 835 (6.2) | 30.77 MB (7.2) | 7 (−) | 34 (−) | 840.06 KB (−) |
∗ All values given in parentheses are percentages.
To educate potential users of the website, live computer demonstrations of the web tool were made at the 2002, 2004, 2005, and 2006 National Immunization Conferences, the major national immunization conference in the United States that brings together approximately 1,500 public health and health care professionals, a committed group of immunization stakeholders in both the private and public sectors, with a common interest in immunization science, policy, education, and planning. These demonstrations have also led to surges of interest in and activity on the website and its capabilities. Moreover, with well over 90,000 pediatricians in the United States, 29,30 not counting family practice physicians and family practice/pediatric residents, and new combination vaccines positioned to become available over the next decade, the potential upside of future users of the website is enormous. Note that the website is also featured on the CDC website at http://www.cdc.gov/od/science/iso/vaxtech/ under Vaccine Safety Home: Vaccine Technology, where it is listed as “Vaccine Formulary Selection Algorithm.”
User statistics suggest that a wide and varied international cross section of the pediatric immunization community, including state and city public health departments, academic institutions, pharmaceutical industry business and marketing firms, pharmaceutical research and development organizations, pediatricians, and family practitioners, may be visiting and using the tools available at the website. In 2007, over 500 optimum pediatric formularies were established through the website. More recently, the website is being used to quantify the economic impact of new vaccines on the pediatric vaccine market, to evaluate the economic value of new pediatric combination vaccine products, and to determine appropriate price levels for such products as they enter the marketplace. For example, when Pediarix, a pediatric combination vaccine that protects against 5 different diseases, gained FDA approval in late 2002, the economic value of this product was assessed using the website by inserting the new product into the list of available vaccines and using a reverse engineering optimization algorithm that incorporates a bisection search procedure outside the website to determine the maximal price at which the new product earns a spot in the lowest overall cost vaccine formulary. 21,31 Both vaccine manufacturers and health care providers can obtain useful descriptive and prescriptive information in this manner. The results of this particular analysis suggested that the new combination vaccine was underpriced relative to the value that it provided. One possible explanation for this pricing decision was that its manufacturer was willing to sacrifice short-term profits to gain market share and to limit future competition for this product. Since that time, the price of this combination vaccine has been significantly increased, effectively removing these marketing barriers and creating a more level competitive environment for competing combination vaccines to enter the market.
Discussion, Limitations, and Future Considerations
As more pediatric combination vaccines gain FDA approval in the United States, the combinatorial explosion of choices and decisions faced by health care providers and public health administrators will grow. Operations research models and algorithms, available at the website www.vaccineselection.com, provide a valuable tool to help the health care industry assess the economic advantages of various vaccine products, and hence determine how to optimally stock pediatric vaccine formularies using factors beyond the individual prices of each vaccine product.
The development of the web tool has provided many lessons. When the original collaboration with CDC personnel began in 1996, they envisioned the need for the web tool to address a vaccine selection problem that would be complicated by a growing complexity in the Recommended Childhood Immunization Schedule and the proliferation of combination vaccines. On the first point, this has been the case, whereas on the second point, this has been much slower to develop because the FDA has been cautious in granting approval for such products in the United States, even though several such products are being used with success in other countries. This has made it less urgent for state purchasers of vaccines through the Vaccine for Children program to optimize their vaccine formulary design because their choices have remained manageable. Indeed, based on anecdotal information, state purchasers are less concerned about saving money and more concerned about providing adequate vaccines for their constituents. Given that the tools at www.vaccineselection.com focus on cost savings, it may be necessary to inform such decision makers that these 2 objectives are inextricably related. An important lesson learned from this experience is that when creating any type of web tool, one must gather sufficient information from potential users regarding what would make them use the tool and how to facilitate their involvement. Moreover, if a web tool is designed to address a problem that stakeholders are unaware of, education may be needed to connect the dots for such individuals.
To illustrate how future decision making for such people may become more complex, Pediarix, the GlaxoSmithKline combination vaccine DTaP-HepB-IPV, gained FDA approval in late 2002. In June 2008, the Sanofi Pasteur combination vaccine DTaP-Hib-IPV gained FDA approval, a time gap of almost 6 years. Since this product gained FDA approval, and is under federal contract, a decision must be made regarding which of these combinations to make as the vaccine formulary backbone. To go one step further, although combination vaccines such as DTaP-HepB-Hib and DTaP-HepB-Hib-IPV are successfully being used in countries around the world, 32 these vaccines have not yet succeeded in gaining FDA approval in the United States. However, once this approval occurs, the United States health care system will be thrust into the debate of which combination vaccines to use, how they should be used, and at what price. Fortunately, www.vaccineselection.com makes it possible to address such questions and concerns in an objective manner. Users can also use the website to see how competing monovalent and combination vaccines fit within their particular immunization environment, and to assess the economic value of these products. This creates a transparent medium for negotiation between vaccine manufacturers and vaccine purchases, which in turn will ultimately serve to moderate vaccine prices by allowing both sides to fairly assess the value of different vaccines. Because each immunization environment (e.g., urban public health clinics, rural community health care centers, and private physician offices) has its own unique set of needs and objectives, the web tool offers the flexibility to assist all these vaccine and immunization stakeholders in making economically sound vaccine procurement decisions well into the future. By making such decisions, and keeping vaccine formularies appropriately stocked, one can argue that this will support higher immunization coverage rates, an important public health concern. 33 Of course, practical issues such as maintaining separate vaccine formulary inventories, one for Vaccines for Children patients and one for private patients, can complicate matters significantly. Varying insurance industry reimbursement policies can also impact which vaccines should be stocked, and nationwide vaccine shortages create their own unique set of challenges. Moreover, the ideal vaccine formulary manager must also take into account local practice issues such as age distribution of the patient population and missed vaccine rates. Nonetheless, web tools such as www.vaccineselection.com are making it possible for medical practice and decision making to be enhanced in both its delivery and its efficiency. 34
Acknowledgments
The authors thank Bruce G. Weniger, MD, MPH, Chief, Vaccine Technology, Immunization Safety Office, Centers for Disease Control and Prevention, for his feedback on this work and his longstanding encouragement on this line of research. His input on all the research efforts in this area has been invaluable and appreciated. The authors also thank Mr. Daniel A. Allwine, President, AES, Inc, for providing up-to-date data and information on the usage of www.vaccineselection.com; Janet A. Jokela, MD, MPH, for her comments on an earlier draft of this article; and the three anonymous reviewers for their numerous comments and suggestions that have led to a significantly improved article.
Footnotes
Supported in part by National Science Foundation grants (DMI-0222554, DMI-0222597, DMI-0457176, DMI-0456945). The Website development was supported in part through contract 200-2002-00789 from the Centers for Disease Control and Prevention to Austral Engineering and Software (AES), Inc. The authors received financial support from AES, Inc., to implement the operations research algorithms for the web-based tool.
References
- 1.Centers for Disease Control and Prevention Notice to Readers Recommended Childhood Immunization Schedule—United States. January 1995. MMWR Morb Mortal Wkly Rep 1995;43:959-960.7799911 [Google Scholar]
- 2.Centers for Disease Control and Prevention Recommended Immunization Schedules for Persons Aged 0–18 Years—United States, 2007 MMWR Morb Mortal Wkly Rep 2007;55:Q1-Q4. [Google Scholar]
- 3.Aguilar F. Combination vaccines are key to achieving complete on-time childhood immunization coverage Curr Pediatr Rev 2007;3:289-292. [Google Scholar]
- 4.Madlon-Kay D, Harper P. Too many shots?. Parent, nurse and physician attitudes toward multiple simultaneous childhood vaccinations. Arch Fam Med 1994;3:610-613. [DOI] [PubMed] [Google Scholar]
- 5.Diekema DS. Responding to parental refusals of immunization of children Pediatrics 2005;115:1428-1431. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhoff AS, Jacobs RJ. Do too many shots due lead to missed vaccination opportunities?. Does it matter?. Prev Med 2005;41:540-544. [DOI] [PubMed] [Google Scholar]
- 7.Parkman PD. Combined and simultaneously administered vaccines: a brief history. In: Combined and Vaccines and Simultaneous Administration: Current Issues and Perspectives. Ann N Y Acad Sci 1995;754:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Combination vaccines for childhood immunization MMWR Morb Mortal Wkly Rep 1999;48:1-15. [Google Scholar]
- 9.Decker MD, Edwards KM. Combination vaccinesIn: Plotkin SA, Orenstein WA, editors. Vaccines. 3rd ed.. Philadelphia: Saunders; 1999. pp. 508-530.
- 10.Jacobson SH, Sewell EC, Deuson R, Weniger BG. An integer programming model for vaccine procurement and delivery for childhood immunization: a pilot study Health Care Manage Sci 1999;2:1-9. [DOI] [PubMed] [Google Scholar]
- 11.Freed GL, Cowan AE, Clark SJ, Santoli J, Bradley J. Use of a new combined vaccine in pediatric practices Pediatrics 2006;118:e251-e257. [DOI] [PubMed] [Google Scholar]
- 12.Goldfarb NI, Patel NM, Clarke JL. Improving quality by encouraging providers to use pediatric combination vaccines Manag Care 2005;14(6 Suppl):3-12. [PubMed] [Google Scholar]
- 13.Dodd D. Benefits of combination vaccines: effective vaccination on a simplified schedule Am J Manag Care 2003;9(1 Suppl):S6-S12. [PubMed] [Google Scholar]
- 14.Flanagan-Klygis EA, Sharp L, Frader JE. Dismissing the family who refuses vaccines: a study of pediatrician attitudes Arch Pediatr Adolesc Med 2005;159:929-934. [DOI] [PubMed] [Google Scholar]
- 15.Lieu TA, Black SB, Ray GT, Martin KE, Shinefield HR, Weniger BG. The hidden costs of infant vaccination Vaccine 2000;19:33-41. [DOI] [PubMed] [Google Scholar]
- 16.Marshall GS. One for all: newer combination vaccines in practice Pediatr Ann 2004;33:501-511. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention General recommendations on immunization: Recommendations of the advisory committee on immunization practices (ACIP) MMWR Morb Mortal Wkly Rep 2006;55:1-48. [PubMed] [Google Scholar]
- 18.Glazner JE, Beaty BL, Pearson KA, Berman S. The cost of giving childhood vaccinations: differences among provider types Pediatrics 2004;113:1582-1587. [DOI] [PubMed] [Google Scholar]
- 19.Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States: days undervaccinated and number of vaccines delayed JAMA 2005;293:1204-1211. [DOI] [PubMed] [Google Scholar]
- 20.Weniger BG, Chen RT, Jacobson SH, et al. Addressing the challenges to immunization practice with an economic algorithm for vaccine selection Vaccine 1998;16:1885-1897. [DOI] [PubMed] [Google Scholar]
- 21.Sewell EC, Jacobson SH. Using an integer programming model to determine the price of combination vaccines for childhood immunization Ann Oper Res 2003;119:261-284. [Google Scholar]
- 22.Miyata H, Katayama S, Nishizawa M, Honjoh K, Kikuchi A, Gemma A. Management of cancer chemotherapy for outpatients—effectiveness of using cancer chemotherapy protocol database for outpatients. Gan to kagaku ryoho. Cancer Chemother 2005;32(Suppl 1):9-11. [PubMed] [Google Scholar]
- 23.Roberts BI, Mathews CL, Walton CJ, Frazier G. A computer-based maintenance reminder and record-keeping system for clinical laboratories Clin Chem 1982;28:1917-1921. [PubMed] [Google Scholar]
- 24.Bramstedt KA, Young LB. Use of the internet by United States heart transplant centers to promote transparency in the process of patient selection Telemed J E Health 2006;129:359-362. [DOI] [PubMed] [Google Scholar]
- 25.Wicks AM, Visich JK, Li S. Radio frequency identification applications in healthcare Int J Healthc Technol Manage 2006;7:522-540. [Google Scholar]
- 26.Jacobson SH, Karnani T, Sewell EC. Assessing the impact of wastage on pediatric vaccine immunization formulary costs using a vaccine selection algorithm Vaccine 2004;22:2307-2315. [DOI] [PubMed] [Google Scholar]
- 27.Setia S, Mainzer H, Washington ML, Coil G, Snyder R, Weniger BG. Frequency and causes of vaccine wastage Vaccine 2002;20:1148-1156. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Use of Diphtheria Toxoid-Tetanus Toxoid-Acellular Pertussis vaccine as a five-dose series: supplemental recommendations of the advisory committee on immunization practices (ACIP) MMWR Morb Mortal Wkly Rep 2000;49:1-8. [PubMed] [Google Scholar]
- 29.Shipman SA, Lurie JD, Goodman DC. The general pediatrician: projecting future workforce supply and requirements Pediatrics 2004;113:435-442. [DOI] [PubMed] [Google Scholar]
- 30.Goodman DC, Anderson MR, Friedman AL, et al. The pediatrician workforce: durrent status and future prospects Pediatrics 2005;116:e156-e173. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson SH, Sewell EC, Karnani T. Engineering the economic value of two pediatric combination vaccines Health Care Manage Sci 2005;8:29-40. [DOI] [PubMed] [Google Scholar]
- 32.Bogaerts H. The future of childhood immunizations: examining the European experience Am J Manag Care 2003;9(1 Suppl):S30-S36. [PubMed] [Google Scholar]
- 33.Goodyear-Smith F, Grant C, York D, et al. Determining immunisation coverage rates in primary health care practices: a simple goal but a complex task Int J Med Inform 2008. in press. [DOI] [PubMed]
- 34.Kohane IS, Greenspun P, Fackler J, et al. Building national electronic medical record systems via the World Wide Web J Am Med Inform Assoc 1996;3:191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]