A key step in the oxidation of water to O2 in the catalytic cycle of Photosystem II is the conversion of a MnOH species to MnO. This transformation is proposed to occur through proton-coupled electron transfer (PCET)1 from the hydroxo ligand to a nearby tyrosyl radical.2 Examples of this transformation in well-characterized systems are rare.3 The reverse reaction, in which a hydrogen atom is abstracted from a substrate by a highly reactive metal oxo intermediate, is more commonly observed.4 Imido ligands, which are often considered to be surrogates for oxos, display similar reactivity. Thus, hydrogen abstraction by imidos is often observed,5,6 but their formation by PCET from an amido complex has not been reported.7 Here we describe the formation of a cobalt(III) imido complex from the corresponding cobalt(II) amido complex, and present computational data consistent with a concerted (PCET) pathway.
The four-coordinate complex LCoCl8 (L = phenyltris(1-tertbutylimidazol-2-ylidene)borato) reacts with LiNHtBu to form the dark green, high spin (S = 3/2) amido complex LCoNHtBu in high yield. This rare example of a monomeric cobalt amido complex has been crystallographically characterized (Fig. 1a). The asymmetric unit consists of three crystallographically independent molecules, all showing identical structural features. The Co-N bond lengths (1.886(7)–1.88(2) Å) and bent Co-N-C linkages (152.5(2)-172.4(9)°) are comparable to those of other three- and four-coordinate cobalt amido complexes.9
Figure 1.
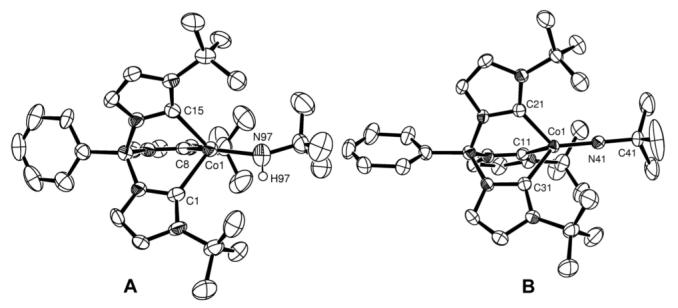
(a) ORTEP diagram of one of the molecules in the asymmetric unit of LCoNHtBu. Thermal ellipsoids shown at 50% probability. Selected bond lengths and angles: Co(1)—N(97) 1.886(7), Co(1)—C(1) 2.128(7), Co(1)—C(8) 2.064(7), Co(1)—C(15) 2.099(6), Co(1)—N(97)—C(28) 153.5(6), C(1)—Co(1)—C(8) 91.6(2), C(1)—Co(1)—C(15) 89.9(2), C(8)—Co(1)—C(15) 93.4(2) (b) ORTEP diagram of LCoNtBu. Thermal ellipsoids shown at 50% probability. Selected bond lengths (Å) and angles (°): Co(1)— N(41) 1.660(3), Co(1)—C(11) 1.988(4), Co(1)—C(21) 1.949(4), Co(1)—C(31) 1.952(4), Co(1)—N(41)—C(41) 179.7(3), C(11)—Co(1)—C(21) 90.6(2), C(11)—Co(1)—C(31) 86.8(2), C(21)—Co(1)—C(31) 87.7(2).
The 1H NMR spectrum of the complex is consistent with the X-ray crystal structure. Seven paramagnetically shifted resonances are observed and can be assigned on the basis of integration. A weak band at 3149 cm-1 in the IR spectrum is assigned to the N-H stretching vibration. Although sensitive to both water and oxygen, this complex has significantly greater thermal stability than most late transition metal alkylamido complexes, remaining unchanged for days at 100 °C.
Reaction of the amido complex with the stable 2,4,6-tri(tert-butyl)phenoxy radical10 results in immediate formation of the lilac cobalt(III) imido complex LCoNtBu in high yield (Scheme 1). The diamagnetic cobalt product has been characterized by X-ray crystallography (Fig. 1b) and 1H NMR spectroscopy, and the 2,4,6-tri(tert-butyl)phenol byproduct has been characterized by 1H NMR spectroscopy. The X-ray crystal structure of LCoNtBu shows similar features to related complexes. In particular, the short Co(1)—N(41) bond length (1.660(3) Å) and linear Co(1)—N(41)— C(41) bond angle (179.7(3)°) are in line with other structurally characterized cobalt(III) imidos.11 This transformation is unique in the synthesis of late metal imido complexes, which are typically prepared via nitrene capture by low valent precursors.
Scheme 1.
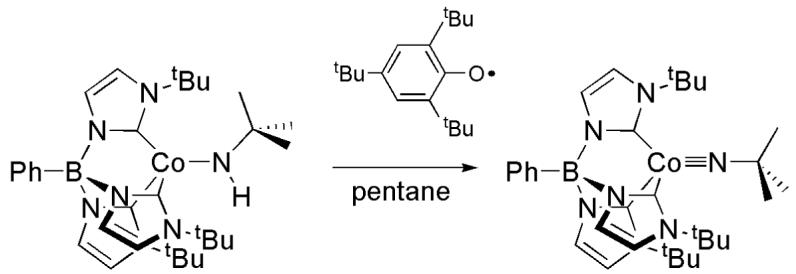
At least three mechanisms for the formation of LCoIIINtBu can be proposed (Scheme 2, the corresponding organic species are not shown).12 The cobalt(II) amido complex could react by electron transfer (ET) to form an intermediate cationic cobalt(III) amido complex, LCoNHtBu+, followed by proton transfer (PT) to form the imido product, a pathway analogous to the route followed in the synthesis of (dtbpe)Ni=N(2,6-iPr2C6H3).13 A second possibility is that the cobalt(III) imido product is formed by successive PT and ET steps via an anionic cobalt(II) imido intermediate LCoNtBu-. Alternatively, the reaction could occur in a concerted PCET step that avoids formation of any of the intermediates.
Scheme 2.
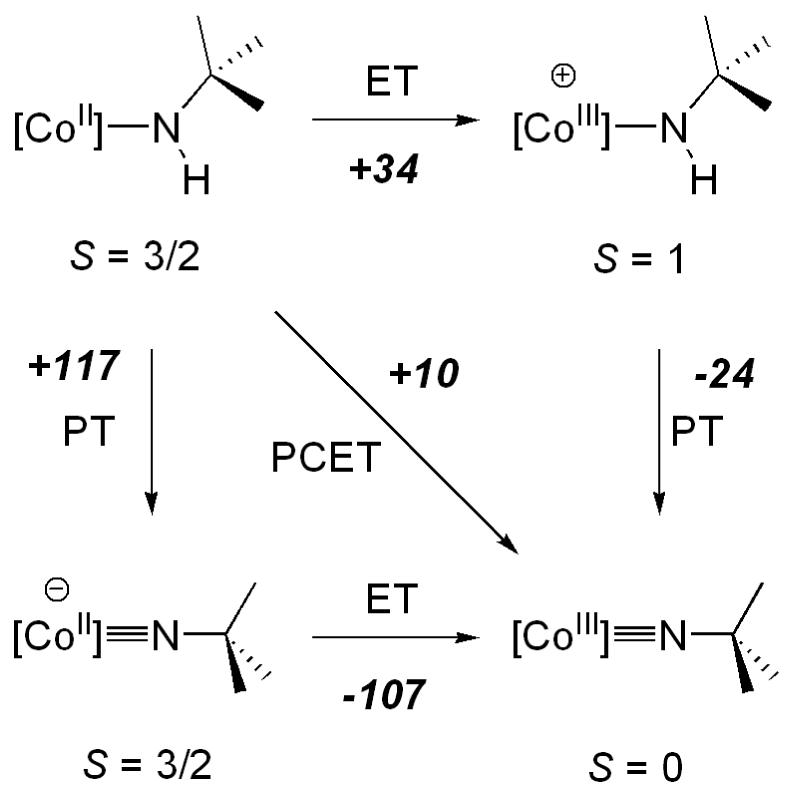
Calculated thermodynamic cycle in diethyl ether (B3LYP/6-31G*, PCM) showing relative free energies (kcal/mol).
We have calculated the relative free energies for all species in the thermodynamic cycle using density functional theory with hybrid functional (B3LYP/6-31G*). In the cases where the spinstates of the proposed intermediate cobalt complexes are not known, the energies of all possible spin states were calculated.14 The lowest energy spin state was used to determine relative free energy barriers (Scheme 2, relative energies in kcal/mol). Diethyl ether solvent was included using the polarizable continuum model (PCM). The free energy barriers obtained using the smaller SDD basis set are comparable (within 5 kcal/mol) to those obtained with the 6-31G* basis set.
The computational results are consistent with a concerted mechanism. The pathway for PCET is lower than for the alternative stepwise mechanisms that involve initial electron or proton transfer. We have also found that the free energy differences are similar to the electronic energy differences, and thus entropic contributions will not alter our conclusion.
Our experimental observations are consistent with the calculations. Thus, we have been unable to access the high energy complex LCoNtBu- by deprotonation of LCoNHtBu. No reaction is observed with LDA, while in the case of MeLi, metathesis occurs to yield the previously characterized complex LCoMe.8 Furthermore, no reduction wave was observed in the cyclic voltammogram of LCoNtBu. Likewise, initial electron transfer from LCoNHtBu to the 2,4,6-tri(tert-butyl)phenoxy radical was found to be thermodynamically uphill.
The relative free energies calculated for LCoNHtBu and LCoNtBu have also allowed us to estimate the gas phase N-H BDE (enthalpy) of LCoNHtBu to be 75 kcal/mol. This is consistent with the spontaneous reaction observed with the 2,4,6-tri(tert-butyl)phenoxy radical (O-H BDE of 2,4,6-tri(tert-butyl)phenol is 81.2 kcal/mol).15 However, we have been unable to experimentally determine a lower limit for the N-H BDE. Even substrates with weak bonds (e.g. TEMPO-H, O-H BDE = 69.7 kcal/mol),16 do not react with LCoNtBu. This may be due to a kinetic barrier resulting from the bulkiness of the ligands, and it is thus notable that TEMPO does not react with LCoNHtBu.
In summary, we have found that a cobalt(III) imido can be prepared from the corresponding cobalt(II) amido complex. A computational investigation of the relative free energies for stepwise and concerted mechanisms is consistent with the concerted pathway involving PCET. Further studies are underway to kinetically investigate the mechanism of this transformation and extend this approach to other metals and metal-ligand multiple bonds.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the ACS-PRF, NMSU and NSF CAREER (CHE 0348956 to HW), OS acknowledges summer support from NIH-BRIDGES (R25 GM048998). The Bruker X8 X-ray diffractometer was purchased via an NSF CRIF:MU award to the University of New Mexico, CHE-0443580. We thank Eileen Duesler for the X-ray data collection and Cindy Zoski for assistance with CV measurements.
References
- 1.PCET is defined as the concerted transfer of an electron and a proton. See:Mayer JM. Annu. Rev. Phys. Chem. 2004;55:363. doi: 10.1146/annurev.physchem.55.091602.094446.
- 2(a).Recent reviews:Carrell TG, Tyryshkin AM, Dismukes GC. J. Biol. Inorg. Chem. 2002;7:2. doi: 10.1007/s00775-001-0305-3.Mukhopadhyay S, Mandal SK, Bhaduri S, Armstrong WH. Chem. Rev. 2004;104:3981. doi: 10.1021/cr0206014.Vrettos JS, Brudvig GW. Oxygen Evolution. In: McCleverty JA, Meyer TJ, editors. Comprehensive Coordination Chemistry II. Elsevier; Oxford: 2004. pp. 507–547.Messinger J. Phys. Chem. Chem. Phys. 2004;6:4764.
- 3(a).Conproportionation of aqua and oxo ligands by PCET: Binstead RA, Stultz LK, Meyer TJ. Inorg. Chem. 1995;34:546.Nemes A, Bakac A. Inorg. Chem. 2001;40:2720. doi: 10.1021/ic0013577.Jensen KB, McKenzie CJ, Pedersen JZ. Inorg. Chem. 2001;40:5066. doi: 10.1021/ic0155380.
- 4(a).For example, in terminal iron oxo complexes: Shan X, Que L. J. Inorg. Biochem. 2006;100:421. doi: 10.1016/j.jinorgbio.2006.01.014.MacBeth CE, Golombek AP, Young VG, Yang C, Kuczera K, Hendrich MP, Borovik AS. Science. 2000;289:938. doi: 10.1126/science.289.5481.938.MacBeth CE, Gupta R, Mitchell-Koch KR, Young VG, Lushington GH, Thompson WH, Hendrich MP, Borovik AS. J. Am. Chem. Soc. 2004;126:2556. doi: 10.1021/ja0305151.Pestovsky O, Bakac A. J. Am. Chem. Soc. 2004;126:13757. doi: 10.1021/ja0457112.Kaizer J, Klinker EJ, Oh NY, Rohde J-U, Song WJ, Stubna A, Kim J, Münck E, Nam W, Que L. J. Am. Chem. Soc. 2004;126:472. doi: 10.1021/ja037288n.Oh NY, Suh Y, Park MJ, Seo MS, Kim J, Nam W. Angew. Chem. Int. Ed. 2005;44:4235. doi: 10.1002/anie.200500623.
- 5(a).Jensen MP, Mehn MP, Que L. Angew. Chem. Int. Ed. 2003;42:4357. doi: 10.1002/anie.200351605. [DOI] [PubMed] [Google Scholar]; (b) Kogut E, Wiencko HL, Zhang L, Cordeau DE, Warren TH. J. Am. Chem. Soc. 2005;127:11248. doi: 10.1021/ja0533186. [DOI] [PubMed] [Google Scholar]; (c) Lucas RL, Powell DR, Borovik AS. J. Am. Chem. Soc. 2005;127:11596. doi: 10.1021/ja052952g. [DOI] [PubMed] [Google Scholar]; (d) Eckert NA, Vaddadi S, Stoian S, Lachicotte RJ, Cundari TR, Holland PL. Angew. Chem. Int. Ed. 2006;45:6868. doi: 10.1002/anie.200601927. [DOI] [PubMed] [Google Scholar]
- 6(a).Thyagarajan S, Shay DT, Incarvito CD, Rheingold AL, Theopold KH. J. Am. Chem. Soc. 2003;125:4440. doi: 10.1021/ja028267g. [DOI] [PubMed] [Google Scholar]; (b) Shay DT, Yap GPA, Zakharov LN, Rheingold AL, Theopold KH. Angew. Chem. Int. Ed. 2005;44:1508. doi: 10.1002/anie.200462529. [DOI] [PubMed] [Google Scholar]
- 7(a).Formation of [OsV]=N-NR2 from [OsIV]-NH-NR2 by PCET: Huynh MHV, Meyer TJ, White PS. J. Am. Chem. Soc. 1999;121:4530.Huynh MHV, Meyer TJ. Proc. Nat. Acad. Sci. 2004;101:13138. doi: 10.1073/pnas.0405086101.
- 8.Cowley RE, Bontchev RP, Duesler EN, Smith JM. Inorg. Chem. 2006;45:9771. doi: 10.1021/ic061299a. [DOI] [PubMed] [Google Scholar]
- 9(a).Murray BD, Power PP. Inorg. Chem. 1984;23:4584. [Google Scholar]; (b) Putzer MA, Neumüller B, Dehnicke K, Magull J. Chem. Ber. 1996;129:715. [Google Scholar]; (c) Panda A, Stender M, Wright RJ, Olmstead MM, Klavins P, Power PP. Inorg. Chem. 2002;41:3909. doi: 10.1021/ic025552s. [DOI] [PubMed] [Google Scholar]; (d) Panda A, Stender M, Olmstead MM, Klavins P, Power PP. Polyhedron. 2003;22:67. [Google Scholar]
- 10.Altwicker ER. Chem. Rev. 1967;67:475. [Google Scholar]
- 11(a).Other cobalt(III) imidos:Jenkins DM, Betley TA, Peters JC. J. Am. Chem. Soc. 2002:11238. doi: 10.1021/ja026852b.Dai X, Kapoor P, Warren TH. J. Am. Chem. Soc. 2004;126:4798. doi: 10.1021/ja036308i.Hu X, Meyer K. J. Am. Chem. Soc. 2004;126:16322. doi: 10.1021/ja044271b.Mehn MP, Brown SD, Jenkins DM, Peters JC, Que L. Inorg. Chem. 2006;45:7417. doi: 10.1021/ic060670r.
- 12(a).Mayer JM, Rhile IJ, Larsen FB, Mader EA, Markle TF, DiPasquale AG. Photosynth. Res. 2006;87:3. doi: 10.1007/s11120-005-8164-3. [DOI] [PubMed] [Google Scholar]; (b) Roth JP, Lovell S, Mayer JM. J. Am. Chem. Soc. 2000;122:5486. [Google Scholar]; (c) Lebeau EL, Binstead RA, Meyer TJ. J. Am. Chem. Soc. 2001;123:10535. doi: 10.1021/ja000517a. [DOI] [PubMed] [Google Scholar]; (d) Soper JD, Mayer JM. J. Am. Chem. Soc. 2003;125:12217. doi: 10.1021/ja036328k. [DOI] [PubMed] [Google Scholar]
- 13.Mindiola DJ, Hillhouse GL. J. Am. Chem. Soc. 2001;123:4623. doi: 10.1021/ja010358a. [DOI] [PubMed] [Google Scholar]
- 14.Alternate spin states were found to be within 12 kcal/mol of the lowest energy spin state. See SI for details.
- 15.Lucarini M, Pedrielli P, Pedulli GF, Cabiddu S, Fattuoni C. J. Org. Chem. 1996;61:9259. [Google Scholar]
- 16(a).Bordwell FG, Cheng J-P, Ji G-Z, Satish AV, Zhang X. J. Am. Chem. Soc. 1991;113:9790. [Google Scholar]; (b) Bordwell FG, Liu W-Z. J. Am. Chem. Soc. 1996;118:10819. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


