Abstract
Ghrelin increases food intake when injected into either the forebrain or hindbrain ventricles. Brain areas activated by ghrelin after forebrain delivery have been examined using Fos immunohistochemistry and include the hypothalamic arcuate (Arc) and paraventricular (PVN) nuclei, and the nucleus of the solitary tract (NTS) in the medulla. It is not clear, however, if ghrelin applied directly to the hindbrain activates forebrain structures. Therefore, we examined Fos expression in the Arc, PVN, and NTS after injecting ghrelin into the fourth ventricle. Animals treated with a hyperphagic dose of ghrelin had greater levels of Fos expression in the NTS at the level of the area postrema than animals injected with vehicle. Ghrelin did not, however, increase Fos expression in the Arc or PVN in rats with open or occluded cerebral aqueducts. Given the importance of caudal brainstem (CBS) catecholamine pathways in the control of food intake, we performed double-labeling experiments to evaluate the potential overlap between tyrosine hydroxylase TH and ghrelin-induced Fos expression. Ghrelin did not increase Fos in TH-positive neurons in the NTS, suggesting that ghrelin delivered to the fourth ventricle does not act through catecholaminergic pathways. Nevertheless, the local (NTS), but not distal (Arc and PVN), induction of Fos suggests the presence of partially independent forebrain and hindbrain circuits that respond to ghrelin. These data support the NTS as a target of ghrelin action by building upon prior findings of increases in food intake in response to third- and fourth-ventricle ghrelin delivery.
Section: Regulatory Systems
Keywords: ghrelin, tyrosine hydroxylase, feeding, hypothalamus, caudal brainstem
1. Introduction
Ghrelin is a 28-amino acid peptide produced in the stomach and small intestine that acts centrally to increase food intake (Arnold et al., 2006; Date et al., 2000; Kojima et al., 1999; Nakazato et al., 2001). Although ghrelin was initially recognized for its role in growth hormone secretion from somatotropes (Arvat et al., 2000; Kojima et al., 1999; Seoane et al., 2000), its independent feeding action arises from binding the growth hormone secretagogue receptor (GHS-R) on neurons within the brain (Cowley et al., 2003; Kojima et al., 1999; Lawrence et al., 2002; Nakazato et al., 2001; Shintani et al., 2001).
The specific CNS sites that mediate the hyperphagic effect of ghrelin have been the subject of a number of studies, the majority of which focused particularly on forebrain structures. GHS-R expression was demonstrated in neurons within the hypothalamus and injections of ghrelin into discrete hypothalamic nuclei or the forebrain ventricles increased food intake (Lawrence et al., 2002; Nakazato et al., 2001; Shintani et al., 2001). The arcuate nucleus of the hypothalamus (Arc) is strongly implicated in the mediation of the hyperphagic response to ghrelin (Cowley et al., 2003; Currie et al., 2005; Hewson and Dickson, 2000; Olszewski et al., 2003a; Olszewski et al., 2003b; Ruter et al., 2003; Wren et al., 2000). Tamura et al., (2002), for example, showed that ablation of Arc neurons by neonatal MSG treatment eliminated the feeding response to lateral intracerebroventricular (i.c.v.) administration of ghrelin. Thus, there is a clear emphasis on the importance of forebrain structures in the feeding response to ghrelin.
In spite of the focus on forebrain areas, there is ample evidence for multiple sites of ghrelin action, including portions of the caudal brainstem (CBS) (Date et al., 2006; Faulconbridge et al., 2003; Faulconbridge et al., 2005; Lin et al., 2004). Several CBS nuclei express GHS-R, including the area postrema, the nucleus of the solitary tract (NTS) and the dorsal motor nucleus of the vagus nerve, which together compose the dorsal vagal complex (DVC) (Guan et al., 1997; Zigman et al., 2006). Comparable hyperphagic effects are obtained when ghrelin is delivered to either the fourth or to the third ventricle (Faulconbridge et al., 2003). Moreover, ghrelin injected directly into or near the NTS increases food intake (Faulconbridge et al., 2003), at very low doses (10 pmol) that are below response threshold for various injection sites within the hypothalamus (Wren et al., 2001). This finding raises two possibilities: first, injections into the CBS lead to activation of forebrain structures known to respond to ghrelin, such as the Arc or the PVN, that in turn generate descending signals driving the behavioral response. Alternatively, it is possible that circuits intrinsic to the hindbrain are sufficient for the observed response, without requiring input from forebrain areas. Support exists for both possibilities. In support of the former case, recruitment of forebrain structures by hindbrain injections has been demonstrated for other feeding-relevant peptides (e.g., urocortin, Daniels et al., 2004) and, with specific respect to ghrelin, fourth-ventricular injections of ghrelin resulted in increased NPY mRNA in the arcuate nucleus (Kinzig et al., 2006). In support of the latter case, we have shown previously that hyperphagic responses to hindbrain injections of ghrelin require NPY Y1 and Y5 receptor activation in the hindbrain, but do not require activation of these receptors in the forebrain (Faulconbridge et al., 2005). As such, the hyperphagic response to fourth-i.c.v. ghrelin may be processed by circuits contained within the CBS.
In addition to functional evidence supporting disparate sites of ghrelin action, anatomical studies demonstrate that centrally applied ghrelin stimulates several distinct brain areas. Delivery of ghrelin to either the forebrain (third or lateral) ventricles, or directly into specific hypothalamic nuclei, induces Fos expression in both forebrain and hindbrain areas associated with the control of feeding behavior, such as the hypothalamic Arc and PVN, and the medullary NTS (Lawrence et al., 2002; Nakazato et al., 2001; Olszewski et al., 2003a; Olszewski et al., 2003b). Unexplored, however, is the extent of Fos expression after delivery of ghrelin to the fourth ventricle. In the present study, we asked if hindbrain ghrelin administration activates neurons within the hypothalamus and/or the caudal brainstem, using a paradigm that we had applied previously to evaluate ingestive responses. Specifically, Fos-immunoreactivity was measured in the NTS, the Arc, and the PVN after fourth-i.c.v injections of ghrelin at a dose (150pmol) shown to reliably induce a hyperphagic response (Faulconbridge et al., 2003; Faulconbridge et al., 2005).
We also used a double-labeling technique to identify Fos-expressing catecholaminergic neurons by presence of both Fos- and tyrosine hydroxylase (TH)-immunoreactivity. Of particular interest are neurons within the NTS that give rise to an ascending noradrenergic pathway to the hypothalamus that has been implicated in the regulation of food intake. Ritter et al. (2001), for example, showed that activation of this pathway is necessary for the hyperphagic response to glucoregulatory challenges. The co-expression of NPY in 81% of the relevant TH neurons in the CBS (Stornetta et al., 1999) makes this system a likely target of ghrelin action, given the dependence of ghrelin hyperphagia on NPY recruitment (Faulconbridge et al., 2005). Several studies directly implicate catecholaminergic pathways arising from the NTS in the hyperphagic response to peripheral or forebrain administration of ghrelin (Abizaid et al., 2006; Brunetti et al., 2002; Date et al., 2006; Jerlhag et al., 2006). For example, Date et al. (2006) showed, with selective immunochemical lesions, that the ingestive response to peripherally administered ghrelin relies on a noradrenergic pathway from brainstem to the hypothalamus. An elevation of Fos expression in NTS TH neurons after brainstem GHS-R stimulation would be consistent with a role for this substrate in the feeding response to ghrelin.
The results of the present experiments show that fourth-ventricle ghrelin delivery increased Fos in the NTS but not in the Arc or PVN. Ghrelin did not, however, increase Fos expression in TH-immunoreactive neurons. Thus the findings support the role of the NTS in the response to ghrelin, but do not support the suggestion that TH neurons, including those co-expressing NPY, are essential for the orexigenic effect of hindbrain GHS-R stimulation.
2. Results
2.1. Fos expression in the NTS after ghrelin administration to the fourth ventricle
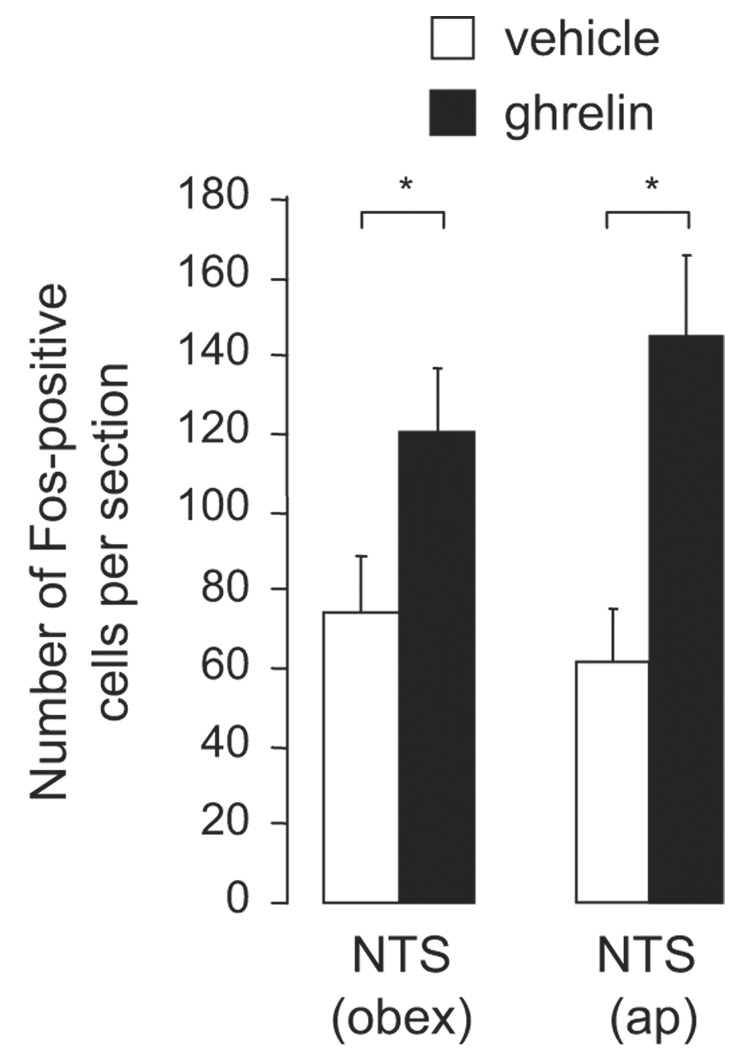
Fos-immunoreactive cells were counted at two levels of the NTS, area postrema and obex, in animals treated with ghrelin or vehicle (see Figure 1 and Figure 2). At the level of the obex, more Fos-positive cells were found in animals who received ghrelin (n=11) than in vehicle-treated animals (n=11) (t=2.23, df=20, p<0.04). Similarly, at the level of the area postrema, sections from animals treated with ghrelin revealed more Fos-positive cells than those from animals who received vehicle (t=3.49, df=20, p<0.001). There was no significant difference in the number of Fos-positive cells between the two different areas of the NTS (obex and area postrema) in either ghrelin- or vehicle-treated animals.
Figure 1.
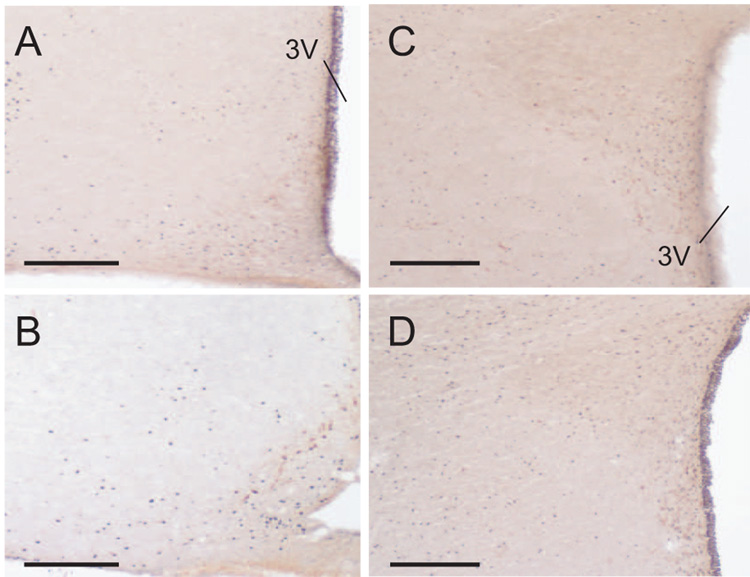
Representative micrographs of coronal brain sections immunohistochemically stained for Fos (black) and TH (brown) 90 min after injection of vehicle (A and C) or ghrelin (B and D) into the fourth ventricle. Sections containing the NTS at the level of obex (A and B) and the area postrema (C and D) are shown. The bars each represent 200 µm. Abbreviations: cc, central canal; ap, area postrema.
Figure 2.
Number of Fos-positive cells after hindbrain (fourth ventricle) injections of ghrelin (150 pmol) or vehicle. Data from the NTS at the level of obex and area postrema (ap) are shown as the mean ± SEM with 11 animals in each treatment group. Asterisks are used to indicate significant difference at p < 0.05.
2.2. Fos expression in the PVN and Arc after ghrelin administration to the fourth ventricle
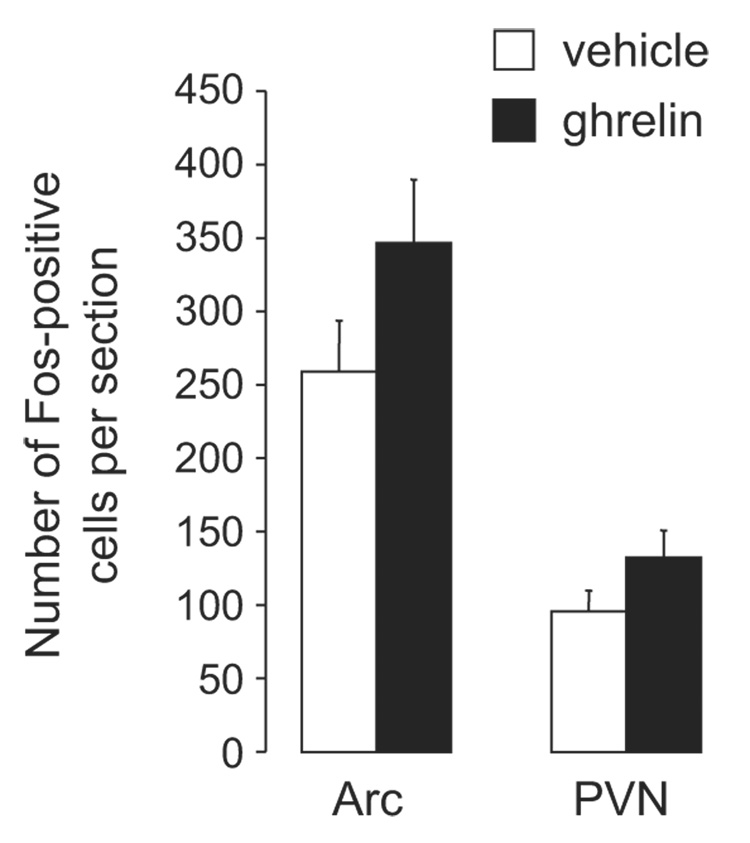
Fos expression was analyzed in sections containing the Arc (ghrelin, n=9; vehicle, n=6) and PVN (ghrelin, n=8; vehicle n=6). In contrast to studies examining Fos expression after forebrain delivery of ghrelin (Lawrence et al., 2002; Nakazato et al., 2001), we failed to detect ghrelin-induced changes in Fos-immunoreactivity in the Arc or PVN (Figure 3 and Figure 4) (Arc: t=1.53, df=13, p=0.12; PVN: t=1.51, df=12, p=0.14, respectively).
Figure 3.
Representative micrographs of coronal brain sections immunohistochemically stained for Fos (black) and TH (brown) 90 min after injection of vehicle (A and C) or ghrelin (B and D) into the fourth ventricle. Sections containing the hypothalamus showing the Arc (A and B) or PVN (C and D) are shown. The bars each represent 200 µm.
Figure 4.
Number of Fos-positive cells in the Arc and PVN after fourth-ventricle injection of ghrelin (150 pmol) or vehicle. No significant differences in Fos were found between the treatment groups in either brain area.
2.3. Effect of aqueduct occlusion on Fos expression in the NTS, Arc, and PVN after fourth ventricle application of ghrelin
The data included in the analyses described above did not parse subjects based on the presence or absence of aqueduct occlusion because aqueduct occlusion had no effect on the number of Fos-immunoreactive neurons in any of the examined brain areas, in both ghrelin- and vehicle-treated animals (all p-values >0.2; data not shown).
2.4. Effect of ghrelin on TH-containing neurons in the NTS
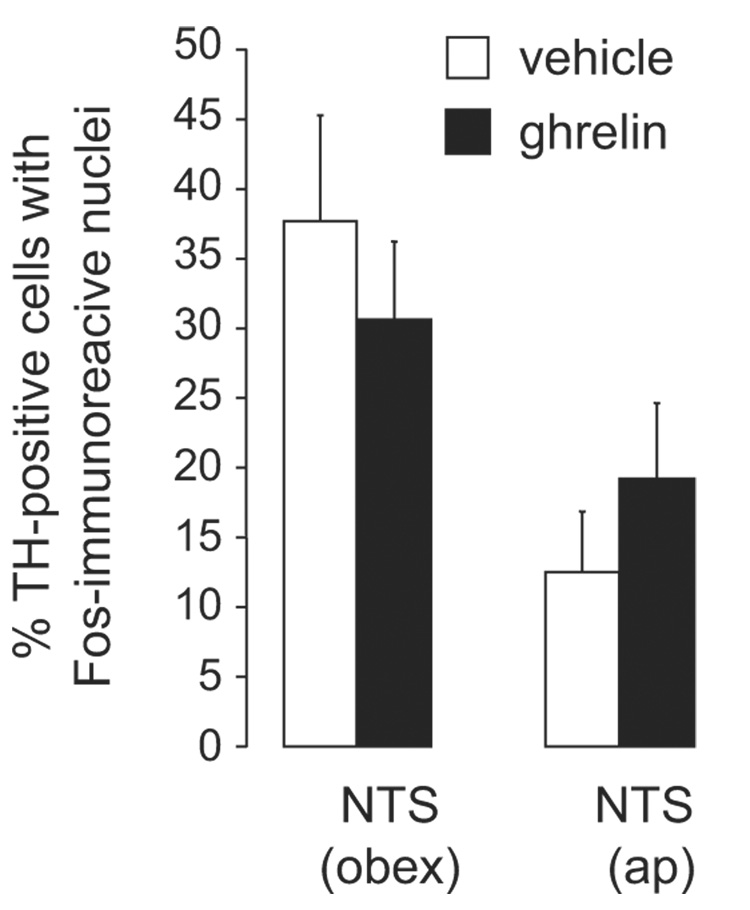
Cells that were immunoreactive for TH were found in the NTS at both the level of the area postrema and at the level of the obex, as expected, (see Figure 5 for a representative micrograph). Ghrelin administration did not affect the number of TH-positive cells in either area (p-values >0.2; data not shown). Moreover, there was no difference between ghrelin- and vehicle-treated animals in the number of TH-positive cells that also stained for Fos in the NTS, either at the level of the obex (t=1.01, df=19, p=0.32), or at the level of the area postrema (t=0.85, df=18, p=0.40, see Figure 6). In the vehicle-treated group, a higher percentage of TH cells that also contained Fos was seen in the obex than in the area postrema (t=3.32, df=18, p<0.004), but in the ghrelin-treated group there was no significant difference (t=1.59, df=19, p=0.13) in the percentage of double-labeled cells in either of the two regions.
Figure 5.
Representative micrographs of a coronal brain section showing double-label immunohistochemistry for Fos and TH 90 min after injection of ghrelin into the fourth ventricle. Fos-immunoreactivity was confined to the nuclear fraction and was visualized using a nickel sulfate-enhanced DAB reaction to generate a black precipitate. Large open arrows are used to highlight examples of cells that were immunoreactive for only Fos. Immunoreactivity of TH was visualized using a reduced concentration of DAB without nickel sulfate to yield a light brown precipitate. Open arrows are used to highlight examples of cells containing immunoreactivity for TH only. Examples of double-labeled cells are highlighted by small, filled arrows. The boxed area is shown at greater magnification in the inset. Bar = 50 µm (inset) or 200 µm.
Figure 6.
The percent of TH-immunoreactive cells that also contained Fos-immunoreactive nuclei were quantified and shown here as the mean ± SEM. No significant differences were found between ghrelin- and vehicle treated animals in the NTS at either the level of the obex or area postrema (ap).
3. Discussion
The caudal brainstem contributes to the hyperphagic response to both central (Faulconbridge et al., 2005) and peripheral (Date et al., 2006) ghrelin delivery. Forebrain ventricular ghrelin injections increase neural activity in the hypothalamus (e.g., Arc and PVN) as well as the hindbrain (e.g., NTS) (Lawrence et al., 2002; Nakazato et al., 2001; Olszewski et al., 2003a; Olszewski et al., 2003b), but the anatomical distribution of Fos expression resulting from hindbrain injection of ghrelin had not been examined. Therefore we evaluated whether or not hindbrain application of ghrelin increases Fos expression in forebrain structures, when delivered at a dose known to elicit hyperphagia (Faulconbridge et al., 2003; Faulconbridge et al., 2005). The present experiments revealed neural activation in the NTS, both at the level of the area postrema and the obex. These results are consistent with previous reports of GHS-R expression in these areas (Guan et al., 1997; Zigman et al., 2006) and the finding that low-dose parenchymal injections in or near the NTS induces robust feeding responses (Faulconbridge et al., 2003). Interestingly, Fos expression was not increased in brainstem TH neurons, leaving open the question of the phenotype of NTS neurons that respond to hindbrain ghrelin delivery.
In addition to evaluating the response in the NTS, we measured Fos expression in forebrain structures following fourth-i.c.v ghrelin injections. In contrast to the increased Fos observed in the NTS, we did not find significant elevation of Fos in either the Arc or the PVN in ghrelin-treated animals, although both of these regions express GHS-R (Guan et al., 1997; Zigman et al., 2006). Taken together with data from studies using forebrain or peripheral injections of ghrelin, these results indicate that the pattern of neuronal activation after orexigenic doses of ghrelin differs markedly as a function of where -- CBS, forebrain, or systemic -- the hormone is delivered.
The present results are consistent with the role of the NTS in the control of feeding behavior. Indeed previous studies demonstrated similar ghrelin-induced increases in Fos expression in the NTS, regardless of the site of administration (e.g., Lawrence et al., 2002; Nakazato et al., 2001; Olszewski et al., 2003a; Olszewski et al., 2003b). Additional work is required, however, to identify the neurochemical phenotype of the NTS neurons that respond to ghrelin and that are presumed to play a role in the resultant hyperphagic response. Several lines of evidence support the hypothesis that an NPY/noradrenergic pathway arising from the NTS plays a significant role in mediating the hyperphagic effects of CBS GHS-R stimulation (Ritter et al., 2001). Our results, however, provide no evidence to suggest a noradrenergic contribution to the hyperphagic effect of ghrelin delivered to the CBS. Despite the robust elevation in the number of Fos-expressing neurons, there was no such increase within TH-expressing neurons. Because the methods used here will detect all cells expressing TH, not just the sub-population co-expressing NPY, a more specific phenotyping of these cells may reveal a subpopulation that is activated selectively by ghrelin administration. Further, it should be emphasized that our tentative negative conclusion about a role for TH neurons in the NTS is limited in scope to the hyperphagic effects induced specifically from the hindbrain site of ghrelin administration. Evidence cited above, in fact, clearly implicates an ascending NPY/noradrenergic pathway in the response to peripherally injected ghrelin (Date et al., 2006). Overall, it is reasonable to conclude that the site of ghrelin administration can uniquely determine the sub-population of GHS-R neurons that are directly stimulated, the set of nearby and distant structures activated via neuronal transition, as well as the pathway(s) required for expression of the ingestive response.
The present data indicate that the ingestive response to CBS ghrelin administration does not depend on activation of neurons in the PVN or Arc, despite the fact that these hypothalamic structures are often considered critical in transmitting the orexigenic influence of ghrelin delivered peripherally or to the forebrain (Cowley and Grove, 2004; Cummings, 2006). The lack of ghrelin-induced Fos expression in the forebrain structures is in clear contrast to the marked elevation in Fos expression observed when ghrelin was delivered via other routes (to the forebrain or to the periphery) (Nakazato et al., 2001; Olszewski et al., 2003a; Olszewski et al., 2003b). Our results are consistent with the suggestion that activation of these structures is not required for a hyperphagic response to ghrelin from every site at which one can be elicited. The negative result, however, must be interpreted with some caution. Indeed, we had expected increases in Fos expression in these forebrain structures based on previous studies. Kinzig et al. (2006) reported increases in NPY mRNA in the Arc after fourth-i.c.v. application of ghrelin, but with a dose six-fold higher than the one used in the present study. (Our decision to include the occluded aqueduct control was based on these findings and would have assured that any response in the forebrain structures did not reflect simple diffusion of injected ghrelin that directly stimulated these substrates.) The possibility remains that a response in the forebrain may be observed with higher doses of ghrelin or with alteration of the background conditions of the present study (e.g., changing the feeding state of the animal or injection timing). Scott et al. (2007), for example, demonstrated differential magnitude of ghrelin-induced Fos expression dependent on the feeding state of the animal. Nevertheless, the relatively low dose (and timing of the injection) used here produces reliable and robust hyperphagic effects (Faulconbridge et al., 2003; Faulconbridge et al., 2005), without increasing Fos in the PVN or Arc. As such, the present lack of Fos expression in these forebrain nuclei after hindbrain administration of ghrelin is in clear contrast to other studies reporting increases in Fos in these structures after peripheral or forebrain delivery.
This study adds support for a distributed, rather than localized, model of ghrelin action (Faulconbridge et al., 2005; Gilg and Lutz, 2006; Grill and Kaplan, 2002). The overall distribution of Fos expression described here after fourth-i.c.v. delivery contrasts with the pattern of expression observed when ghrelin was delivered to the periphery or to other CNS locations (Lawrence et al., 2002; Nakazato et al., 2001; Olszewski et al., 2003a; Olszewski et al., 2003b). The difference in Fos expression is not surprising given previous work indicating that there are several sites, across different levels of the neuraxis, at which local GHS-R stimulation drives a hyperphagic response (Faulconbridge et al., 2005). It is also clear that pathways required for expression of feeding responses also can vary as a function of the site at which the hormone is delivered. For example, Date et al. (2006) found that peripherally administered ghrelin requires central catecholaminergic pathways for the induced hyperphagia, but that these catecholaminergic cells are not required for the hyperphagic response to centrally applied ghrelin. The lack of Fos expression in TH neurons after central delivery of ghrelin in the present study is consistent with the failure to disrupt ghrelin-induced feeding by destroying ascending catecholamine pathways when ghrelin was administered centrally (Date et al., 2006). Caution, therefore, must be exercised in generalizing interpretations based on single-site pharmacological treatment. Nevertheless, the current findings complement previous research by highlighting the multiple mechanisms through which ghrelin may act in the CNS to regulate food intake and may aid in the generation of a satisfactory model for the central networks that underlie feeding responses to physiological hormone levels.
4. Experimental Procedures
4.1. Animals
Twenty-two male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 295–330 g at surgery were housed in hanging stainless steel cages under a 12:12 hour light: dark cycle (lights on 9 am). Pelleted chow (Ralston Purina Co., St. Louis, MO) and water were available ad libitum. The experimental protocols used conform to institutional standards of animal care and the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
4.2. Surgery
Rats were anesthetized with ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) delivered intramuscularly. A 22G guide cannula (Plastics One; Roanoke, VA) was implanted 2 mm above an injection site in the fourth ventricle (coordinates were on the midline, 2.5 mm anterior to the occipital suture and 5.2 mm below the skull surface). A second guide cannula, aimed at the cerebral aqueduct, was angled 11° in the lateral-midline direction and guided to a position on the midline, 7 mm posterior to bregma and 5 mm below the dura. The cannulas were attached to the skull with jeweler’s screws and dental acrylic and closed with obturators.
4.3. Verification of fourth-ventricular cannula positions
Ventricular cannula placements were evaluated functionally after at least 7 d of recovery from surgery through measurement of the sympathetically-mediated hyperglycemic response to 210 µg of 5-Thio-D-glucose (5TG) in 2 µl artificial CSF (aCSF) (Ritter et al., 1981). Only rats that showed at least a two-fold increase of plasma glucose level in response to this treatment were used in the experiments.
4.4. Aqueduct occlusion: delivery and verification
For those animals who received aqueduct occlusion (n=10), 5 ul of a 50:50 ratio of High Vacuum grease (Dow Corning) and Dialectic Connector grease (VersaChem) mixed with 0.1 ug of fast green dye (for visualization) was injected via a 100ul Hamilton syringe into the cerebral aqueduct 1 h before drug injections on the test day. Complete occlusion was verified histologically by inspection of the location of the injected green dye.
4.5. Drug Preparation and Injection
Rat ghrelin (Phoenix Pharmaceuticals, Belmont, CA) was dissolved in aCSF and stored at −80° C. Ghrelin (150 pmol) or vehicle (2ul aCSF) was delivered via a Hamilton microsyringe (Reno, NV) connected by polyethylene tubing to a 28-G injector that extended beyond the guide cannula and into the ventricle. The 150-pmol ghrelin dose is known to be a moderate, supra-threshold dose for the feeding response upon forebrain or brainstem ventricular delivery (Faulconbridge et al., 2003; Faulconbridge et al., 2005; Nakazato et al., 2001). Final experimental injections were made during the early hours of the light portion of the light-dark cycle, consistent with previous studies using this paradigm (Faulconbridge et al., 2003; Faulconbridge et al., 2005). On at least two occasions before experimental testing, rats were given vehicle injections in order to habituate them to the injection procedure. In all experiments, food was available until the injection time, after which the animals had no access to food or water.
4.6. Perfusions and tissue preparation
Ninety minutes after vehicle (occluded, n=5; non-occluded n=6) or ghrelin (occluded, n=5; non-occluded n=6) was injected into the fourth ventricle, the animals were anesthetized with ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) and transcardially perfused with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed from the crania and postfixed for no more than 24 hr. After being submerged in 20% sucrose in 0.1 M PB for at least 48 hr, brains were frozen and cut by microtome into four sets of 30 µm coronal sections. Sections were stored in cryoprotectant (Watson et al., 1986) at −20° until processed by immunohistochemistry as described below.
4.7. Immunohistochemistry
Immunohistochemistry for Fos and for TH was performed on one set of free-floating sections from each animal. Sections were removed from cryoprotectant, washed in Tris-buffered saline (TBS), pH 7.4, and incubated for 15 min in TBS containing 0.3% H2O2 before being washed again and incubated overnight at room temperature in rabbit anti-Fos (1:5,000; Calbiochem, San Diego, CA) and mouse anti-TH (1:5000, Chemicon, Temecula, CA) diluted in TBS containing 0.2% Triton X-100 and 3% normal donkey serum. Sections then were washed in TBS and incubated for 2 hr with biotinylated donkey anti-rabbit IgG (1:1000; Jackson ImmunoResearch, West Grove, PA). After a brief wash with TBS, sections were incubated for 1 hr in an avidin–biotin–peroxidase complex (1:333; Elite kit; Vector Laboratories, Burlingame, CA). Sections were washed again with TBS and then with 50 mM Tris, pH 7.4, before immunoreactivity was visualized by incubation for 5 min with 3,3-diaminobenzidine (0.2 mg/ml) and 0.025% H2O2 in 50 mM Tris with 25mg/ml nickel sulfate. The reaction was stopped with TBS washes, after which the sections were incubated in H2O2 to quench remaining peroxidases before incubation for 2 hr with biotinylated donkey anti-mouse (1:1000; Jackson ImmunoResearch). Sections were washed and incubated in avidin-biotin-peroxidase complex (1:333; Vector Laboratories) before being washed and reacted with 3,3-diaminobenzidine (0.1 mg/ml) and 0.025% H2O2 in 50 mM Tris for 5 min. The protocol was developed to produce dark, blue-black nuclear Fos-immunoreactivity and light brown somatic TH-immunoreactivity. Sections were floated onto Superfrost plus slides (Fisher, Pittsburgh, PA), dehydrated with increasing concentrations of alcohol followed by Hemo-De (Fisher Scientific, Pittsburgh, PA), and coverslipped with Permount (Fisher Scientific).
4.8. Data analysis
Immunohistochemically stained sections were visualized and imaged using a Nikon Eclipse 80i microscope (Nikon USA, Melville, NY) with attached RT-KE Spot digital camera (Diagnostic Instruments, Sterling Heights, MI). Double-labeled cells were quantified with care taken to ensure that the black, nuclear precipitate was in the same focal plane as the brown, somatic precipitate. Fos immunoreactivity was quantified by counting labeled nuclei on printed digital micrographs that had been coded such that the treatment condition was unknown. Two to four hemisections per brain area from each animal were quantified in duplicate with an error rate lower than 5%. Somatic labeling was ignored in this analysis and the number of double-labeled cells from the microscopic analysis was added later to insure that the total number of Fos-labeled nuclei was represented by the final count. The average number of cells per section or hemisection was determined for each brain area from each animal and statistical comparisons were made using SigmaStat (Systat Software Inc, San Jose, CA). Data for each brain nuclei were analyzed separately with a two-tailed t-test and expressed graphically as the mean ± SEM number of cells per section or hemisection.
Acknowledgements
We thank Katelyn Rood and Ethan Gable for help with data collection. This work was supported by N.I.H grants DK-73800 (DD), DK-21397 (HJG), and DK-42284 (JMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–495. doi: 10.1007/BF03343763. [DOI] [PubMed] [Google Scholar]
- Brunetti L, Recinella L, Orlando G, Michelotto B, Di Nisio C, Vacca M. Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur J Pharmacol. 2002;454:189–192. doi: 10.1016/s0014-2999(02)02552-9. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Grove KL. Ghrelin--satisfying a hunger for the mechanism. Endocrinology. 2004;145:2604–2606. doi: 10.1210/en.2004-0346. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol. 2005;289:R353–R358. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–331. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes. 2005;54:1985–1993. doi: 10.2337/diabetes.54.7.1985. [DOI] [PubMed] [Google Scholar]
- Gilg S, Lutz TA. The orexigenic effect of peripheral ghrelin differs between rats of different age and with different baseline food intake, and it may in part be mediated by the area postrema. Physiol Behav. 2006;87:353–359. doi: 10.1016/j.physbeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Lateral ventricular ghrelin and fourth ventricular ghrelin induce similar increases in food intake and patterns of hypothalamic gene expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1565–R1569. doi: 10.1152/ajpregu.00785.2005. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43:977–982. doi: 10.1161/01.HYP.0000122803.91559.55. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-Fos immunoreactivity. Peptides. 2003a;24:919–923. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003b;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- Ruter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Wiedenmann B, Tache Y, Monnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res. 2003;991:26–33. doi: 10.1016/j.brainres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Scott V, McDade DM, Luckman SM. Rapid changes in the sensitivity of arcuate nucleus neurons to central ghrelin in relation to feeding status. Physiol Behav. 2007;90:180–185. doi: 10.1016/j.physbeh.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Seoane LM, Tovar S, Baldelli R, Arvat E, Ghigo E, Casanueva FF, Dieguez C. Ghrelin elicits a marked stimulatory effect on GH secretion in freely-moving rats. Eur J Endocrinol. 2000;143:R7–R9. doi: 10.1530/eje.0.143r007. [DOI] [PubMed] [Google Scholar]
- Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat medulla. J Comp Neurol. 1999;415:482–500. doi: 10.1002/(sici)1096-9861(19991227)415:4<482::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143:3268–3275. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]