Abstract
The viscous seed mucilage of flax (Linum usitatissimum) is a mixture of rhamnogalacturonan I and arabinoxylan with novel side group substitutions. The rhamnogalacturonan I has numerous single nonreducing terminal residues of the rare sugar l-galactose attached at the O-3 position of the rhamnosyl residues instead of the typical O-4 position. The arabinoxylan is highly branched, primarily with double branches of nonreducing terminal l-arabinosyl units at the O-2 and O-3 positions along the xylan backbone. While a portion of each polysaccharide can be purified by anion-exchange chromatography, the side group structures of both polysaccharides are modified further in about one-third of the mucilage to form composites with enhanced viscosity. Our finding of the unusual side group structures for two well-known cell wall polysaccharides supports a hypothesis that plants make a selected few ubiquitous backbone polymers onto which a broad spectrum of side group substitutions are added to engender many possible functions. To this end, modification of one polymer may be accompanied by complementary modifications of others to impart functions to heterocomposites not present in either polymer alone.
The primary cell walls of plants are dynamic composites. A fundamental scaffold of cellulose microfibrils is coated and interlaced with cross-linking glycans and embedded in a coextensive and interactive pectin matrix (McCann and Roberts, 1991; Carpita and Gibeaut, 1993). The major cross-linking glycans xyloglucan, glucuronoarabinoxylan, and glucomannan are found in all angiosperms, but they vary greatly in relative abundance and side group substitution in a species-dependent manner (Carpita and McCann, 2000). Two major pectins, homogalacturonan and rhamnogalacturonan I (RG I), are ubiquitous among angiosperms but exhibit great variation in side group substitution that is cell and developmental stage specific (Willats et al., 2001).
The structural and functional relationships and dynamics of the side group substitutions of pectins are not completely established, but there is general agreement that pectins establish wall porosity, provide the milieu for control of charge density and pH, and constitute a major determinant of the rheological properties of the wall (Jarvis, 1984; Baron-Epel et al., 1988; Willats et al., 2001). Seed mucilages provide a system to examine the physical properties of pectin gels in the absence of cellulose and its cross-linking glycans. The epidermal cells of the seed coats of certain plants release large quantities of mucilage that form a gel-like capsule surrounding the seed upon imbibition (Frey-Wyssling, 1976; Esau, 1977). The functions of mucilage are still matters of speculation, but they are proposed to aid in dispersal by sticking to animals, to resist being swept away by wind or rain by adhering to soil, to facilitate seed hydration or resist desiccation during brief drought after imbibition, and/or to provide a nutrient reserve material during germination (Esau, 1977; Franz, 1989; Gutterman and Shemtov, 1996).
Seed mucilages vary in their chemical composition, but RG I appears to be a common constituent (Bailey, 1935; Smith and Montgomery, 1959; Gutterman and Shemtov, 1996). The RG I has a backbone structure of repeating dimers of →2)-α-l-rhamnose-(1→4)-β-d-galactosyluronic acid-(1→, in which variable amounts of neutral polymers of branched or unbranched 5-linked α-arabinans, 4-linked β-galactans, or type I arabinogalactans are attached to the O-4 position of the rhamnose residues in a cell and developmentally specific pattern (Talmadge et al., 1973; Darvill et al., 1980; McCann and Roberts, 1991; Carpita and Gibeaut, 1993; Willats et al., 2001). Okra (Abelmoschus esculentus) seed pods are rich in d-GalA, l-Rha, and d-Gal, with d-GalA attached to the O-2 of l-Rha (Whistler and Conrad, 1954a, 1954b); mucilage from seeds of white mustard (Brassica alba; Bailey and Norris, 1932) and cress (Lepidium sativum; Bailey, 1935) are also rich in l-Rha and d-GalA, but they also contain l-Ara and d-Gal. In contrast, seeds of the plantain family (Plantago psyllium and subspecies) have two distinct polysaccharides in the seed mucilage, one highly acidic and rich in l-Rha and d-GalA and one relatively neutral, with l-Ara and d-Xyl (Jones and Albers, 1955). The Arabidopsis (Arabidopsis thaliana) mucilage is composed almost exclusively of RG I, with a low frequency of side branching with both 5-arabinans and nonreducing terminal galactosyl residues (Western et al., 2000, 2001; Penfield et al., 2001; Dean et al., 2007).
Because of its traditional health benefits and the commercial utility of its viscous mucilage, the chemical composition of the seed mucilage of flax (Linum usitatissimum) was investigated early in the last century. Acidic hydrolysates of flax mucilage yielded primarily an aldobiouronic acid (Neville, 1912), shown later to be a dimer of d-GalA and l-Rha (Anderson and Crowder, 1930). Identification of this aldobiouronic acid as α-d-galactosyluronic acid-(1→2)-l-rhamnose (Tipson et al., 1939) is the earliest report in the literature of the repeating unit of the backbone of RG I. Flax mucilage was found to be a surprisingly good source of the rare sugar l-Gal (Anderson, 1933), and l-Ara and d-Xyl were also identified as significant neutral sugars in its composition (Anderson and Lowe, 1947; Easterby and Jones, 1950). More recently, the flax mucilage was characterized as a mixture of neutral arabinoxylans and strongly acidic rhamnose-containing polymers (Muralikrishna et al., 1987; Cui et al., 1994; Fedeniuk and Biliaderis, 1994; Warrand et al., 2005a, 2005b).
Here, we report that the arabinoxylan backbone of the mucilage is typical of many dicotyledonous species, but it is unusual because of the high degree to which t-Araf residues are attached to both the O-2 and O-3 of the (1→4)-β-d-xylan chain to form doubly branched residues. The arabinoxylan is weakly acidic, owing to the t-GlcA residues also attached to the xylan chain. The side group structure of the flax RG I is also atypical, with single nonreducing terminal l-Gal and l-Fuc residues attached to the O-3 position instead of the O-4 position. While some of the arabinoxylan and RG I can be separated chromatographically, a composite of the two polymers not separated by anion-exchange chromatography was found to have enhanced viscosity unable to be reproduced in mixtures of the two isolated polymers. These findings support a concept that plants synthesize a few common polymer backbone compositions; however, depending on the developmental context, the side group constituent linkage structure is modified in diverse ways to fine-tune their physical, biological, and physiological functions. Flax mucilage also serves as an example that alterations in the fundamental structure of one polymer are complemented by alterations in others to form composites to yield a property not present in either polymer alone.
RESULTS
Arabidopsis and Flax Mucilages Exist in Two States
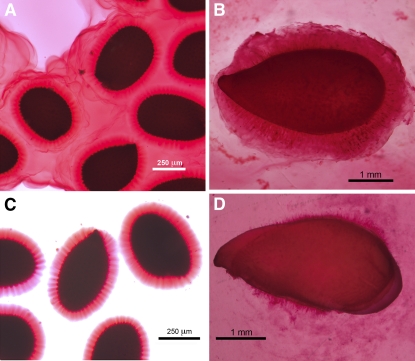
The mucilages of flax and Arabidopsis exist in two states, one that is water soluble and is easily separated from the seed coat and a second that tightly adheres to the special secretory cell walls. If the seeds of each are submerged in water containing ruthenium red, then the water-soluble mucilage is gelled by the stain and becomes visible (Fig. 1, A and B). However, when seeds are soaked in water overnight and gently swirled and then stained with ruthenium red, only the tightly adhering mucilage is observed (Fig. 1, C and D). Whereas the Arabidopsis water-soluble mucilage disperses in the surrounding medium, the flax mucilage is more abundant and precipitates into masses of ruthenium red-positive material (Fig. 1, B and D). The water-soluble mucilage of flax represents about 2% of the total seed mass.
Figure 1.
Micrographs of fresh samples of Arabidopsis and flax seeds in the presence or absence of 0.2% (w/v) ruthenium red (Sigma) in water containing 0.1% Tween 20 for 18 h. Samples without ruthenium red were then stained with the reagent 3 h before photography. A, Arabidopsis seeds placed directly in ruthenium red solution. B, Flax seeds placed directly in ruthenium red solution. C, Arabidopsis seeds incubated in water overnight, swirled gently, and stained with ruthenium red. D, Flax seeds incubated in water overnight, swirled gently, and stained with ruthenium red.
Linkage Composition of Flax and Arabidopsis Water-Soluble Mucilages
The water-soluble mucilages of both Arabidopsis and flax were dialyzed extensively against deionized water and freeze dried. Neither the flax nor Arabidopsis mucilage was methyl esterified when measured by either methanol release upon saponification (Wood and Siddiqui, 1971) or a selective reduction method with NaBD4 (Carpita and McCann, 1996). However, the degree of acetylation, determined by the Hestrin assay (1949), was substantial for Arabidopsis, whereas acetylation of the flax mucilage was below detection. Degrees of acetylation for the Arabidopsis mucilage ranged from 3 to 7 mol % of total sugar, or 5 to 14 mol % of uronic acid residues, depending on the batch of seeds.
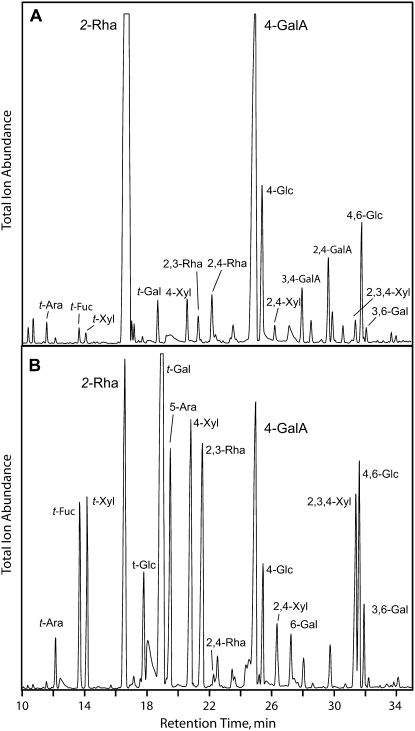
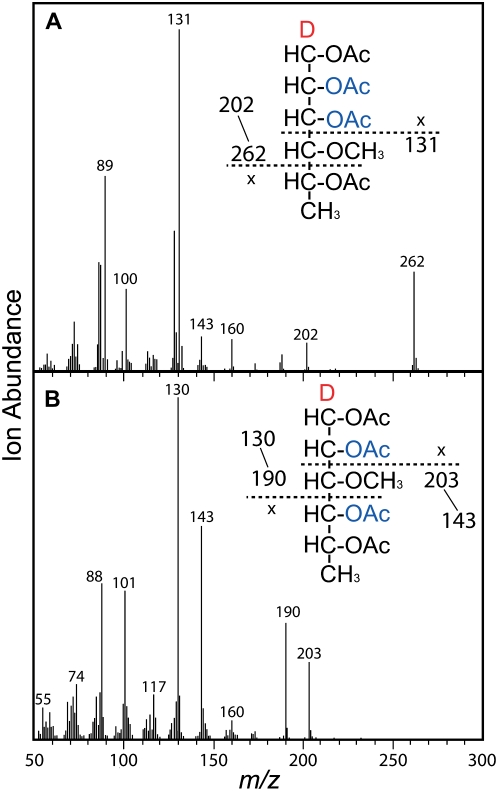
For linkage analysis, the glycosyluronic acid residues in water-soluble mucilage fractions from both Arabidopsis and flax were activated with a water-soluble carbodiimide and reduced with NaBD4 to generate 6,6-dideutero derivatives of their respective neutral sugars. The additional 2 atomic mass units permitted the former uronic acids to be differentiated from their respective neutral sugars by gas-liquid chromatography electron-impact mass spectrometry (GLC-EIMS) of alditol acetates. Linkage analysis showed the enhanced complexity of the flax mucilage over that from Arabidopsis (Fig. 2, A and B). The Arabidopsis mucilage is dominated by 2-Rha and 4-GalA residues of RG I, with small amounts of 2,3-Rha and 2,4-Rha, indicating a low degree of branching. The most likely corresponding branch point residues are nonreducing t-Gal units. Linkages typical of arabinoxylans, namely t-Ara, 4-Xyl, 2,4-Xyl, and the doubly branched 2,3,4-Xyl, are also detected in small quantities. In contrast, the flax mucilage comprises an abundant highly branched arabinoxylan, with more of the residues doubly branched than singly branched (Fig. 2B). RG I is also a major constituent of the flax mucilage, but in contrast to that of Arabidopsis, it is more highly branched. The appearance of 2,3-Rha instead of 2,4-Rha branch point residues indicated that side group constituents of the flax RG I were attached at the rhamnosyl O-3 position instead of the expected O-4 position. The 2,3-Rha and 2,4-Rha branch point residues are easily distinguished by EIMS because of their characteristic fragmentation patterns (Fig. 3, A and B). Arabidopsis RG I shows 2,3-Rha residues as well, but they are in lower abundance than the 2,4-Rha residues (Fig. 2A). In flax mucilage RG I, the 2,4-Rha residues are barely detectable (Fig. 2B). The principal side group constituent sugars of the flax mucilage RG I are t-Fuc and t-Gal.
Figure 2.
Separation of partly methylated alditol acetates by gas chromatography reveals greater complexity of the flax mucilage. A, Arabidopsis mucilage. B, Flax mucilage. Partly methylated alditol acetates were made after carboxyl reduction with CMC and NaBD4 to generate 6,6-dideutero-hexitols of Gal and Glc to differentiate the respective uronic acid from its neutral sugar. Peaks designated as GalA are 6,6-dideuterogalactosyl residues. Elution profiles and linkage structures were determined as described previously (Carpita and Shea, 1989).
Figure 3.
Electron-impact mass spectra confirm the 2,3-Rha (A) and 2,4-Rha (B) branch point residues. The anomeric carbons of all partly methylated sugars are reduced with NaBD4 to tag all fragments containing the C-1. Cleavage on either side of the C-4 methoxylated carbon gives m/z 262 and m/z 131 as major primary fragments, unequivocally showing the C-2 and C-3 acetylations indicative of the 2,3-Rha branch point residue. In contrast, cleavage on either side of the C-3 methoxylated carbon gives m/z 190 and m/z 203 as major primary fragments, unequivocally showing the C-2 and C-4 acetylations indicative of the typical 2,4-Rha branch point residue. [See online article for color version of this figure.]
Fractionation of the Water-Soluble Flax Mucilage
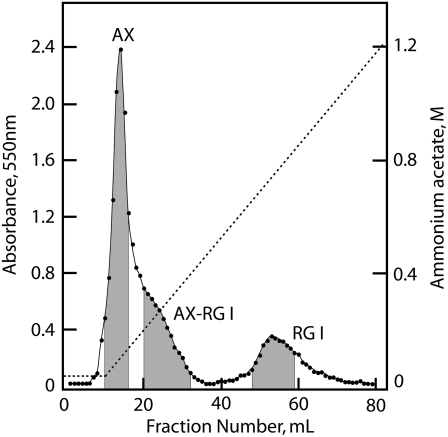
Size fractionation of the flax mucilage by gel-permeation chromatography on columns of Sepharose 4B-CL gave a void peak of about 5,000 kD and a broad distribution of small polysaccharides to less than 50 kD, with no clear separation of distinct polymer fractions (data not shown). Anion-exchange chromatography on columns of DEAE A-25 in a gradient of ammonium acetate, pH 5.2, to 1.2 m gave three major fractions: a sharp peak voiding the column, a long tailing fraction of the void fraction, and an included peak eluting with high salt (Fig. 4). The uronosyl residues in each of these three fractions were carboxyl reduced with NaBD4 as described previously, and monosaccharide and linkage analyses were performed (Table I). The void fraction was predominantly arabinoxylan and the late-eluting fraction was RG I, but the tailing fraction was an unresolved composite of both polysaccharides. A feature of both flax and Arabidopsis RG I that remains unexplained is the great abundance of Rha over GalA (Table I). The classical ratio of 1:1 is not maintained, and no other linkage group is present to interrupt the expected disaccharide backbone units. The isolated RG I fraction was highly branched, with a 2,3-Rha:2-Rha ratio of nearly 7:1, and the enrichment of t-Fuc and t-Gal in this fraction confirms them both as the major side groups.
Figure 4.
Separation of three fractions of the flax mucilage by anion-exchange chromatography. The mucilage polysaccharides dissolved in 10 mL of 30 mm ammonium acetate, pH 5.2, were loaded on a 2.5-cm × 10-cm column of Sephadex A-25 (Sigma) equilibrated in the same buffer and, after a 5-mL elution in 30 mm running buffer, were separated in an 80-mL gradient of ammonium acetate, pH 5.2, from 30 mm to 1.2 m. Carbohydrate in 1-mL samples was determined by the phenol-sulfuric acid assay (Dubois et al., 1956). Three fractions were pooled as designated in gray, dialyzed against deionized water, and freeze dried for further analysis.
Table I.
Linkage analysis of the three primary fractions of flax seed mucilage
The three major mucilage fractions recovered from DEAE chromatography were carboxyl reduced with a water-soluble carbodiimide and NaBD4 to produce 6,6-dideuteroglycosides that could be distinguished from their respective neutral monosaccharide by EIMS. Amounts of each monosaccharide were determined by GC-EIMS of alditol acetates, and the mole percentage of each linkage group was determined relative to total monosaccharide recovered. Values are means of two samples, with less than 5% variance for all linkage groups. nd, Not determined; tr, trace.
| Linkage | Arabinoxylan | Arabinoxylan-RG I | RG I |
|---|---|---|---|
| mol % | |||
| t-Fuc | 3.0 | 6.2 | 15.0 |
| t-Rha | tr | tr | tr |
| 2-Rha | 4.4 | 10.5 | 4.9 |
| 2,3-Rha | 6.8 | 12.8 | 32.9 |
| 2,4-Rha | 0.5 | 0.5 | 0.5 |
| t-Araf | 2.6 | 1.0 | 0.2 |
| 3-Araf | 4.3 | 2.6 | 0.3 |
| 5-Araf | 12.4 | 6.8 | 0.6 |
| 2,5-Araf | 0.4 | 0.3 | 0.2 |
| 3,5-Araf | 0.3 | 0.3 | 0.2 |
| t-Xyl | 9.5 | 5.0 | tr |
| 4-Xyl | 16.3 | 10.9 | 1.1 |
| 2,4-Xyl | 3.8 | 2.1 | 0.4 |
| 3,4-Xyl | 0.6 | 0.3 | tr |
| 2,3,4-Xyl | 13.3 | 9.0 | 0.5 |
| t-Man | tr | tr | tr |
| 4-Man | 0.8 | 1.3 | 0.7 |
| 4,6-Man | 0.1 | 0.1 | tr |
| t-Gal | 9.0 | 16.2 | 25.2 |
| 3-Gal | 0.2 | 0.4 | 0.7 |
| 6-Gal | 0.4 | 0.8 | 1.2 |
| 3,6-Gal | 0.1 | 0.3 | 0.3 |
| t-Glc | 1.5 | 0.8 | 0.1 |
| 4-Glc | 2.5 | 1.4 | 1.6 |
| 4,6-Glc | 0.6 | 0.3 | tr |
| t-GalA | tr | tr | tr |
| 4-GalA | 5.5 | 9.0 | 12.5 |
| 3,4-GalA | 0.7 | 0.9 | 0.9 |
| t-GlcA | 0.4 | 0.2 | nd |
The arabinoxylan is typical of those of type I walls, in which singly attached t-Ara and much smaller amounts of t-GlcA are attached to the O-2 position of the 4-linked xylan backbone (Table I). However, the arabinoxylan has significant amounts of doubly branched residues at both the O-2 and O-3 positions. The total ratio of branched to unbranched residues of the isolated arabinoxylan is relatively high at 1.1:1.0, with doubly branched residues constituting 75% of the branch point residues of the xylan backbone (Table I). Associated with the arabinoxylan are 3- and 5-linked arabinosyl units, and a portion of the t-Gal and t-Xyl residues must also constitute side group residues of this polysaccharide.
The linkage analysis reveals some interesting distinctions in the structures of both polymers when they join to make up the composite. For the arabinoxylan component, the ratio of branched to unbranched xylosyl residues decreases slightly to 0.96:1.00, primarily from decreases in singly branched 2,4-Xyl residues and the nonreducing terminal and linked arabinosyl residues. More marked decreases in the degree of branching of RG I are observed, dropping from nearly 7:1 of the isolated RG I to only 1:1 (Table I).
Both l- and d-Gal Are Found in the Flax Mucilage
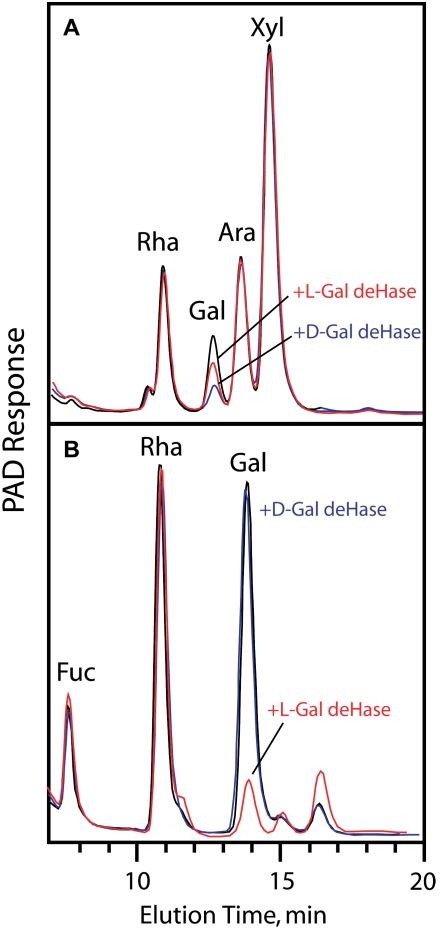
Flax mucilage is known to be a good source of l-Gal (Anderson, 1933). Amounts of l- and d-Gal associated with the arabinoxylan and RG I were determined enzymatically using their respective l- and d-Gal dehydrogenases. The isolated arabinoxylans and RG I were each hydrolyzed with trifluoroacetic acid (TFA), and the acid was evaporated. The residue was dissolved in water, and l- or d-Gal dehydrogenase was used, in paired samples, to oxidize the Gal to an unrecoverable sugar, as detected by high-performance anion-exchange chromatography (Fig. 5) or as alditol acetates by GLC (data not shown). The enzyme-catalyzed reduction of NAD was used to quantify the absolute amounts of each sugar in each fraction. The vast majority of the l-Gal is associated with RG I, whereas the d-Gal is associated with the arabinoxylan (Fig. 5). Linkage analysis indicates that both of them are mostly nonreducing terminal residues.
Figure 5.
l-Gal is a side group substituent of the mucilage RG I, and d-Gal is associated with the arabinoxylan. A, Arabinoxylan. B, RG I. Arabinoxylan and RG I were hydrolyzed to monosaccharide by TFA, and the Gal in dry residues dissolved in water was oxidized by l-Gal and d-Gal dehydrogenase. The resultant monosaccharides were separated by high-performance anion-exchange chromatography to show the relative amounts of each Gal moiety remaining. PAD, Pulsed-amperometric detection. [See online article for color version of this figure.]
The Arabinoxylan-RG I Composite Exhibits Enhanced Viscosity
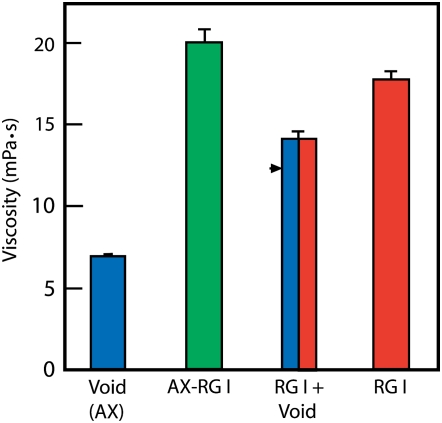
Arabinoxylan, RG I, and the arabinoxylan-RG I composite was each dialyzed extensively against deionized water, freeze dried, and brought to 1 mg mL−1 in water. Viscosity relative to water at 20°C was determined by a spinning-disc viscometer, with spacing of 0.2 mm at variable shear rates. All three exhibited Newtonian behavior, as the viscosity measured was independent of shear rate. The composite had a viscosity substantially higher than that of either the low-viscosity arabinoxylan or the high-viscosity RG I (Fig. 6). Mixtures of the arabinoxylan and RG I gave slightly higher viscosities than the predicted individual contributions, but not as high as the natural composite.
Figure 6.
A tight association of the arabinoxylan and RG I enhances mucilage viscosity. About 100 μL of 1 mg mL−1 solutions in water was assayed by spinning-disc viscometry. The arabinoxylan, RG I, and composite materials were collected after anion-exchange chromatography as described for Figure 4. Values (in millipascals per second at 20°C) are relative to water (1 mPa s−1 at 20°C) and were independent of shear rate. The arrowhead represents the expected additive contributions of arabinoxylan (AX)-void and RG I in the in vitro mixture. [See online article for color version of this figure.]
DISCUSSION
Our finding of the unusual side group structures for two well-known cell wall polysaccharides supports the hypothesis that plants make a selected few ubiquitous backbone polymers onto which a broad spectrum of side group substitutions are added to engender many possible functions. This concept is not restricted to the pectic polysaccharides. One of the better examples is xyloglucan, a principal cross-linking glycan in all angiosperms but enriched in dicots and noncommelinoid monocots. The simple (1→4)-β-d-glucan backbone is typically substituted with three contiguous Xyl units (Fry et al., 1993), but variants have been found with just two (Sims et al., 1996; Gibeaut et al., 2005; Hoffman et al., 2005) or up to five (Tiné et al., 2006) contiguous xylosylations. Upon this fundamental xyloglucan scaffold are added several other sugars in a species- and cell-specific manner. In most xyloglucan units with three contiguous xylosylations, d-Gal is added to one or both of the xylosyl units closer to the reducing end of the unit, and a portion of those at the first position are extended further by l-Fuc units. However, considerable species-specific diversity exists in the decoration of xyloglucan by l-Ara, d-Xyl, and l-Gal residues in addition to, or in place of, d-Gal (Hoffman et al., 2005; Hilz et al., 2007). Without these modifications, xyloglucans are poorly soluble in water, and d-Gal enhances their solubility (Shirakawa et al., 1998) and recognition as substrates by xyloglucan endotransglucosylases (Peña et al., 2004). As addition of d-Gal to either the first or second Xyl is sufficient for physiological function (Vanzin et al., 2002; Peña et al., 2004), the additional roles for other subtending sugars, such as d-Xyl or l-Fuc, are still to be resolved. The degree of galactosylation of galactomannans is developmentally regulated and produces different physical states of polymer behavior (Edwards et al., 1992; Redgwell et al., 2003). Galactosylation needs only to be above 10% for good solubility, but higher degrees of galactosylation result in enhancements of viscosity and gel formation (McCleary et al., 1981). Thus, addition of a single type of sugar in differing amounts and patterns of substitution can impart multiple functionalities.
Whereas the arabinoxylan contains a (1→4)-β-d-xylan backbone found in all angiosperms and the nonreducing t-Araf residues are attached O-2 of the xylosyl units, as typical for dicots, the flax arabinoxylan is unique for the high degree of doubly substituted t-Araf units on the O-2 and O-3 of the xylosyl units. High degrees of arabinosyl substitution of xylans are typical in the primary walls of grasses (Carpita and Whittern, 1986), but mostly as singly substituted xylosyl residues and at the O-3 position, not the O-2. However, like flax mucilage, arabinoxylans of the caryopsis endosperm also contain substantial amounts of doubly substituted xylosyl units (Wilkie, 1979). Like with d-Gal, l-Ara substitutions enhance water solubility of the polysaccharide, and trimming of these residues results in increased tenacity of xylan binding into the wall matrix (Carpita, 1984).
The two major pectins also display significant alterations in side group constituencies that are functionally relevant. It is expected that dynamic changes to the constituents alter the mechanical and rheological properties of the wall to suit function (Brummell, 2006; Thompson, 2008). To homogalacturonans are added xylosyl units at certain stages of development (Willats et al., 2001), and the elaboration of four complex side groups of RG II functions to stabilize the borate didiester cross-linking essential for wall tensile strength (Ryden et al., 2003) and control of porosity (Fleischer et al., 1999). RG I, which appears to hold a central function as a scaffold for the pectin network (Vincken et al., 2003; Ulvskov et al., 2005), bears a range of oligomeric and polymeric substitutions to the Rha O-4 position in cell- and developmental stage-specific patterns. The branched and unbranched (1→5)-α-l-arabinans are enriched in meristematic cells, whereas (1→4)-β-d-galactans appear during elongation (Bush et al., 2001). The Arabidopsis mucilage represents one extreme, in which the RG I is minimally branched (Penfield et al., 2001), to the other extreme in flax soluble β-galactan, in which almost every Rha unit is branched (Gorshkova et al., 1996). The depolymerization of these side chains is developmentally regulated, as well documented in fruit ripening (Brummell, 2006). Loss of β-galactans is an early event in the ripening of tomato (Solanum lycopersicum; Gross and Sams, 1984) and apple (Malus domestica; Peña and Carpita, 2004), and subsequent loss of α-arabinans prestages the debranching of an RG I associated with the loss of firm texture in apple (Peña and Carpita, 2004). The appearance of β-galactans during pea (Pisum sativum) cotyledon development is implicated in increased tissue firmness (McCartney et al., 2000), whereas α-arabinans are also implicated in wall flexibility required for the reversible opening and closing of guard cells (Jones et al., 2003). Lines of transgenic potato (Solanum tuberosum) with reduced β-galactan or α-arabinan lost water more rapidly from tuber discs exposed to strain, indicating that these particular side groups enhance not only polymer solubility but also water retention (Ulvskov et al., 2005). However, significant differences were detected in response to deformation at different strain rates, indicating that reduction in the β-galactans and α-arabinans differ in the way they reduce wall stiffness (Ulvskov et al., 2005).
For the flax mucilage, the fact that both the arabinoxylan and RG I polymers have been altered from structures normally found in the primary wall suggests that each contains complementary alterations from typical to accommodate new functions unrelated to wall physics. That seeds have at least two forms of mucilage suggests that multiple physical properties provide multiple functions. Arabidopsis and flax produce a water-soluble mucilage not connected to the seed coat that can extend the viscous matrix a considerable distance from the seed (Western et al., 2000; Macquet et al., 2007). Whereas RG I, or at least polymers containing l-Rha and d-GalA, are abundant in all plant mucilages (Smith and Montgomery, 1959), flax mucilage is a mixture of RG I and arabinoxylan. Ironically, flax provided the first evidence for the repeating dimer of the RG I backbone (Anderson and Crowder, 1930; Tipson et al., 1939), yet its side group constitution is remarkably different from the descriptions in textbooks and major reviews of (1→4)-β-d-galactans, branched and unbranched (1→5)-α-l-arabinans, and type I arabinogalactans, all attached invariably at the O-4 position of the l-rhamnosyl residues of the RG I backbone (Darvill et al., 1980; McCann and Roberts, 1991; Carpita and Gibeaut, 1993; Carpita and McCann, 2000). In flax mucilage RG I, l-Fuc and the rare sugar, l-Gal, are attached almost exclusively at the O-3, not the O-4, position.
The rheological properties vary among sources and fractions of the flax mucilage, from a viscous fluid to a viscoelastic fluid and to an elastic element, with elasticity and viscosity increasing with increasing proportions of arabinoxylan to RG I (Wannerberger et al., 1991; Cui et al., 1994). Warrand et al. (2005a, 2005b) found three populations of arabinoxylan-rich polymers based on molecular size ranging from 200,000 g mol−1 to over 5,000,000 g mol−1. Each had a similar Ara:Xyl ratio of 0.24 but varied in the apparent degree of substitution with additional Gal and Fuc side chain residues. Higher order aggregation through hydrogen bonding of the larger arabinoxylans was hypothesized to provide a basis for the high viscosity and elasticity of the mucilage (Warrand et al., 2005a). The size of the RG I molecule is considered too small to contribute substantially to the viscosity (Goh et al., 2006), but the contribution of RG I in the rheological properties of the mucilage has not been studied empirically. Our results show that both polymers contribute to the physical properties.
The observation that the structures of both RG I and arabinoxylan in the composite are different from the isolated forms indicates that both synthesis and hydrolysis contribute to the construction of a functional composite. We do not know if the interaction of arabinoxylan and RG I involves covalent interactions, but the lack of increase in the proportion of t-GlcA residues in the composite suggests a close interaction of the arabinoxylan to the RG I that cannot be mimicked by mixing the two pure fractions together. Our observation of substantial enhancement of viscosity of the native composite in deionized water at a physiological temperature of 25°C could be one of several physical properties dependent on the environment, and examination of the physical behavior of the mucilage in a range of ionic environments, pH, and temperatures is warranted.
We have estimated that plants devote 10% of their genomes to wall biogenesis, including dozens of glycosyltransferase gene families involved in synthesis and polysaccharide-modifying enzymes involved in trimming and degradation (McCann and Carpita, 2005; Yong et al., 2005). Only a handful of the products of these genes have been functionally characterized, and scant information is available on their cell-specific expression. Finding novel structures such as these helps answer the question of why there are so many (Coutinho et al., 2003). One could argue that most glycosyltransferases and hydrolases are cryptic and unused in any given species. However, a strong counterargument, for which the flax and Arabidopsis mucilages serve as examples, is that all walls may be constructed of a broad range of building materials, but a select few are enhanced in any given cell type. While Arabidopsis mucilage is predominantly a matrix of acetylated unbranched RG I backbones, one also finds, like in flax, significant amounts of 2,3-linked Rha in addition to 2,4-Rha, nonreducing t-Gal, and traces of an arabinoxylan with high degrees of doubly substituted xylosyl units. Our findings exemplify the fact that we have a long way to go to complete the inventory of all possible cell wall polysaccharides made by any plant, let alone completing the functional analysis of the enzymes that construct and modify them.
MATERIALS AND METHODS
Extraction of Flax Seed Mucilage
Flax seeds (Linum usitatissimum; Bob's Red Mill) were purchased from a local market, and Arabidopsis (Arabidopsis thaliana ecotype Columbia-0) seeds were from multiple stocks propagated annually in our laboratory. The seeds (100-g batches) were suspended in 800 mL of Nanopure (Barnstead) deionized water and stirred gently on a magnetic plate for 24 h at ambient temperature. The extract was filtered through nylon mesh and dialyzed extensively against running deionized water for 36 h, followed by an additional 12 h in the Nanopure deionized water, and then freeze dried.
Anion-Exchange Chromatography of the Whole Mucilage
Freeze-dried flax mucilage (100 mg) was dissolved in 10 mL of Nanopure deionized water, and a small amount of insoluble material was removed by centrifugation. The soluble part was loaded on a 2.5-cm × 10-cm column of DEAE Sephadex A-25 resin equilibrated in 30 mm ammonium acetate, pH 5.2. After an initial 5-mL elution with 30 mm ammonium acetate, pH 5.2, an 80-mL linear gradient of ammonium acetate, pH 5.2, from 30 mm to 1.2 m, was applied at a flow rate of 1 mL min−1 maintained by a Bio-Rad model 385 gradient former coupled with Alltech reciprocal pump model 3101. Fractions (1 mL) were collected, and 50 μL of each fraction was assayed for sugars by phenol-sulfuric acid assay (Dubois et al., 1956).
Determination of the Degree of Methyl Esterification and Acetylation
The degree of methyl esterification was also determined by double reduction of the uronosyls as described by Kim and Carpita (1992), as modified by Carpita and McCann (1996). Briefly, methyl-esterified uronosyl residues in two matched sets of samples were reduced with either NaBD4 or NaBH4. After dialysis, the remaining uronosyl residues were activated by 1-cyclo-3-(2-morpholinoethyl)-carbodiimide metho p-toluene-sulfonate (CMC) and reduced by NaBH4, if NaBD4 was used for reduction of methyl-esterified uronosyl residues, and by NaBD4, if NaBH4 was used for ester reduction. Separately, total uronosyl residues of the total mucilage, and its separated arabinoxylans and RG I, were reduced by NaBD4 after CMC activation. The degree of methylation was determined from GLC-EIMS of their alditol acetates as described below. Alternatively, the degree of methylation was assayed by determination of methanol released upon saponification (Wood and Siddiqui, 1971) and uronic acid (Filisetti-Cozzi and Carpita, 1991).
The degree of acetylation was determined according to Hestrin (1949) as modified. Briefly, to samples of mucilage in water (0.5 mL) was added 1 mL of alkaline hydroxylamine (1 m hydroxylamine in 1.75 m NaOH), and the mixture was incubated for 30 min before addition of 0.5 mL each of 4 m HCl and 0.37 m FeCl3 in 0.1 m HCl. Absorbance was read at 504 nm (true maximum) and compared with ethyl acetate standards in methanol.
Determination of the Sugar Composition of Polysaccharide Fractions
Fractions were hydrolyzed with 2 m TFA containing 1 μmol of myoinositol (internal standard) for 90 min at 120°C. The TFA containing the soluble sugars was evaporated under a stream of air, and the sugars were reduced with NaBH4 and converted to alditol acetates as described previously (Gibeaut and Carpita, 1991). The alditol acetates were separated by GLC on a 0.25-mm × 30-m vitreous silica capillary column of SP-2330 (Supelco). The temperature was held at 80°C for 1 min upon injection, then programmed from 80°C to 170°C at 25°C min−1, then to 240°C at 5°C min−1, with a 6-min hold at the upper temperature. Monosaccharide identity, as pentose, hexose, and deoxyhexose, was confirmed by EIMS (Carpita and Shea, 1989). Equimolar standards were also converted to alditol acetates to calculate response factors for the quantitation of mol % relative to the myoinositol standard. The 6,6-dideuterogalactosyl units, diagnostic of GalA, were determined (after correction for 13C spillover) by the shift of 2 atomic mass units of the secondary fragments from EIMS from those of Gal. Diagnostic pairs used were m/z 187/189, m/z 217/219, and m/z 289/291. The degree of methylation was calculated from comparison of esterified GalA (NaBD4 used in the initial reduction) and nonesterified GalA (NaBD4 used in the second reduction) to total Gal and GalA, as described by Carpita and McCann (1996).
Methylation Analysis
Total mucilage and polysaccharide samples with all uronosyl residues reduced to their respective 6,6-dideutero neutral sugars were per-O-methylated with n-butyllithium and methyl iodide as described by Gibeaut and Carpita (1991). The per-O-methylated polymers were recovered after addition of water to the mixture and partitioning into chloroform. The chloroform extracts were washed five times with a 3-fold excess of water, and the chloroform was evaporated. The partly methylated polymers were hydrolyzed in 2 m TFA for 90 min at 120°C, the TFA was evaporated in a stream of nitrogen, and the sugars were then reduced with NaBD4 and acetylated. The partly methylated alditol acetates were separated by GLC on a 0.25-mm × 30-m vitreous silica capillary column of SP-2330 (Supelco). The temperature was held at 80°C for 1 min upon injection, then programmed from 80°C to 170°C at 25°C min−1, then to 210°C at 2°C min−1, then to 240°C at 5°C min−1, with a 10-min hold at the upper temperature. Linkage composition was deduced from EIMS (Carpita and Shea, 1989).
Determination of l-Gal and d-Gal
Portions of the carboxyl-reduced arabinoxylan and RG I mucilage fractions were hydrolyzed with 2 m TFA for 90 min, and the acid was evaporated in a stream of nitrogen. The residue was dissolved in water, and aliquots were incubated with either d-Gal dehydrogenase (Sigma) or l-Gal dehydrogenase, produced recombinantly in Escherichia coli BL21 cells transformed with a kiwifruit (Actinidia deliciosa) gene fused to a maltose-binding protein sequence in a pMAL2cx plasmid (New England Biolabs; gift of from William Laing). We found no cross-activity of either enzyme preparation against its respective enantiomer. The relative amounts were determined by increase in A340 from reduction of NAD+ to NADH (Bergmeyer and Klotzsch, 1965). Alternatively, as described by Roberts and Harrer (1973), the relative destruction of l- and d-Gal by their respective dehydrogenases was determined by the Gal remaining, either by high-performance anion-exchange chromatography or as alditol acetates quantified by GLC as described earlier.
Determination of Viscosity
The viscosities of 1 mg mL−1 solutions in water of arabinoxylan, arabinoxylan-RG I composite, RG I, and a 1:1 (v/v) mixture of arabinoxylan and RG I were measured with a mechanical spectrometer (Reologica Instruments) using a spinning-disc method. The upper plate had a radius of 20 mm, and samples (approximately 100 μL) were placed between the two plates with a 0.2-mm gap at 20°C. Because of the relatively low viscosity of the samples, the viscosity measurements were conducted in a high shear rate range of 30 to 150 s−1 to reduce noise. Viscosities measured for all samples of all solutions were independent of shear rate and, hence, showed Newtonian behavior. All measurements were conducted in triplicate.
Acknowledgments
We thank Dr. William Laing (HortResearch, New Zealand) for the gift of the l-Gal dehydrogenase plasmid and Dr. Osvaldo Campanella (Department of Agricultural and Biological Engineering, Purdue University) for helpful discussions. This is Journal Paper Number 2008–18332 of the Purdue University Agricultural Experiment Station.
This work was supported by the National Science Foundation Plant Genome Research Program (to N.C.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Nicholas C. Carpita (carpita@purdue.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Anderson E (1933) The preparation of l-galactose from flaxseed mucilage. J Biol Chem 100 249–253 [Google Scholar]
- Anderson E, Crowder JA (1930) The composition of an aldobionic acid from mucilage. J Biol Chem 52 3711–3715 [Google Scholar]
- Anderson E, Lowe HJ (1947) The composition of flaxseed mucilage. J Biol Chem 168 289–297 [PubMed] [Google Scholar]
- Bailey K (1935) Cress seed mucilage. Biochem J 29 2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, Norris FW (1932) The nature and composition of the mucilage of the seed of white mustard (Brassica alba). Biochem J 26 1609–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Epel O, Gharyal PK, Schindler M (1988) Pectins as mediators of wall porosity in soybean cells. Planta 175 389–395 [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Klotzsch H (1965) l-Galactose. In HU Bergmeyer, ed, Methods of Enzymatic Analysis. Academic Press, New York, pp 131–133
- Brummell DA (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33 103–119 [DOI] [PubMed] [Google Scholar]
- Bush MS, Marry M, Huxam IM, Jarvis MC, McCann MC (2001) Developmental regulation of pectic epitopes during potato tuberization. Planta 213 869–880 [DOI] [PubMed] [Google Scholar]
- Carpita NC (1984) Cell wall development in maize coleoptiles. Plant Physiol 76 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3 1–30 [DOI] [PubMed] [Google Scholar]
- Carpita NC, McCann MC (1996) Some new methods to study plant polyuronic acids and their esters. In R Townsend, A Hotchkiss, eds, Progress in Glycobiology. Marcel Dekker, New York, pp 595–611
- Carpita NC, McCann MC (2000) The cell wall. In R Buchanan, R Jones, W Gruissem, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Biologists, Rockville, MD, pp 52–108
- Carpita NC, Shea EM (1989) Linkage structure by gas chromatography-mass spectrometry of partially-methylated alditol acetates. In CJ Biermann, GD McGinnis, eds, Analysis of Carbohydrates by GLC and MS. CRC Press, Boca Raton, FL, pp 155–216
- Carpita NC, Whittern D (1986) A highly substituted glucuronoarabinoxylan from developing maize coleoptiles. Carbohydr Res 146 129–140 [Google Scholar]
- Coutinho PM, Starn M, Blanc E, Henrissat B (2003) Why are there so many carbohydrate-active enzyme-related genes in plants? Trends Plant Sci 8 563–565 [DOI] [PubMed] [Google Scholar]
- Cui W, Mazza G, Biliaderis CG (1994) Chemical structure, molecular size distributions, and rheological properties of flaxseed gum. J Agric Food Chem 42 1891–1895 [Google Scholar]
- Darvill A, McNeil M, Albersheim P, Delmer DP (1980) The primary cell walls of flowering plants. In NE Tolbert, ed, The Biochemistry of Plants, Vol 1. Academic Press, New York, pp 91–162
- Dean GH, Zheng H, Tewari J, Huang J, Young DS, Hwang YT, Western TL, Carpita NC, McCann MC, Mansfield SD, et al (2007) The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19 4007–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles DA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28 350–356 [DOI] [PubMed] [Google Scholar]
- Easterby DG, Jones JKN (1950) Composition of linseed mucilage. Nature 165 614. [DOI] [PubMed] [Google Scholar]
- Edwards M, Scott C, Gidley MJ, Reid JSG (1992) Control of mannose galactose ratio during galactomannan formation in developing legume seeds. Planta 187 67–74 [DOI] [PubMed] [Google Scholar]
- Esau K (1977) Anatomy of Seed Plants, Ed 2. John Wiley & Sons, New York
- Fedeniuk RW, Biliaderis CG (1994) Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J Agric Food Chem 42 240–247 [Google Scholar]
- Filisetti-Cozzi TMCC, Carpita NC (1991) Measurement of uronic acids without interference from neutral sugars. Anal Biochem 197 157–162 [DOI] [PubMed] [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol 121 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G (1989) Polysaccharides in pharmacy: current applications and future concepts. Planta Med 55 493–497 [DOI] [PubMed] [Google Scholar]
- Frey-Wyssling A (1976) The plant cell wall. In Encyclopedia of Plant Anatomy, Ed 3. Gebruder Borntraeger, Berlin
- Fry SC, York WS, Albersheim P, Darvill A, Hayashi T, Joseleau JP, Kato Y, Lorences EP, MacLachlan GA, McNeil M, et al (1993) An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol Plant 89 1–3 [Google Scholar]
- Gibeaut DM, Carpita NC (1991) Tracing the biosynthesis of the cell wall in intact cells and plants: selective turnover and alteration of cytoplasmic and cell wall polysaccharides of proso millet cells in liquid culture and Zea mays seedlings. Plant Physiol 97 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB (2005) Changes in cell wall polysaccharides in developmental barley (Hordeum vulgare) coleoptiles. Planta 221 729–738 [DOI] [PubMed] [Google Scholar]
- Goh KKT, Pinder DN, Hall CE, Hemar Y (2006) Rheological and light scattering properties of flaxseed polysaccharide aqueous solutions. Biomacromolecules 7 3098–3103 [DOI] [PubMed] [Google Scholar]
- Gorshkova TA, Wyatt SE, Salnikov VV, Gibeaut DM, Ibragimov MR, Lozovaya VV, Carpita NC (1996) Cell-wall polysaccharides of developing flax plants. Plant Physiol 110 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross KC, Sams CE (1984) Changes in cell wall neutral sugar composition during fruit ripening: a species survey. Phytochemistry 23 2457–2461 [Google Scholar]
- Gutterman Y, Shemtov S (1996) Structure and function of the mucilaginous seed coats of Plantago coronopus inhabiting the Negev Desert of Israel. Isr J Plant Sci 44 125–133 [Google Scholar]
- Hestrin S (1949) The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem 180 249–261 [PubMed] [Google Scholar]
- Hilz H, de Jong LE, Kabel MA, Verhoef R, Schols HA, Voragen AGJ (2007) Bilberry xyloglucan: novel building blocks containing β-xylose within a complex. Carbohydr Res 342 170–181 [DOI] [PubMed] [Google Scholar]
- Hoffman M, Jia Z, Peña MJ, Cash M, Harper A, Blackburn AR II, Darvill A, York WS (2005) Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydr Res 340 1826–1840 [DOI] [PubMed] [Google Scholar]
- Jarvis MC (1984) Structure and properties of pectin gels in plant cell walls. Plant Cell Environ 7 153–164 [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA 100 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MJ, Albers CC (1955) Further studies on Texas plantago seeds. J Am Pharm Assoc 44 100–105 [DOI] [PubMed] [Google Scholar]
- Kim JB, Carpita NC (1992) Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiol 98 646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM (2007) In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 48 984–999 [DOI] [PubMed] [Google Scholar]
- McCann MC, Carpita NC (2005) Looking for invisible phenotypes in cell-wall mutants of Arabidopsis thaliana. Plant Biosyst 139 80–83 [Google Scholar]
- McCann MC, Roberts K (1991) Architecture of the primary cell wall. In CW Lloyd, ed, Cytoskeletal Basis of Plant Growth and Form. Academic Press, New York, pp 109–129
- McCartney L, Ormerod AP, Gidley MJ, Knox JP (2000) Temporal and spatial regulation of pectic (1→4)-β-D-galactan in cell walls of developing pea cotyledons: implications for mechanical properties. Plant J 22 105–113 [DOI] [PubMed] [Google Scholar]
- McCleary BV, Amado R, Waibel R, Neukom H (1981) Effect of galactose content on the solution and interaction properties of guar and carob galactomannans. Carbohydr Res 92 269–285 [Google Scholar]
- Muralikrishna G, Salimath PV, Tharanathan RN (1987) Structural features of an arabinoxylan and a rhamno-galacturonan derived from linseed mucilage. Carbohydr Res 161 265–271 [Google Scholar]
- Neville A (1912) Linseed mucilage. J Agric Sci 5 113–128 [Google Scholar]
- Peña MJ, Carpita NC (2004) Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple. Plant Physiol 135 1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña MJ, Ryden P, Madson M, Smith A, Reiter WD, Carpita NC (2004) Galactosylation of xyloglucans is essential for maintenance of cell wall tensile strength during cell growth in plants. Plant Physiol 134 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW (2001) MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13 2777–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell RJ, Curti D, Rogers J, Nicolas P, Fischer M (2003) Changes to the galactose/mannose ratio in galactomannans during coffee bean (Coffea arabica L.) development: implications for in vivo modification of galactomannan synthesis. Planta 217 316–326 [DOI] [PubMed] [Google Scholar]
- Roberts RM, Harrer E (1973) Determination of L-galactose in polysaccharide material. Phytochemistry 12 2679–2682 [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC (2003) Tensile properties of Arabidopsis cell walls depend on both xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol 132 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa M, Yamatoya K, Nishinari K (1998) Tailoring of xyloglucan properties using an enzyme. Food Hydrocolloids 12 25–28 [Google Scholar]
- Sims IM, Munro SLA, Currie G, Craik D, Bacic A (1996) Structural characterization of xyloglucan secreted by suspension cultured cells of Nicotiana plumbaginifolia. Carbohydr Res 293 147–172 [DOI] [PubMed] [Google Scholar]
- Smith F, Montgomery R (1959) The Chemistry of Plant Gums and Mucilages. Monograph Series No. 141. American Chemical Society, Reinhold, NY
- Talmadge KW, Keegstra K, Bauer WD, Albersheim P (1973) The structure of plant cell walls. I. The macromolecular components of the walls of suspension-cultured sycamore cells with a detailed analysis of the pectic polysaccharides. Plant Physiol 51 158–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DS (2008) Space and time in the plant cell wall: relationships between cell type, cell wall rheology and cell function. Ann Bot (Lond) 101 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiné MAS, Clovis CO, de Lima DU, Carpita NC, Buckeridge MS (2006) Fine structure of a mixed-oligomer storage xyloglucan from seeds of Hymenaea courbaril. Carbohydr Polym 66 444–454 [Google Scholar]
- Tipson RS, Christman CC, Levene PA (1939) The structure of the aldobionic acid from flaxseed mucilage. J Biol Chem 128 609–620 [Google Scholar]
- Ulvskov P, Wium H, Bruce D, Jørgensen B, Qvist KB, Skjøt M, Hepworth D, Borkhardt B, Sørensen SO (2005) Biophysical consequences of remodeling the neutral side chains of rhamnogalacturnonan I in tubers of transgenic potatoes. Planta 220 609–620 [DOI] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter WD (2002) The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc Natl Acad Sci USA 99 3340–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincken JP, Schols HA, Oomen RJFJ, McCann MC, Ulvskov P, Voragen AGJ, Visser RGF (2003) If homogalacturonan were a side-chain of rhamnogalacturonan I: implications for cell wall architecture. Plant Physiol 132 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannerberger K, Nylander T, Nyman M (1991) Rheological and chemical properties of mucilage in different varieties from linseed (Linum usitatissimum). Acta Agric Scand 41 311–319 [Google Scholar]
- Warrand J, Michaud P, Picton L, Muller G, Courtois B, Ralainirina R, Courtois J (2005. a) Structural investigations of the neutral polysaccharide of Linum usitatissimum L. seeds mucilage. Int J Biol Macromol 35 121–125 [DOI] [PubMed] [Google Scholar]
- Warrand J, Michaud P, Picton L, Muller G, Courtois B, Ralainirina R, Courtois J (2005. b) Contributions of intermolecular interactions between constitutive arabinoxylans to the flaxseeds mucilage properties. Biomacromolecules 6 1871–1876 [DOI] [PubMed] [Google Scholar]
- Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, Haughn GW (2001) Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 127 998–1011 [PMC free article] [PubMed] [Google Scholar]
- Western TL, Skinner DJ, Haughn GW (2000) Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 122 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler RL, Conrad HE (1954. a) A crystalline galactobiose from acid hydrolysis of okra mucilage. J Am Chem Soc 76 1673–1674 [Google Scholar]
- Whistler RL, Conrad HE (1954. b) 2-O-(D-Galactopyranosyluronic acid)-L-rhamnose from okra mucilage. J Am Chem Soc 76 3544–3546 [Google Scholar]
- Wilkie KCB (1979) The hemicelluloses of grasses and cereals. Adv Carbohydr Chem Biochem 36 215–264 [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47 9–27 [PubMed] [Google Scholar]
- Wood PJ, Siddiqui IR (1971) Determination of methanol and its application to measurement of pectin ester content and pectin methyl esterase activity. Anal Biochem 39 418–428 [DOI] [PubMed] [Google Scholar]
- Yong W, Link B, O'Malley R, Tewari J, Hunter CT III, Lu CA, Li X, Bleecker AB, Koch KE, McCann MC, et al (2005) Genomics of plant cell wall biogenesis. Planta 221 747–751 [DOI] [PubMed] [Google Scholar]