Abstract
Viroids are small self-replicating RNAs that infect plants. How these noncoding pathogenic RNAs interact with hosts to induce disease symptoms is a long-standing unanswered question. Recent experimental data have led to the suggestive proposal of a pathogenic model based on the RNA silencing mechanism. However, evidence of a direct relation between key components of the RNA silencing pathway and symptom expression in infected plants remains elusive. To address this issue, we used a symptomatic transgenic line of Nicotiana benthamiana that expresses and processes dimeric forms of Hop stunt viroid (HSVd). These plants were analyzed under different growing temperature conditions and were used as stocks in grafting assays with the rdr6i-Nb line, in which the RNA-dependent RNA polymerase 6 (RDR6) is constitutively silenced. Here, we show that the symptom expression in N. benthamiana plants is independent of HSVd accumulation levels but dependent on an active state of the viroid-specific RNA silencing pathway. The scion of rdr6i-Nb plants remained asymptomatic when grafted onto symptomatic plants, despite an accumulation of a high level of mature forms of HSVd, indicating the requirement of RDR6 for viroid-induced symptom production. In addition, the RDR6 requirement for symptom expression was also observed in wild-type N. benthamiana plants mechanically infected with HSVd. These results provide biological evidence of the involvement of the viroid-specific RNA silencing pathway in the symptom expression associated with viroid pathogenesis.
One of the most intriguing and still unsolved topics in the study of pathogen-host interactions is how these agents, on their way to suppress host defense, cause undesirable pathological effects. Pathogens recruit host routes/factors to self-perpetuate within the invaded cell and to translocate to neighboring groups of cells. Plant viruses have the potential to use from five to 30 viral origin proteins to accomplish these functions, whereas viroids, the smallest known infectious agents in plants, must take advantage of their structurally informative circular RNA molecule to do so (Hull, 2002; Tabler and Tsagris, 2004; Daròs et al., 2006; Ding and Itaya, 2007). In view of the recent picture emerging with the noncoding RNAs as key components of the regulation mechanisms of developmental and defense processes, viroids have become one of the most productive tools to unravel some of the fundamental principles of life processes (Ding and Itaya, 2007).
Viroid genomes consist of a single-stranded, covalently closed, circular, noncoding, and nonencapsidated RNA, ranging from 246 to 401 nucleotides long (Tabler and Tsagris, 2004; Flores et al., 2005; Daròs et al., 2006; Flores and Pallás, 2006; Ding and Itaya, 2007). The search for plant cell components involved in the regulation of the biological processes of viroid diseases has identified different host factors involved in viroid replication (Daròs and Flores, 2002) and movement (Gómez and Pallás, 2001, 2004; Owens et al., 2001; Gómez et al., 2005). Despite intensive research, however, the basic question of how these pathogenic RNAs alter, when they do, host development and physiology to induce disease symptoms remains unanswered.
It has long been accepted that the genomic RNA acts as the primary pathogenic effector via a direct interaction between specific viroid motifs and cellular factors (Diener, 1999; Flores et al., 2004, 2005). However, the identification of a group of small RNAs that regulate host and nonhost gene expression in eukaryotes has led to a new hypothesis based on the viroid pathogenesis possibly being mediated by the interference of specific host-mRNA expression via viroid-induced RNA silencing (Papaefthimiou et al., 2001; Conejero, 2003; Markarian et al., 2004; Wang et al., 2004).
RNA silencing is a sequence-specific RNA-inactivation mechanism described in diverse eukaryotes that guides gene regulation, chromatin modification, and defense against viruses (Baulcombe, 2002; Vogt et al., 2004; Brodersen and Voinnet, 2006; Ding and Voinnet, 2007). These different biological processes share three biochemical characteristics: (1) formation of double-stranded RNAs (dsRNAs); (2) processing of dsRNAs to 20- to 26-nucleotide small RNAs (sRNAs); and (3) inhibitory action of sRNAs on complementary RNA or DNA (Brodersen and Voinnet, 2006). The sRNAs, the central molecules of the RNA silencing pathway, are broadly classified into microRNAs (miRNAs) and small interfering RNAs (siRNAs), which have similar chemical structures but differ in their function and mode of biogenesis. Production of both types of sRNAs depends on the activity of RNase III-type enzymes known as Dicer (Llave et al., 2002; Brodersen and Voinnet, 2006; Vaucheret, 2006; Ding and Voinnet, 2007).
In plants, a RNA-dependent RNA polymerase, initially identified as RNA-dependent RNA polymerase 6 (RDR6), was shown to be essential for posttranscriptional gene silencing (PTGS) induced by sense transgene and by some viruses but not required for inverted repeat dsRNA-induced PTGS (Dalmay et al., 2000, Mourrain et al., 2000; Vance and Vaucheret, 2001). RDR6 is also involved in the transitivity phenomenon (Vaistij et al., 2002). Transitivity can be explained as the transition of primary siRNAs to secondary siRNAs that serves as an amplification mechanism of the initial RNA silencing reaction (Brodersen and Voinnet, 2006) and accounts for their systemic spreading (Himber et al., 2003; Schwach et al., 2005). Accumulation of secondary siRNAs during PTGS of exogenous nucleic acids, including many viruses and transgene transcripts, is dependent on RDR6 in Arabidopsis (Arabidopsis thaliana) plants. In addition, RDR6 is functionally involved in the biogenesis of trans-acting siRNAs (tasiRNAs; Peragine et al., 2004, Vazquez et al., 2004; Allen et al., 2005) and natural antisense transcript siRNAs (Brodersen and Voinnet, 2006; Chapman and Carrington, 2007). Consequently, diverse small RNA pathways in plants have an RDR6 requirement.
RNA silencing is also affected by environmental factors, especially temperature. High temperatures are frequently associated with enhanced RNA silencing activity, whereas low temperatures inhibit this regulatory mechanism (Szittya et al., 2003; Qu et al., 2005).
Increasing evidence indicates that viroid genomic RNAs and/or their replicative intermediates are inductors and potential targets of RNA silencing (Martínez de Alba et al., 2002; Vogt et al., 2004; Wang et al., 2004; Gómez and Pallás, 2007a; Itaya et al., 2007; Martín et al., 2007). Moreover, it was recently demonstrated that mature forms of Potato spindle tuber viroid (PSTVd) and Hop stunt viroid (HSVd) can simultaneously elicit and resist this plant defense mechanism in tomato (Solanum lycopersicum; Itaya et al., 2007) and Nicotiana benthamiana (Gómez and Pallás, 2007a) plants, respectively. Interestingly, these results have provided a unified picture in which these noncoding RNAs are inductors, potential targets, and evaders of RNA silencing at the same time (Wang et al., 2004; Ding and Itaya, 2007; Gómez and Pallás, 2007a; Itaya et al., 2007).
The possibility that viroid-induced RNA silencing could be associated with the symptom expression in infected plants was originally suggested by Papaefthimiou et al. (2001). This idea was reinforced by the observation that the symptom severity in Gynura aurantiaca plants infected with Citrus exocortis viroid (CEVd) correlated with CEVd-specific siRNA accumulation but not with the viroid titer (Markarian et al., 2004). Later, Wang et al. (2004) showed that transgenic tomato plants expressing a hairpin construct of a partial-length sequence of PSTVd accumulated specific siRNAs and developed symptoms similar to those of PSTVd infection. They suggested that RNA silencing plays an important role in the pathogenesis and evolution of these subviral RNAs. Based on these findings, they proposed that the viroid-specific siRNAs might function like miRNAs to down-regulate the expression of physiologically important host genes and induce disease-associated symptoms by means of the RNA silencing pathway (Wang et al., 2004). In addition, there have been recent reports showing that high levels of PSTVd-specific siRNAs were associated with the expression of strong symptoms in N. benthamiana plants after biolistic infection (Matoušek et al., 2007). Even though the involvement of viroid-specific RNA silencing is currently accepted as a plausible explanation for viroid-induced pathogenesis (Tabler and Tsagris, 2004; Ding and Itaya, 2007), biological evidence of a direct correlation between the viroid-specific RNA silencing pathway and symptom expression is scarce.
We reasoned that if the symptoms developed by a viroid infection are mediated by RNA silencing, then the following predictions should be fulfilled: (1) the inhibition of the RNA silencing activity would be associated with a decrease in symptom severity; and (2) infected plants with deficiencies in the RNA silencing pathway would either be asymptomatic or show a reduction in the severity of symptoms. In an attempt to validate these predictions, we used a symptomatic transgenic line of N. benthamiana (HSVd-Nb) expressing and processing a dimeric HSVd RNA into the biologically active monomeric circular and linear forms (Gómez and Pallás, 2006). In these plants, the mature forms of the HSVd could resist the HSVd-induced RNA silencing-mediated degradation, whereas the identical full-length HSVd sequences fused to the mRNA GFP could not. Consequently, the HSVd-GFP construct could be used as a reporter of the HSVd-specific RNA silencing activity (Gómez and Pallás, 2007a). The HSVd-Nb plants were analyzed under different growth temperature conditions and used as stocks in grafting assays with the rdr6i-Nb line, in which the Nb-RDR6 is constitutively silenced (Schwach et al., 2005). In addition, HSVd agroinoculation assays were performed in wild type (Wt-Nb) and rdr6i-Nb plants to analyze the interrelation between RNA silencing and pathogenesis in a conventional infection. The findings presented here demonstrate that in N. benthamiana plants, the symptoms induced by viroid infection are dependent on RDR6 activity, a key component of diverse RNA silencing pathways, and their severity is associated with efficient viroid-specific RNA silencing activity.
RESULTS
The HSVd Symptom Expression Is Dependent on the Temperature But Independent of the Accumulation Levels of HSVd Mature Forms
Stunting was recently observed as the most characteristic symptom induced by the expression of a dimeric form of HSVd in four independent transgenic lines of HSVd-Nb plants (Gómez and Pallás, 2006; Martinez et al., 2008). Thus, this phenotypic characteristic was selected to determine symptom intensity. To test whether the HSVd-induced symptoms in N. benthamiana are temperature dependent, HSVd-Nb plants were grown at different temperatures (28°C, 20°C, and 14°C) and symptoms were evaluated as severe, moderate, or null.
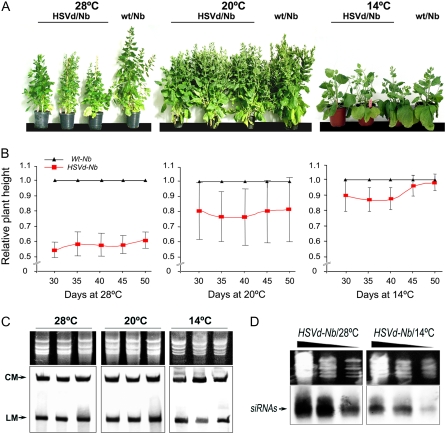
Striking differences in symptom expression were observed. As Figure 1A shows, the average height of HSVd-Nb plants at 28°C was severely reduced, only 60% of the height of Wt-Nb plants at 50 d postincubation (dpi; Fig. 1B). Stunting was moderate in the HSVd-Nb plants incubated at 20°C (Fig. 1A), representing 80% of the height of control plants on average (Fig. 1B). On the other hand, although the plants that were incubated at 14°C presented a slight reduction in growth (11%–14% on average) at 30 to 35 dpi (Fig. 1B), the HSVd-induced stunting in relation to controls was absent at 50 dpi (Fig. 1, A and B). These results indicate that, as observed for the naturally infected hosts, the HSVd-induced symptoms in HSVd-Nb are temperature dependent, being severe at high temperatures and decreased at low temperatures until they eventually disappear.
Figure 1.
Symptoms induced by HSVd in transgenic N. benthamiana plants (HSVd-Nb). A, HSVd-Nb plants grown for 50 d at 28°C, 20°C, and 14°C compared with an untransformed control kept under the same growing conditions. B, Influence of temperature on the dynamics of HSVd-Nb growth. The relative heights of 10 HSVd-Nb plants were measured at 30, 35, 40, 45, and 50 dpi at 28°C, 20°C, and 14°C. Error bars indicate sd values among the 10 analyzed plants. C, Northern-blot assays of total RNA from three different HSVd-Nb plants grown at 28°C, 20°C, and 14°C. The levels of circular (CM) and linear (LM) monomeric forms of HSVd were kept constant at the different temperatures. D, Small RNA-enriched RNAs were extracted from three pooled HSVd-Nb plants maintained at 28°C (symptomatic) and 14°C (asymptomatic). The RNAs were diluted 1:1, 1:5, and 1:25 and analyzed by northern-blot assays to detect HSVd-specific siRNAs. RNAs were quantified by spectrometry and their concentrations equalized. Ethidium bromide-stained gels are shown as RNA loading controls (in C and D).
To check whether the variation in symptom severity observed in the HSVd-Nb plants grown at different temperatures was associated with alterations at the level of accumulation of the viroid, we analyzed the levels of HSVd in plants incubated at 28°C, 20°C, and 14°C. They presented severe, moderate, or null stunting, respectively. Hybridization assays revealed that HSVd circular and linear monomeric forms accumulated at similar levels in N. benthamiana plants at the tested temperatures (Fig. 1C), indicating that the differences observed in symptom intensity in HSVd-Nb incubated at 28°C, compared with the plants incubated at 20°C and 14°C, were independent of HSVd accumulation levels. Interestingly, hybridization assays revealed a reduction of the HSVd-specific siRNA concentration in the asymptomatic HSVd-Nb plants grown at 14°C compared with the symptomatic HSVd-Nb plants grown at 28°C (Fig. 1D), suggesting a possible association between the HSVd-specific siRNA levels and viroid-induced symptoms.
Low Temperatures Affect the HSVd RNA-Induced RNA Silencing
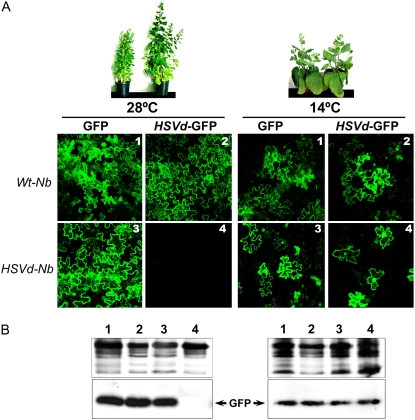
It was previously demonstrated that the defense pathway mediated by RNA silencing was inhibited at low temperatures in diverse plant-virus interactions (Szittya et al., 2003). Therefore, we went on to determine whether the lack of symptoms in the HSVd-Nb plants grown at 14°C could be associated with alterations in the HSVd RNA-induced RNA silencing pathway. To test this possibility, agroinfiltration assays using an HSVd-GFP reporter (Gómez and Pallás, 2007b) were carried out in the HSVd-Nb plants incubated at 28°C (symptomatic) and 14°C (asymptomatic). Consistent with previous results (Gómez and Pallás, 2007a), the expression of the HSVd-GFP reporter was suppressed in the HSVd-Nb plants incubated at 28°C but not in the Wt-Nb control plants, which indicates an HSVd-specific RNA silencing activity in symptomatic HSVd-Nb plants incubated at 28°C (Fig. 2, A and B, left). In contrast, when the reporter was infiltrated in the asymptomatic HSVd-Nb plants incubated at 14°C, the GFP gene was not suppressed and the fluorescence level was similar to that observed in control plants (Fig. 2, A and B, right). These results demonstrate that plants grown under conditions in which RNA silencing is less efficient did not develop any symptoms at all, whereas plants grown under conditions in which RNA silencing was active underwent severe stunting. These results establish a strong correlation between the activity level of viroid-specific RNA silencing and symptom expression.
Figure 2.
HSVd-GFP expression is not suppressed in asymptomatic HSVd-Nb plants grown at 14°C. A, Wt-Nb and HSVd-Nb plants, kept at 28°C (left) and 14°C (right), were agroinfiltrated with the HSVd-GFP reporter. As observed in panel 4 (left), the reporter expression was suppressed in symptomatic HSVd-Nb plants incubated at 28°C. In contrast, when the silencing reporter was infiltrated in the asymptomatic HSVd-Nb incubated at 14°C (panel 4, right), GFP fluorescence was similar to that observed in the control plants. B, Detection of the HSVd-GFP reporter by western-blot analysis of total proteins extracted from agroinfiltrated Wt-Nb and HSVd-Nb plants. Silver-stained gels are shown as protein loading controls.
The Expression of HSVd-Induced Symptoms Depends on RDR6 Activity
It was recently demonstrated that the reduced expression of Nb-RDR6 results in hypersusceptibility to some viruses (Schwach et al., 2005). In addition, Nb-RDR6 has been mechanistically implicated in transitive silencing and diverse small RNA pathways (Brodersen and Voinnet, 2006; Chapman and Carrington, 2007). To investigate whether the symptoms observed in infected plants are related to the viroid-induced RNA silencing pathway, we examined the symptom development in transgenic rdr6i-Nb grafted onto HSVd-Nb plants.
We reasoned that if RDR6-dependent RNA silencing pathways mediate viroid pathogenesis, HSVd would infect the silence-defective rdr6i scions without developing the characteristic symptoms. Conversely, in the wild-type scions, HSVd infection would develop the characteristic pathogenic effects. To test this prediction, the scion length was monitored and measured at 30 and 45 d after grafting (dag).
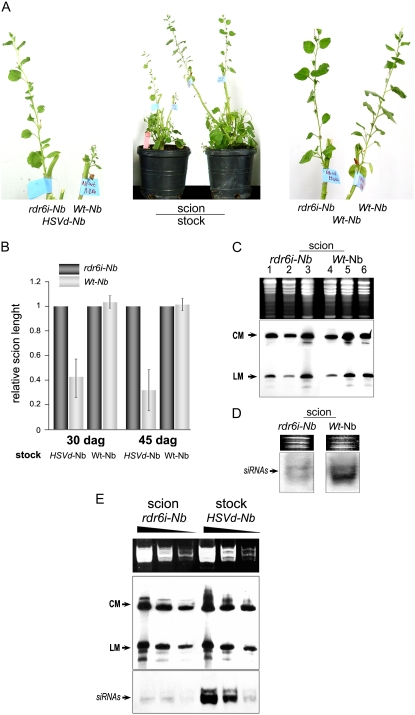
Wild-type scions showed moderate stunting at 17 dag, whereas rdr6i scions did not (Supplemental Fig. S1). Size reduction was more pronounced (44% of the size of the corresponding rdr6i scions) at later stages of the incubation, as observed in Figure 3B, which represents the average length of Wt-Nb and rdr6i-Nb scions at 30 dag. Severe stunting in the wild-type scions was observed at 45 dag, representing 32% of the size of the rdr6i scions on average (Fig. 3, A and B). Moreover, the leaves of wild-type scions presented a considerable size reduction and chlorosis followed by necrosis compared with the normal development of the rdr6i scions grafted onto identical stocks (Fig. 3A; Supplemental Figs. S2 and S3). Consistent with previous results (Gómez and Pallás, 2006), the sequence of the HSVd RNA isolated from scion tissue was identical to the HSVd sequence present in the stock (data no shown).
Figure 3.
Effects of HSVd accumulation in Wt-Nb and rdr6i-Nb plants. Two-week-old Wt-Nb and rdr6i-Nb scions were grafted onto symptomatic transgenic HSVd-Nb stocks and kept at 28°C. A (middle), General symptoms induced by HSVd in a representative Wt-Nb scion compared with an rdr6i-Nb scion grafted onto an identical HSVd-Nb stock at 45 dag. The left and right panels show magnified images of the grafts. B, Relative length of Wt-Nb and rdr6i-Nb scions grafted onto Wt-Nb and HSVd-Nb plants. The scion length was measured at 30 and 45 dag. Error bars indicate sd values between six analyzed samples. C, Total RNAs were extracted from three different scions at 45 dag and analyzed by northern-blot assays. The HSVd RNA circular (CM) and linear (LM) monomeric form levels were significantly similar in symptomless rdr6i-Nb (lanes 1–3) and symptomatic Wt-Nb (lanes 4–6) scions. D, Small RNA-enriched RNAs were extracted from three pooled rdr6i-Nb and Wt-Nb scions at 45 dag. The RNAs were analyzed by northern-blot assays to detect HSVd-specific siRNAs. E, Total RNAs and small RNA-enriched RNAs were extracted from three pooled rdr6i-Nb scions and their respective HSVd-Nb stocks at 45 dag. The RNAs were diluted 1:1, 1:5, and 1:25 and analyzed by northern-blot assays to detect mature forms and specific siRNAs of HSVd. RNAs were quantified by spectrometry and their concentrations equalized. Ethidium bromide-stained gels are shown as RNA loading controls (in C–E). [See online article for color version of this figure.]
To test whether the presence or absence of symptoms observed in scions was associated with a differential concentration of the viroid, we analyzed the levels of HSVd in symptomatic Wt-Nb and asymptomatic rdr6i-Nb scions. Hybridization assays revealed that the HSVd accumulated at similar levels in both scion types (Fig. 3C), demonstrating that the viroid-induced symptoms observed in the infected Wt-Nb scions are dependent on the RDR6 activity but not on the HSVd accumulation level. Furthermore, a significant reduction of the siRNA concentration in the asymptomatic rdr6i scions compared with the symptomatic wild-type scions was observed (Fig. 3D), reinforcing the possible association between the HSVd-specific siRNA level and viroid-induced symptoms. In order to obtain additional evidence of the relation between HSVd-specific RNA silencing and symptom expression, we analyzed the accumulation of HSVd-specific siRNAs and HSVd mature forms in the HSVd-Nb (stocks) and rdr6i-Nb (scions) plants. The difference in siRNA accumulation was also observed when the rdr6i scions were compared with HSVd-Nb stocks, even though the viroid titer in the two sources was similar (Fig. 3E). The observation that the levels of mature forms of HSVd were similar in asymptomatic scions and symptomatic stocks (Fig. 3E) indicates that the symptoms observed in infected N. benthamiana plants are also independent of HSVd accumulation levels in the graft assays.
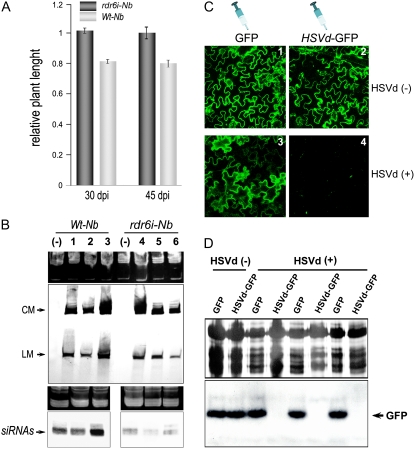
To further dispell the notion that HSVd transgene-derived transcripts could influence the observed differences, we performed an alternative experiment using Wt-Nb and rdr6i-Nb plants infected by mechanical agroinoculation of HSVd RNA, a recently described efficient method to induce PSTVd (Carbonell et al., 2008) and HSVd (R. Flores, personal communication) infection in N. benthamiana plants. Inoculated Wt-Nb plants showed moderate stunting at 25 dpi compared with the noninoculated controls, whereas inoculated rdr6i-Nb plants did not (data no shown). Size reduction was more significant at 30 and 45 dpi, representing 81% of the size of the uninoculated controls on average (Fig. 4A; Supplemental Fig. S4). Both the stunting and leaf size reduction were induced by HSVd in the infected Wt-Nb plants as well as in the HSVd-Nb plants, indicating that the symptomatic phenotype described in the HSVd-Nb plants was the consequence of a viroid-induced pathogenesis process. The slight differences in stunting intensity between HSVd-infected N. benthamiana plants (approximately 80% of the control) and HSVd-Nb plants (approximately 60% of the control) maintained at the same temperature can be explained by considering that in transgenic plants, the HSVd RNA input is constitutively provided from the first development stage. Thus, the symptom expression is enhanced in comparison with a conventional pathogenesis process.
Figure 4.
Influence of HSVd on the growth of mechanically infected N. benthamiana plants compared with noninoculated controls. A, The relative heights with respect to mock-inoculated controls of six Wt-Nb and six rdr6i-Nb HSVd-infected plants were measured at 30 and 45 dpi. Error bars indicate sd values between the analyzed plants. B, Total RNAs and small RNA-enriched RNAs were extracted from three different symptomatic Wt-Nb plants (lanes 1–3) and symptomless rdr6i-Nb plants (lanes 4–6) at 45 dpi and analyzed by northern-blot assays to detect mature forms (top) and specific siRNAs (bottom) of HSVd. RNAs were quantified by spectrometry and their concentrations equalized. Ethidium bromide-stained gels are shown as RNA loading controls. C, HSVd-infected Wt-Nb plants were agroinfiltrated with the HSVd-GFP reporter. As observed in panel 4, the reporter expression was suppressed in symptomatic HSVd-infected Wt-Nb plants. D, Detection of the HSVd-GFP reporter by western-blot analysis of total proteins extracted from three HSVd-infected Wt-Nb plants. Silver-stained gels are shown as protein loading controls. [See online article for color version of this figure.]
To check whether the lack of symptoms in the rdr6i-Nb plants was related to alterations in the accumulation of the HSVd, we analyzed the levels of viroid in inoculated plants. Hybridization assays showed that HSVd accumulated at similar levels in both Wt-Nb and rdr6i-Nb plants (Fig. 4B), indicating that in a conventional pathogenic process, the symptom expression is also independent of HSVd accumulation levels but dependent on the RDR6 activity. In agreement with the results described in the grafting assays, a significant reduction of the HSVd-specific siRNA concentration was observed in the asymptomatic rdr6i-Nb plants compared with the symptomatic Wt-Nb plants (Fig. 4B, bottom).
Next, we went on to determine, by means of agroinfiltration assays, whether the HSVd RNA-induced RNA silencing pathway is active in the infected N. benthamiana plants. Consistent with the results observed in HSVd-Nb plants, the expression of the HSVd-GFP reporter was suppressed in the infected Wt-Nb plants but not in the uninoculated control plants (Fig. 4, C and D). These results indicate an HSVd-specific RNA silencing activity in the symptomatic N. benthamiana plants, reinforcing the strong correlation between the activity level of the viroid-specific RNA silencing and the symptom expression observed in the HSVd-Nb plants.
DISCUSSION
Since the discovery of the PSTVd (Diener, 1971), viroids have always been puzzling exceptions to the rules that have been commonly accepted for other plant pathogenic RNAs (Tabler and Tsagris, 2004; Daròs et al., 2006; Flores and Pallás, 2006; Ding and Itaya, 2007). Their genome consists of a naked RNA characterized by the absence of any functional open reading frame. In the “viroid world,” however, noncoding does not signify nonfunctional. Thus, a viroid contains sufficient genetic information to establish infection in susceptible hosts. Very little information is currently known regarding how these sui generis RNAs elicit the pathogenic process.
An emergent view in viroid research links the RNA silencing pathway with viroid pathogenesis (Papaefthimiou et al., 2001; Conejero, 2003; Markarian et al., 2004; Wang et al., 2004). Much of the evidence that supports this association is based on the observation of a positive correlation among the levels of small RNAs, the hallmarks of the RNA silencing mechanisms, and symptom severity (Itaya et al., 2001; Papaefthimiou et al., 2001; Markarian et al., 2004; Matoušek et al., 2007). However, this correlation is not fulfilled in some viroid-host combinations (Papaefthimiou et al., 2001; Markarian et al., 2004; Sano and Matsuura, 2004), which raises significant doubts about this attractive hypothesis. The most robust evidence linking RNA silencing and viroid pathogenesis is the observation made by Wang et al. (2004) that tomato plants expressing hairpin RNAs, derived from PSTVd, developed symptoms similar to those of PSTVd infection. Nonetheless, direct evidence for the involvement of the RNA silencing pathway in viroid pathogenesis has not yet been reported. Here, we present solid evidence that RDR6 must be active for a characteristic pathogenic response due to viroid infection. N. benthamiana plants that accumulate high levels of HSVd but have a reduced RDR6 expression, or that were grown under environmental conditions in which the RNA silencing pathway is less efficient, did not develop the characteristic stunting induced by the HSVd.
Low temperature has been demonstrated to inhibit the RNA silencing-mediated defense (Szittya et al., 2003; Chellappan et al., 2005; Qu et al., 2005). Thus, we analyzed symptom expression in the HSVd-Nb plants growing at different temperatures. The HSVd-Nb plants kept at 28°C, which is the optimal temperature for RNA silencing (Szittya et al., 2003; Chellappan et al., 2005; Qu et al., 2005), presented the characteristic symptoms previously described in N. benthamiana plants expressing HSVd (Gómez and Pallás, 2006; Martinez et al., 2008). This phenotypic characteristic was reduced in HSVd-Nb plants kept at 20°C and 14°C until it eventually disappeared (Fig. 1, A and B), temperatures at which the RNA silencing-based defense has been shown to have a diminished activity in N. benthamiana plants (Szittya et al., 2003; Qu et al., 2005). Interestingly, the accumulation of circular and linear forms of HSVd was similar in the plants growing at the three different temperatures (Fig. 1C). This indicates that the symptoms observed in the HSVd-Nb plants growing at 28°C do not correlate with HSVd accumulation. These results are in accordance with previous reports showing that, in general, symptom severity was not correlated with accumulation levels of viroid RNAs (Schnölzer et al., 1985; Gruner et al., 1995; Gora et al., 1996; Rodio et al., 2006). In addition, the symptom expression in G. aurantiaca plants infected with CEVd was associated with a specific temperature-sensitive response without any alteration to viroid sequences or structures (Skoric et al., 2001). Although these findings demonstrate a direct relation between temperature and symptom intensity, irrespective of genomic HSVd-RNA accumulation, they do not provide evidence to support the involvement of RNA silencing in the viroid-associated pathogenesis process.
The first suggestion that the lack of symptom expression in the HSVd-Nb plants grown at lower temperatures should be related to deficiencies in the RNA silencing-based pathway was provided by the results obtained after transitory expression of an HSVd-GFP reporter in HSVd-Nb plants. This reporter was normally expressed in the HSVd-Nb plants at 14°C (Fig. 2, A and B), indicating that the HSVd-specific RNA silencing was inhibited or severely affected in these asymptomatic plants, whereas the reporter was suppressed in symptomatic plants. In addition, a significant reduction in the HSVd-specific siRNA levels was observed in the asymptomatic HSVd-Nb plants. These results show a clear association between symptom expression and the activity of the HSVd-specific RNA silencing pathway. However, the possibility that low temperatures would block the direct interaction between the HSVd and cellular factors that serve as targets for symptom expression cannot be ruled out.
The most robust evidence supporting the involvement of RNA silencing in the expression of HSVd symptoms was provided by the findings obtained in the grafting assays using HSVd-Nb plants as stocks and Wt-Nb or rdr6i-Nb plants as scions. Here, we demonstrate that the infected rdr6i-Nb scions were unable to develop HSVd-characteristic symptoms under identical growing temperature conditions (Fig. 3, A and B). The symptomless phenotype of the infected rdr6i-Nb scions contrasts with the severe stunting observed in the infected Wt-Nb scions grafted onto identical HSVd-Nb stocks. Remarkably, this RDR6 dependence of viroid-induced symptom development was also observed in Wt-Nb plants infected by HSVd agroinoculation (Fig. 4A). Interestingly, while the symptomatic N. benthamiana plants presented a significant amount of HSVd-specific siRNAs, the symptomless rdr6i-Nb plants did not.
Taking into account that the rdr6i-Nb plants down-regulated the expression of an enzyme playing a key role in sense transgene-induced PTGS and the transitive RNA silencing pathway, and that the biogenesis of tasiRNAs and natural antisense transcript siRNAs is RDR6 dependent, we propose that the asymptomatic phenotype exhibited by these infected plants is a direct consequence of deficiencies in the RNA-mediated silencing mechanism. Interestingly, the accumulation of biological forms of the viroid molecule in RNA silencing-deficient host plants that would not develop symptoms was previously suggested to be a requirement for the pathogenicity model based on the RNA silencing pathway (Tabler and Tsagris, 2004).
An intriguing question that emerges from our results is which of the RDR6-dependent pathways could be associated with the expression of viroid-induced symptoms. Several remarkable observations prompt us to speculate that the pathogenic process induced by the viroid infection could be a consequence of interference in the regulatory pathway involving tasiRNA biogenesis, possibly due to fortuitous matches between HSVd-derived siRNAs and the N. benthamiana genome. The existence in the Nicotiana genus database of sequences presenting a high similarity to specific regions of the HSVd sequence (Supplemental Table S1) is in agreement with this idea. Indeed, both tasiRNA precursors and HSVd RNA replication intermediates are nuclear noncoding RNAs dependent on RNA polymerase II activity (Peragine et al., 2004; Allen et al., 2005; Flores et al., 2005). In addition, it has been suggested that tasiRNAs could regulate transcription factors involved in growth, development, and flowering in Arabidopsis plants (Remington et al., 2004; Allen et al., 2005; Adenot et al., 2006; Xu et al., 2006), resembling the morphological alterations associated with HSVd infection in diverse hosts (Diener et al., 1988; Shikata, 1990; Martinez et al., 2008). The potential involvement of other relevant players in this regulatory pathway would shed light on the role of RDR6 in viroid pathogenesis.
In short, our results demonstrate the requirement of RDR6 activity, a key factor in diverse RNA silencing pathways, for the production of viroid-induced symptoms, reinforcing the emergent view that the viroid-specific RNA silencing-based pathway modulates symptom expression in infected plants (Papaefthimiou et al., 2001; Conejero, 2003; Markarian et al., 2004; Wang et al., 2004).
MATERIALS AND METHODS
Plant Material
HSVd-transgenic Nicotiana benthamiana (line HSVd-Nb/6), expressing and correctly processing dimeric (+)HSVd cDNA, has been described previously (Gómez and Pallás, 2006). Seeds of rdr6i-Nb were kindly provided by Dr. D. Baulcombe (Sainsbury Laboratory, John Innes Centre). The plants were grown at 28°C under natural light with supplementary illumination to maintain a 16-h photoperiod. For temperature treatment, plants were grown in climate boxes (Versatile Environmental Test Chambers; Sanyo) under a 16-h-light (50 μE m−2 s−1) and 8-h-dark regime at 14°C and 20°C.
RNA Extraction and Northern-Blot Analysis
Total RNA was extracted using the TRI reagent (Sigma) according to the manufacturer's instructions. Briefly, 250 mg of leaves from N. benthamiana plants was ground in 2 mL of TRI reagent, 400 μL of chloroform was added, and the sample was vigorously vortexed, followed by centrifugation. The supernatant was recovered, and the total RNAs were precipitated with isopropanol and resuspended in 50 μL of sterile water. The total RNA preparations were quantified by spectrometry, and their concentrations were equalized. To analyze the mature forms of HSVd by northern-blot analysis, 1.5 μg of the total RNA preparations was electrophoresed under denaturing conditions on a 5% polyacrylamide gel with 0.25× Tris-borate/EDTA and 8 m urea (Pallás et al., 1987). The RNA was blotted to nylon membranes (Roche Diagnostics) and hybridized as described previously (Gómez and Pallás, 2007a).
To analyze the small RNAs, total nucleic acids were extracted from 4 g of leaves as described previously. The pellets obtained were used as starting material to purify the small RNAs (<200 nucleotides) enriched for siRNA using miRACLE (miRNA isolation kit; Stratagene) according to the manufacturer's instructions. Equal amounts of small RNAs (25 μg) were loaded onto 20% polyacrylamide gels with 0.25× Tris-borate/EDTA and 8 m urea. The RNA was transferred to nylon membranes (Roche Diagnostics). Hybridization was performed at 32.5°C for 14 to 16 h using a digoxigenin-labeled negative strand-specific HSVd RNA as a probe. The membrane was washed with 2× SSC, 0.1% SDS for 15 min at 24°C and for 15 min at 32.5°C. Chemiluminescent detection was performed as described previously (Gómez and Pallás, 2007a).
Agroinfiltration
Binary pMOG800 plasmids carrying the HSVd-GFP reporter (Gómez and Pallás, 2007b) were transformed into Agrobacterium tumefaciens strain C58C1 (Hamilton et al., 1996). The N. benthamiana plants were agroinfiltrated in basal leaves as described previously (Gómez and Pallás, 2007b). Three days after agroinfiltration, leaf discs (three per leaf) were obtained. GFP expression was analyzed with a Leica TCS SL confocal laser-scanning microscope, with excitation at 488 nm and emission at 510 to 560 nm, and by western-blot assays as described previously (Gómez and Pallás, 2007b).
Agroinoculation
The N. benthamiana plants were agroinoculated with Agrobacterium strain C58C1 transformed with a binary pMOG800 vector carrying a head-to-tail infectious dimeric HSVd cDNA (Gómez and Pallás, 2006) as described previously (Carbonell et al., 2008). The agroinfiltrated leaves were eliminated at 6 dpi. The plants were maintained in environmentally controlled growing chambers at 30°C for 16 h with fluorescent light and at 25°C for 8 h in darkness and analyzed at 30 and 45 dpi.
Grafting Assays
Two-week-old Wt-Nb and rdr6i-Nb scions were cut to a wedge shape that was then inserted into a vertical slit cut into HSVd-Nb/6 and Wt-Nb stocks. The grafting junction was wrapped with Parafilm, and grafted plants were kept at 22°C for 10 d under controlled humidity conditions under plastic covers and later moved to environmentally controlled growth chambers (28°C/14 h of light). The scion length was measured at 30 and 45 dag. Total RNAs were obtained from the excised scions and stocks as described above.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Wt-Nb and rdr6i-Nb scions grafted onto symptomatic HSVd-Nb stocks.
Supplemental Figure S2. HSVd-induced symptoms in Wt-Nb scions.
Supplemental Figure S3. Symptomatic Wt-Nb and asymptomatic rdr6i-Nb scions grafted onto the same stock branch.
Supplemental Figure S4. Symptoms in HSVd-infected Wt-Nb plants.
Supplemental Table S1. Nb mRNAs with high similarity with HSVd.
Supplementary Material
Acknowledgments
We thank Dr. D. Baulcombe for providing the seeds of RDR6i-Nb and Drs. C. Llave, S.F. Elena, and J.A. Daròs for their valuable contribution in the critical reading of the manuscript. We are indebted to Dr. M.D. Gómez-Jimenez for assistance in the observation of GFP expression with a confocal microscope. We also thank an anonymous reviewer for his/her valuable suggestions.
This work was supported by the Spanish granting agency Dirección General de Investigacion Cientifica y Technica (grant no. BIO2005–07331) and by the Generalitat Valenciana (grant no. GV05–238). G.G. is the recipient of a contract from the Consejo Superior de Investigaciones Científicas. G.M. is the recipient of a fellowship from the Ministry of Education and Science.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Vicente Pallás (vpallas@ibmcp.upv.es).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouché N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16 927–932 [DOI] [PubMed] [Google Scholar]
- Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121 207–221 [DOI] [PubMed] [Google Scholar]
- Baulcombe DC (2002) RNA silencing. Curr Biol 12 82–84 [DOI] [PubMed] [Google Scholar]
- Brodersen P, Voinnet O (2006) The diversity of RNA silencing pathways in plants. Trends Genet 22 268–280 [DOI] [PubMed] [Google Scholar]
- Carbonell A, Martínez de Alba AE, Flores R, Gago S (2008) Double-stranded RNA interferes in a sequence-specific manner with the infection of representative members of the two viroid families. Virology 371 44–53 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet 8 884–896 [DOI] [PubMed] [Google Scholar]
- Chellappan P, Vanitharani R, Ogbe F, Fauquet CM (2005) Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol 138 1828–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero V (2003) Viroids and gene silencing. In A Hadidi, R Flores, JW Randles, JS Semancik, eds, Viroids. CSIRO Publishing, Collingwood, Australia, pp 67–70
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101 543–553 [DOI] [PubMed] [Google Scholar]
- Daròs JA, Elena SF, Flores R (2006) Viroids: an Ariadne's thread into the RNA labyrinth. EMBO Rep 7 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daròs JA, Flores R (2002) A chloroplast protein binds a viroid RNA in vivo and facilitates its hammerhead-mediated self-cleavage. EMBO J 21 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener TO (1971) Potato spindle tuber “virus”: replicating low molecular weight RNA. Virology 45 411–428 [DOI] [PubMed] [Google Scholar]
- Diener TO (1999) Viroids and the nature of viroid diseases. Arch Virol 15 203–220 [DOI] [PubMed] [Google Scholar]
- Diener TO, Smith D, Hammond R, Albanese G, La Rosa R, Davino M (1988) Citrus B viroid identified as a strain of hop stunt viroid. Plant Dis 72 691–693 [Google Scholar]
- Ding B, Itaya A (2007) Viroid: a useful model for studying the basic principles of infection and RNA biology. Mol Plant Microbe Interact 20 7–20 [DOI] [PubMed] [Google Scholar]
- Ding SW, Voinnet O (2007) Antiviral immunity directed by small RNAs. Cell 130 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores R, Delgado S, Gas ME, Carbonell A, Molina D, Gago S, de la Peña M (2004) Viroids: the minimal non-coding RNAs with autonomous replication. FEBS Lett 567 42–48 [DOI] [PubMed] [Google Scholar]
- Flores R, Hernández C, Martínez de Alba AE, Daròs JA, Di Serio F (2005) Viroids and viroid-host interactions. Annu Rev Phytopathol 43 117–139 [DOI] [PubMed] [Google Scholar]
- Flores R, Pallás V (2006) Viroids. In JA Khan, J Dijkstra, eds, Handbook of Plant Virology. Haworth Press, New York, pp 93–104
- Gómez G, Pallás V (2001) Identification of an in vitro ribonucleoprotein complex between a viroid RNA and a phloem protein from cucumber plants. Mol Plant Microbe Interact 14 910–913 [DOI] [PubMed] [Google Scholar]
- Gómez G, Pallás V (2004) A long-distance translocatable phloem protein from cucumber forms a ribonucleoprotein complex in vivo with Hop stunt viroid RNA. J Virol 78 10104–10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez G, Pallás V (2006) Hop stunt viroid is processed and translocated in transgenic N. benthamiana plants. Mol Plant Pathol 7 511–517 [DOI] [PubMed] [Google Scholar]
- Gómez G, Pallás V (2007. a) Mature monomeric forms of Hop stunt viroid resist RNA silencing in transgenic plants. Plant J 51 1041–1049 [DOI] [PubMed] [Google Scholar]
- Gómez G, Pallás V (2007. b) A peptide derived from a single-modified viroid-RNA can be used as an “in vivo” nucleolar marker. J Virol Methods 144 169–171 [DOI] [PubMed] [Google Scholar]
- Gómez G, Torres H, Pallás V (2005) Identification of translocatable RNA-binding phloem proteins from melon, potential components of the long-distance RNA transport system. Plant J 41 107–116 [DOI] [PubMed] [Google Scholar]
- Gora A, Candresse T, Zagorski W (1996) Use of intramolecular chimeras to map molecular determinants of symptom severity of Potato spindle tuber viroid. Arch Virol 141 2045–2055 [DOI] [PubMed] [Google Scholar]
- Gruner R, Fels A, Qu F, Zimmat R, Steger G, Riesner D (1995) Interdependence of pathogenicity and replicability with Potato spindle tuber viroid. Virology 209 60–69 [DOI] [PubMed] [Google Scholar]
- Hamilton CM, Frary A, Lewis C, Tanksley SD (1996) Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc Natl Acad Sci USA 93 9975–9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O (2003) Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J 22 4523–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R (2002) Virus movement through the plant. In Matthews' Plant Virology, Ed 4. Academic Press, San Diego
- Itaya A, Folimonov A, Matsuda Y, Nelson RS, Ding B (2001) Potato spindle tuber viroid as inducer of RNA silencing in infected tomato. Mol Plant Microbe Interact 14 1332–1334 [DOI] [PubMed] [Google Scholar]
- Itaya A, Zhong X, Bundschuh R, Qi Y, Wang Y, Takeda R, Harris AR, Molina C, Nelson RS, Ding B (2007) A structured viroid RNA is substrate for Dicer-Like cleavage to produce biologically active small RNAs but is resistant to RISC-mediated degradation. J Virol 81 2980–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C, Kasschau KD, Rector MA, Carrington JC (2002) Endogenous and silencing-associated small RNAs in plants. Plant Cell 14 1605–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markarian N, Li HW, Ding SW, Semancik JS (2004) RNA silencing as related to viroid induced symptom expression. Arch Virol 149 397–406 [DOI] [PubMed] [Google Scholar]
- Martín R, Arenas C, Daròs JA, Covarrubias A, Reyes JL, Chua N (2007) Characterization of small RNAs derived from Citrus exocortis viroid in infected tomato plants. Virology 367 135–146 [DOI] [PubMed] [Google Scholar]
- Martinez G, Pallás V, Gómez G (2008) Analysis of the symptoms developed in N. benthamiana plants expressing dimeric forms of the Hop stunt viroid. J Plant Pathol 90 121–124 [Google Scholar]
- Martínez de Alba AE, Flores R, Hernández C (2002) Two chloroplastic viroids induce the accumulation of the small RNAs associated with post-transcriptional gene silencing. J Virol 76 13094–13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoušek J, Kozlová P, Orctová L, Schmitz A, Pesina K, Bannach O, Diermann N, Steger G, Riesner D (2007) Accumulation of viroid-specific small RNAs and increase in nucleolytic activities linked to viroid-caused pathogenesis. Biol Chem 388 1–13 [DOI] [PubMed] [Google Scholar]
- Mourrain P, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel JB, Jouette D, Lacombe AM, Nikic S, Picault N, et al (2000) Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101 533–542 [DOI] [PubMed] [Google Scholar]
- Owens RA, Blackburn M, Ding B (2001) Possible involvement of the phloem lectin in long-distance viroid movement. Mol Plant Microbe Interact 14 905–909 [DOI] [PubMed] [Google Scholar]
- Pallás V, Navarro A, Flores R (1987) Isolation of a viroid-like RNA from Hop different to HSVd. J Gen Virol 68 3201–3205 [Google Scholar]
- Papaefthimiou I, Hamilton AJ, Denti MA, Baulcombe DC, Tsagris M, Tabler M (2001) Replicating Potato spindle tuber viroid RNA is accompanied by short RNA fragments that are characteristic of post-transcriptional gene silencing. Nucleic Acids Res 29 2395–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS (2004) SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev 18 2368–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu F, Ye XH, Hou GC, Sato S, Clemente TE, Morris TJ (2005) RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in N. benthamiana. J Virol 79 15209–15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington DL, Vision TJ, Guilfoyle TJ, Reed JW (2004) Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol 135 1738–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodio ME, Delgado S, Flores R, Di Serio F (2006) Variants of Peach latent mosaic viroid inducing peach calico: uneven distribution in infected plants and requirements of the insertion containing the pathogenicity determinant. J Gen Virol 87 231–240 [DOI] [PubMed] [Google Scholar]
- Sano T, Matsuura Y (2004) Accumulation of short interfering RNAs characteristics of RNA silencing precedes recovery of tomato plants from severe symptoms of Potato spindle tuber viroid infection. J Gen Plant Pathol 70 50–53 [Google Scholar]
- Schnölzer M, Haas B, Ramm K, Hofmann H, Sänger HL (1985) Correlation between structure and pathogenicity of Potato spindle tuber viroid (PSTV). EMBO J 4 2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwach F, Vaistij FE, Jones L, Baulcombe DC (2005) An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol 138 1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata E (1990) New viroids from Japan. Semin Virol 1 107–115 [Google Scholar]
- Skoric D, Conerly M, Szychowski JA, Semancik JS (2001) CEVd-induced symptom modification as a response to a host-specific temperature-sensitive reaction. Virology 280 115–123 [DOI] [PubMed] [Google Scholar]
- Szittya G, Silhavy D, Molnar A, Havelda Z, Lovas A, Lakatos L, Banfalvi Z, Burgyan J (2003) Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J 22 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M, Tsagris M (2004) Viroids: petite RNA pathogens with distinguished talents. Trends Plant Sci 9 339–348 [DOI] [PubMed] [Google Scholar]
- Vaistij FE, Jones L, Baulcombe DC (2002) Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell 14 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V, Vaucheret H (2001) RNA silencing in plants: defense and counterdefense. Science 292 2277–2280 [DOI] [PubMed] [Google Scholar]
- Vaucheret H (2006) Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 20 759–771 [DOI] [PubMed] [Google Scholar]
- Vazquez F, Vaucheret H, Rajagopalan R, Lepers C, Gasciolli V, Mallory AC, Hilbert JL, Bartel DP, Crété P (2004) Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Mol Cell 16 69–79 [DOI] [PubMed] [Google Scholar]
- Vogt U, Pelissier T, Putz A, Razvi F, Fischer R, Wassenegger M (2004) Viroid-induced RNA silencing of GFP-viroid fusion transgenes does not induce extensive spreading of methylation or transitive silencing. Plant J 38 107–118 [DOI] [PubMed] [Google Scholar]
- Wang MB, Bian XY, Wu LM, Liu LX, Smith NA, Isenegger D, Wu RM, Masuta C, Vance VB, Watson JM, et al (2004) On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. Proc Natl Acad Sci USA 101 3275–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yang L, Pi L, Liu Q, Ling Q, Wang H, Poethig RS, Huang H (2006) Genetic interaction between the AS1-AS2 and RDR6-SGS3-AGO7 pathways for leaf morphogenesis. Plant Cell Physiol 47 853–863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.