Abstract
Symbiotic nitrogen fixation is sensitive to dark chilling (7°C–15°C)-induced inhibition in soybean (Glycine max). To characterize the mechanisms that cause the stress-induced loss of nodule function, we examined nodule structure, carbon-nitrogen interactions, and respiration in two soybean genotypes that differ in chilling sensitivity: PAN809 (PAN), which is chilling sensitive, and Highveld Top (HT), which is more chilling resistant. Nodule numbers were unaffected by dark chilling, as was the abundance of the nitrogenase and leghemoglobin proteins. However, dark chilling decreased nodule respiration rates, nitrogenase activities, and NifH and NifK mRNAs and increased nodule starch, sucrose, and glucose in both genotypes. Ureide and fructose contents decreased only in PAN nodules. While the chilling-induced decreases in nodule respiration persisted in PAN even after return to optimal temperatures, respiration started to recover in HT by the end of the chilling period. The area of the intercellular spaces in the nodule cortex and infected zone was greatly decreased in HT after three nights of chilling, an acclimatory response that was absent from PAN. These data show that HT nodules are able to regulate both respiration and the area of the intercellular spaces during chilling and in this way control the oxygen diffusion barrier, which is a key component of the nodule stress response. We conclude that chilling-induced loss of symbiotic nitrogen fixation in PAN is caused by the inhibition of respiration coupled to the failure to regulate the oxygen diffusion barrier effectively. The resultant limitations on nitrogen availability contribute to the greater chilling-induced inhibition of photosynthesis in PAN than in HT.
The predictability of crop yields, income to the farmer, and reliable sources for agroindustries are severely hampered by environmental factors, particularly extremes of temperature. Over the last 50 years, plant breeders have improved the yield potential, but the yield gap, defined as the difference between the theoretical yield potential of a crop and the actual yield achieved, remains. The yield gap occurs because plants grow best within certain ranges of environmental conditions and stop growing in other conditions, for example, at high and low temperatures; hence, yield is reduced. As with other major crops, grain legume production can be severely restricted by environmental stresses. Soybean (Glycine max) is an important crop and a key source of proteins for human and animal consumption (Keyser and Li, 1992) and is also used as a natural nitrogen source in agriculture, particularly in Africa, because of the presence of nitrogen-fixing bacteria in specialized organs called “root nodules.” The symbiotic association between the plant and nitrogen-fixing bacteria in nodule formation has been the subject of intensive study, but much less information is available on the mechanisms that cause the breakdown of symbiosis, particularly during stress. New nodules have to be formed to take the place of the chilled organs, causing nitrogen deficits and extra carbon and nitrogen costs to the chilling-sensitive plants. Although the symptoms and progression of nodule senescence have been described (Puppo et al., 2005), much remains to be discovered regarding the mechanisms that trigger the end of symbiosis and the genes and proteins that underpin nodule senescence.
Like many other warm-climate crop species (e.g. cucumber [Cucumis sativus], tomato [Solanum lycopersicum], and maize [Zea mays]), soybean is sensitive to suboptimal growth temperatures. Plants vary greatly in their ability to tolerate low growth temperatures, and there is much interspecific variation in the capacity to maintain optimal metabolism under these conditions. Plants can be classified as chilling sensitive or chilling tolerant, or freezing sensitive or freezing tolerant, according to the threshold below which injury is observed. Soybean is denoted as chilling sensitive, as the growth of soybean plants is usually negatively affected upon exposure to temperatures below 15°C. Low-night-temperature-induced (dark chilling) injury to crops can cause problems even in subtropical agriculture. For example, many soybean-producing regions in South Africa are located at high altitudes where dark chilling limits soybean production potential (Smith, 1994). The sensitivity of soybean to night temperatures below 15°C is reflected in the changes that occur in metabolism, growth, development, and yield (Musser et al., 1983, 1984; Van Heerden et al., 2003). A single night of dark chilling, with minimum temperatures of 8°C, is sufficient to inhibit pod formation (Hume and Jackson, 1981). Genotypes also respond differently to dark chilling, with some exhibiting a degree of tolerance while others are extremely sensitive (Lawn and Hume, 1985; Seddigh et al., 1989).
We have previously characterized the adverse effects of dark chilling on soybean leaf photosynthesis and carbon metabolism (Van Heerden et al., 2003) as well as the respective roles of shoots and root parameters (Strauss et al., 2006, 2007). Exposure to dark chilling had a direct effect on photosynthesis, as determined by the polyphasic (OKJIP, the letters denoting the positions of various peaks) rise in chlorophyll a fluorescence. Specifically, dark chilling resulted in the appearance of a J-peak (Strauss et al., 2006, 2007). However, the J-peak was induced by dark chilling in all of the 30 soybean genotypes that were screened under conditions in which root temperatures were maintained at optimal values (shoot chilling treatment), regardless of their relative sensitivities to dark chilling (Strauss et al., 2006). The only difference that was observed between the 30 genotypes analyzed was the magnitude of the J-peak. Genotypes such as Highveld Top (HT) showed a much smaller J-peak than chilling-sensitive genotypes such as PAN809 (PAN). However, when the roots and the shoots were exposed simultaneously to dark chilling (whole plant chilling), the OKJIP kinetic change induced in PAN was absent from HT. The typical J-peak that formed in PAN was accompanied by the generation of a K-peak that appeared as leaf ureide contents fell (Strauss et al., 2007). Since to date only heat stress (Lazár et al., 1997; Lazár, 2006) and nitrogen limitation (Strasser et al., 2004) have been implicated in the formation of the K peak in the OKJIP transient, the observation of this kinetic change in PAN, in combination with a greater inhibition of CO2 assimilation rates, suggested that chilling sensitivity in the shoot results more from a dark chilling-dependent inhibition of symbiotic nitrogen fixation (SNF) than a direct effect on shoot parameters per se.
SNF is exquisitely sensitive to metabolic and environmental perturbations such as defoliation, water deficit, continuous darkness, nitrate fertilization, and chilling (Duke et al., 1979; Walsh and Layzell, 1986; Matamoros et al., 1999). Exposure to even relatively mild temperatures (15°C) can inhibit SNF by up to 45% (Walsh and Layzell, 1986). However, despite the adverse effects of this phenomenon on grain legume yield, the mechanisms underpinning the chilling-induced inhibition of SNF have received relatively little attention. Relatively few studies to date have investigated genotypic variations in chilling-induced inhibition of SNF (Lynch and Smith, 1994). Recently, Strauss et al. (2006) evaluated the dark chilling response of 30 South African soybean genotypes on the basis of changes induced in fast fluorescence rise kinetics during exposure to dark chilling stress. Large genotypic differences in dark chilling response were observed, especially in the two genotypes PAN and HT, which were subsequently classified as dark chilling “sensitive” and “tolerant,” respectively (Strauss et al., 2006).
Some types of stress (e.g. drought) decrease nodule permeability to O2 with an associated lowering in symbiosome O2 concentrations (Oi), which in turn inhibits nitrogenase activity indirectly because of lower nodule respiratory activity (Vessey et al., 1988; Serraj and Sinclair, 1996). Chilling stress, on the other hand, modifies nodule O2 homeostasis through an increase in Oi and the fractional oxygenation of leghemoglobin (Kuzma et al., 1995). High Oi favors inhibition of nitrogenase activity, as the enzyme is extremely sensitive to O2-induced inactivation. Other processes within the plant partner rather than the bacterial partner can contribute to the chilling sensitivity of SNF (Lynch and Smith, 1994). Such results have led to the hypothesis that inoculation with Bradyrhizobium japonicum strains from cold environments will have little benefit in alleviating the sensitivity of soybean to chilling stress, although contrasting evidence also exists (Zhang et al., 2003). In order to explore the relationship between plant and bacterial processes that underpin the sensitivity of SNF to dark chilling, we have characterized the dark chilling-induced responses of metabolism in the chilling-sensitive soybean genotype PAN and the chilling-resistant genotype HT.
RESULTS
Dark Chilling Effects on Shoot Phenotype
After three nights of chilling, no visual effects on growth or differences in leaf chlorophyll contents were observed in either genotype (data not shown). Prolonged exposure to dark chilling involving 12 consecutive nights of exposure to low temperatures led to severe inhibition of shoot growth and visible chlorosis in PAN, while there were no visual symptoms in the chilling-tolerant HT (Fig. 1). The chlorophyll content of the youngest fully expanded PAN leaves was 57% lower than those of plants maintained at optimal growth temperatures after 12 nights of chilling. In contrast, the chlorophyll contents were only decreased by 15% in HT after 12 nights of chilling (data not shown). Since preliminary tests showed that differences in nodule function occurred between genotypes after three consecutive nights of dark chilling, we used this treatment regime in the following experiments as the optimal period with which to observe inhibition and recovery responses.
Figure 1.
A comparison of shoot development in HT (chilling tolerant) and PAN (chilling sensitive). The plants were either maintained under optimal growth conditions (Control) or exposed to 12 consecutive nights of dark chilling (7°C; Chilled).
Dark Chilling Effects on CO2 Gas Exchange Parameters
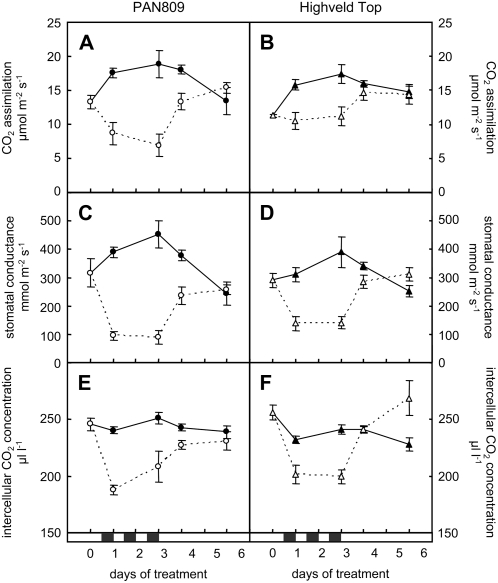
Dark chilling led to more severe inhibition of photosynthesis in PAN (Fig. 2A) than in HT (Fig. 2B). CO2 assimilation rates were inhibited by up to 63% in PAN during the period (days 1–3) of dark chilling, whereas the inhibition never exceeded 35% in HT. PAN also took longer than HT to fully recover CO2 assimilation rates during the postchilling period (days 4–6). Photosynthesis rates were similar to those of controls maintained at optimal temperatures after only 1 d following the cessation of dark chilling in HT. In contrast, photosynthetic rates were still inhibited by 26% in PAN at this time point. The inhibition of photosynthesis was closely associated with decreases in stomatal conductance (Fig. 2, C and D) and concomitant decreases in intercellular CO2 concentrations (Fig. 2, E and F) in both genotypes.
Figure 2.
A comparison of the responses of photosynthesis in PAN (left graphs) and HT (right graphs) to dark chilling. Photosynthetic CO2 assimilation rates (A and B), stomatal conductance (C and D), and intercellular CO2 concentration (E and F) were measured in the central leaflet of the youngest fully expanded trifoliate leaf before the onset of chilling (day 0), at 5 h after return to the greenhouse following each night of dark chilling (days 1–3), and after 3 d of recovery under optimal day/night temperatures (days 4–6). The black horizontal bars above the x axis indicate the three consecutive nights of chilling. Black and white symbols represent control and dark chilled plants, respectively. Each data point represents the mean of three replicates ± se.
Dark Chilling Effects on Nodulation and SNF
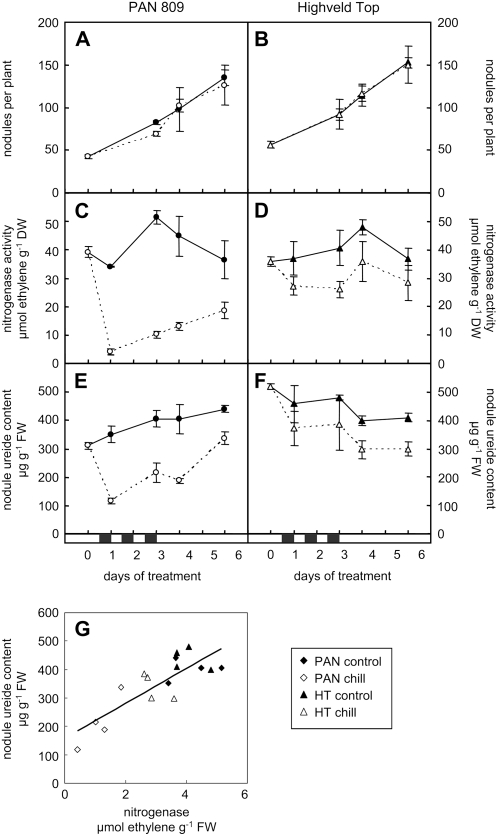
The two genotypes exhibited similar nodule emergence rates (measured as the number of new nodules present per day) at optimal growth temperatures. Dark chilling did not affect nodule emergence rates in either genotype, at least not during the 6 d of the experiment (Fig. 3, A and B), presumably because the new nodules that emerged during this time were already initiated prior to the chilling treatment.
Figure 3.
A comparison of the effects of dark chilling on nodule numbers, nitrogenase activity, and nodule ureide content in PAN and HT (top) and the relationship between nodule ureide contents and nitrogenase activities (bottom). Nodules per plant (A and B), nitrogenase activity of whole root systems with attached nodules (C and D), and nodule ureide content (E and F) were determined in samples collected at midday (noon). The relationship between nodule ureide contents and nitrogenase activities is shown in G. DW, Dry weight; FW, fresh weight. The black horizontal bars above the x axis indicate the three consecutive nights of chilling. Black and white symbols represent control and dark chilled plants, respectively. Each data point represents the mean of three replicates ± se.
Under optimal growth conditions, similar specific nitrogenase activities were measured in the nodules of both genotypes (Fig. 3, C and D). However, the response of nitrogenase activity to dark chilling differed drastically between the two genotypes. PAN nodule nitrogenase activity was inhibited by 87% at 6 h after the return to optimal temperatures following the first night of chilling. Conversely, the HT nitrogenase activities in chilled plants were not statistically significantly different (P > 0.05) from those of controls that had been maintained at optimal temperatures. Similar trends were observed following consecutive nights of chilling. In contrast to the observed recoveries in photosynthesis following dark chilling, PAN nitrogenase activities never fully recovered from the chilling stress and remained inhibited (by about 50%) even 3 d after termination of the chilling treatments.
PAN nodule ureide contents fell by over 60% following dark chilling, whereas no significant decreases in nodule ureides were observed in HT (Fig. 3, E and F). The responses of nodule ureide contents to dark chilling closely paralleled those observed in nitrogenase activity (Fig. 3, C and D), such that a positive correlation (r2 > 0.68) was obtained between nitrogenase activity and nodule ureide content in response to this stress (Fig. 3G). Although the inherent limitations associated with the acetylene reduction assay (Minchin et al., 1983, 1986) are acknowledged, the close correlation that existed between nitrogenase activity and nodule ureide content suggests that the method did resolve genotype-specific effects of dark chilling on nitrogenase activity accurately enough to be useful.
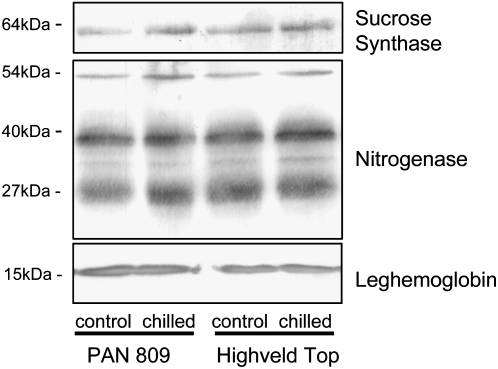
Dark Chilling Effects on Nodule Proteins and Transcripts
Nodule protein contents were similar in both genotypes under optimal growth conditions (Table I), and they did not vary significantly as a result of chilling. The abundance of the nitrogenase protein was similar in HT and PAN nodules under all conditions (Fig. 4). Similarly, the abundance of nodule Suc synthase and leghemoglobin was not decreased in response to dark chilling treatments (Fig. 4). Unlike the HT nodules, which showed no change in the abundance of Suc synthase protein, levels were slightly increased in the PAN nodules after three nights of chilling compared with controls (Fig. 4).
Table I.
A comparison of the total soluble protein contents (mg g−1 fresh weight) of PAN and HT nodules
Samples were harvested from either plants maintained under optimal growth conditions (Optimal) or from plants exposed to three nights of dark chilling (7°C). Values represent means of three replicates ± se per experiment.
| Time (d) | Treatment | Genotype
|
|
|---|---|---|---|
| PAN | HT | ||
| 0 | Optimal | 9.9 ± 0.5 | 10.9 ± 0.9 |
| 7°C | – | – | |
| 3 | Optimal | 11.9 ± 1.1 | 10.8 ± 1.4 |
| 7°C | 10.0 ± 1.1 | 10.7 ± 0.05 | |
Figure 4.
The effects of dark chilling on the abundance of nodule Suc synthase, nitrogenase, and leghemoglobin protein in PAN and HT. Proteins were extracted from the nodules of plants either maintained under optimal growth conditions (control) or exposed to three consecutive nights of dark chilling (chilled).
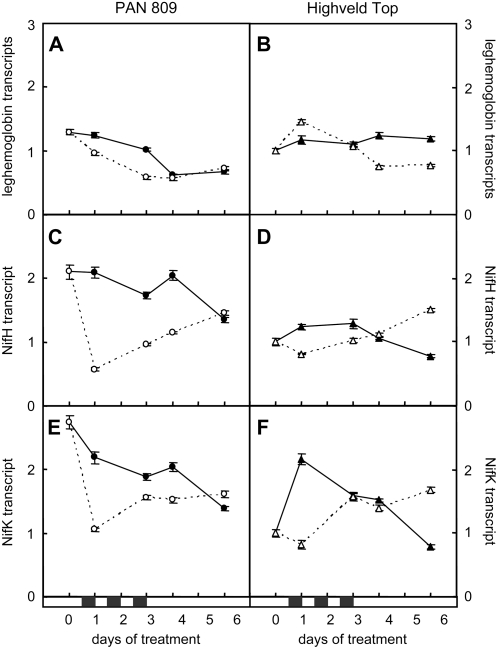
The abundance of transcripts encoding the nitrogenase subunits NifH (Fig. 5, C and D) and NifK (Fig. 5, E and F) was decreased in both genotypes after only one night of dark chilling. However, the level of transcripts recovered soon after the return to optimal night temperatures. The chilling-induced decrease in NifH and NifK transcripts was more pronounced in PAN than in HT. Similarly, the recovery of NifH and NifK transcripts in PAN was less rapid following the termination of dark chilling treatment. The response of leghemoglobin transcripts to dark chilling was genotype-dependent (Fig. 5, A and B), being lower than controls during the chilling period in PAN and lower during the recovery period after chilling in HT (Fig. 5, A and B).
Figure 5.
A comparison of the effects of dark chilling on the abundance of transcripts encoding leghemoglobin, NifH, and NifK in PAN and HT. Data are expressed relative to values obtained in HT unchilled samples at the start of the experiment. Black and white symbols represent control and dark chilled plants, respectively. Each data point represents the mean of three replicates ± se.
Dark Chilling Effects on Nodule Carbohydrate Contents
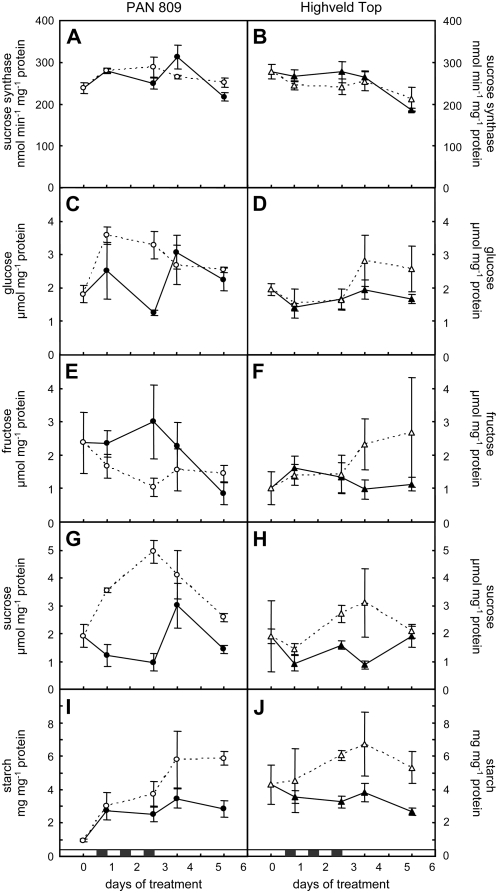
A small increase in Suc synthase activity was observed in the PAN nodules after three nights of chilling (Fig. 6A), which is consistent with the slight increase in the abundance of nodule Suc synthase protein at this time point (Fig. 4). However, PAN nodule Suc synthase activity decreased rapidly to below control levels within 1 d of return to optimal growth temperatures. The Suc synthase activity of the HT nodules was similar in both treatments over the whole treatment and recovery period and was unaffected by dark chilling (Fig. 6, A and B). However, the chilling treatment increased nodule Glc (Fig. 6, C and D), Suc (Fig. 6, G and H), and starch (Fig. 6, I and J). While Glc accumulated to higher levels during the dark chilling period in PAN than in HT, it increased only in the recovery period in HT nodules (Fig. 6, C and D). In contrast, Fru decreased in PAN but not in HT nodules during the chilling period (Fig. 6E) and was higher in chilled HT nodules in the recovery period (Fig. 6F). Hence, as the decrease in Fru offset the increase in Glc in chilled PAN nodules, the total hexose pool was unchanged during the chilling period in either genotype (data not shown). In contrast, nodule Suc levels were increased during the chilling treatment in both genotypes, being most marked in PAN, and chilled nodules maintained higher Suc levels than controls even in the recovery period (Fig. 6, G and H).
Figure 6.
A comparison of the effects of dark chilling on PAN and HT nodule Suc synthase activity and carbohydrate contents. Suc synthase activity (A and B), Glc (C and D), Fru (E and F), Suc (G and H), and starch (I and J) were measured in nodules harvested at midday. Each data point represents the average of five independent measurements ± se. Black and white symbols represent control and dark chilled plants, respectively. Each data point represents the mean of three replicates ± se.
Dark Chilling Effects on Nodule Respiration
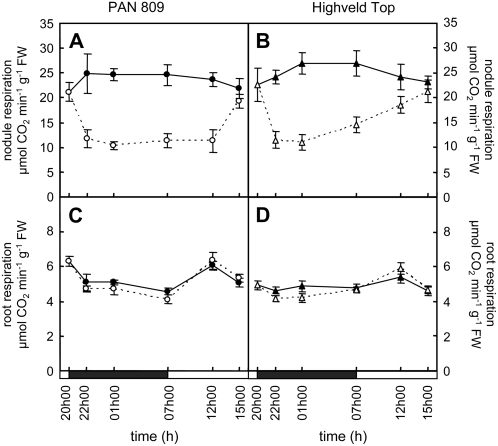
In contrast to root respiration rates, which were unaffected by dark chilling (Fig. 7, C and D), nodule respiration showed a rapid and cultivar-specific pattern of inhibitory response and recovery (Fig. 7, A and B). The results on the respiration of attached root nodules shown in Figure 7 represent pooled data obtained during and after one or three nights of exposure to dark chilling. After only 2 h of exposure to chilling night temperatures, a large inhibition of nodule respiration rates was observed in both genotypes. The chilling-dependent inhibition of respiration was still present after 4 h of chilling (Fig. 7, A and B). However, unlike PAN nodules, in which respiration rates remained low throughout the duration of the chilling treatment, the respiration rates of HT nodules were significantly increased once more after 10 h of exposure to dark chilling (7h00 in Fig. 7). After 5 h of recovery at optimal growth temperatures (12h00 in Fig. 7), the point at which measurements of growth and metabolism were performed, nodule respiration rates no longer exhibited significant differences (P > 0.05) from the controls maintained at optimal growth temperatures in HT, whereas the degree of inhibition of respiration remained high in PAN nodules. PAN nodules thus showed an absence of the acclimation in respiration to chilling observed in HT. Respiration rates only recovered to control values in PAN nodules after 8 h of exposure to optimal day temperatures. The acclimation of respiration to dark chilling was observed in HT nodules even after repeated chilling stress exposures.
Figure 7.
A comparison of the effects of dark chilling on diurnal nodule and root respiration rates in PAN and HT. Respiration rates were measured in nodules (A and B) and roots (C and D) before the onset of dark chilling treatment (20h00), during dark chilling treatment (22h00, 01h00, and 07h00), and during the day following dark chilling (12h00 and 15h00). The black horizontal bars above the x axis indicate the dark chilling period. Black and white symbols represent control and dark chilled plants, respectively. Each data point represents the mean of six replicates ± se.
Dark Chilling Effects on the Oxygen Diffusion Barrier within Nodules
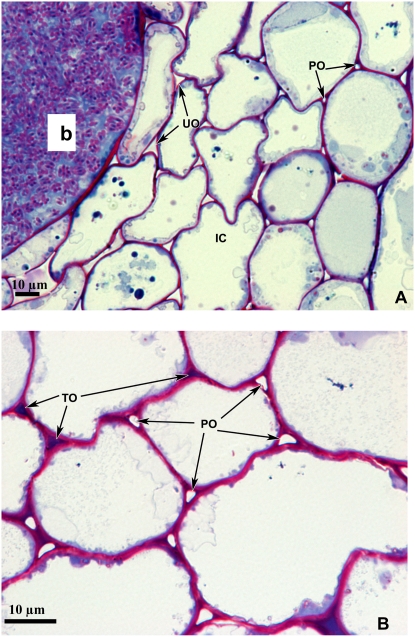
The surface area of the individual intercellular air spaces in the middle cortex, inner cortex, and infected zones of the nodule were similar in the genotypes in the absence of chilling (Table II, control). While the area of the intercellular air spaces was unchanged in PAN nodules after chilling, the area was decreased by about 40% as a result of the chilling treatment in all zones of the HT nodules (Table II, chilled). The decrease in area appeared to be mainly caused by the widespread formation of electron-dense occlusions within intercellular air spaces of chilled HT nodules (Fig. 8). After three nights of recovery at optimal growth temperatures, the area of intercellular spaces in previously chilled HT nodules returned to values comparable to those of unchilled controls. The values obtained for the air space volume in the inner cortex were 8.2 ± 0.2 and 8.6 ± 0.7 μm2 for the unchilled controls and recovering nodules, respectively. The values obtained for the air space volume of the middle cortex were 16.3 ± 3 and 20.8 ± 4 μm2 for the unchilled controls and recovering nodules, respectively.
Table II.
A comparison of the effects of dark chilling on the average area (μm2) of individual intercellular spaces (n = 50) in the middle cortex, inner cortex, and infected zone regions of nodules collected from PAN and HT plants that had been either maintained at optimal night temperatures or subjected to three nights of dark chilling
Values in parentheses give percentage decreases in the area of intercellular spaces in chilled HT plants compared with those maintained at optimal night temperatures. All values are means ± se of data from nodules of three to five plants per treatment.
| Region | PAN
|
HT
|
||
|---|---|---|---|---|
| Control | Chilled | Control | Chilled | |
| Middle cortex | 14.0 ± 2.1 | 15.5 ± 1.8 | 14.7 ± 3.1 | 9.4 ± 0.5 (−36%) |
| Inner cortex | 7.0 ± 1.3 | 9.1 ± 2.6 | 8.1 ± 1.1 | 5.0 ± 0.2 (−38%) |
| Infected zone | 25.4 ± 1.1 | 22.4 ± 5.9 | 24.4 ± 1.6 | 14.1 ± 3.7 (−42%) |
Figure 8.
Micrographs of cross sections through chilled HT nodules showing the infected zone, inner cortex (IC; A), and middle cortex (B). The presence of unoccluded (UO), partially occluded (PO), and totally occluded (TO) intercellular air spaces is indicated by arrows. b, Bacteroids. Bars = 10 μm.
DISCUSSION
Soybean genotypes vary greatly in their ability to tolerate low growth temperatures, and there is much interspecific variation in the capacity to maintain optimal metabolism under such conditions (Strauss et al., 2006). The high degree of natural genetic variation in dark chilling tolerance existing within South African soybean germplasm allows a precise characterization of the metabolic factors that underpin this important trait. Our analysis of the responses of carbon/nitrogen relationships associated with SNF to dark chilling suggests that nitrogenase activity is a major target for this stress-induced inactivation. Soybean SNF and nodule respiration rates are inhibited by low soil temperatures under field conditions (Duke et al., 1979; Kuzma et al., 1995). In the experiments reported here, whole plants were exposed to chilling night temperatures. While the abrupt transition of the roots in the pots to low soil temperatures may appear artificial compared with temperature changes experienced by roots under field conditions, the chilling-induced symptoms observed in the soybean plants in this study are comparable to those observed under field cultivation in various regions of the world (Layzell et al., 1984; Walsh and Layzell, 1986; Legros and Smith, 1994). Moreover, the first nodules appear in cluster formation at the base of the primary root, just below the soil surface, where they experience similar suboptimal temperatures to the shoot under dark chilling, even when deeper soil layers do not experience such low temperatures.
The significant genotype-specific differences in the magnitude of the chilling-induced inhibition of photosynthesis and the capacity for recovery reported here are consistent with previous results on these genotypes (Strauss et al., 2006). The similar patterns of chilling-induced inhibition of photosynthetic CO2 assimilation observed in HT and PAN in this study can be explained simply in terms of the comparable degrees of stomatal closure and hence intercellular CO2 availability in response to dark chilling. The effects of shoot chilling alone on photosynthesis and leaf ureide contents have been compared with those of whole plant chilling for the two genotypes studied here, the chilling sensitivity in PAN being specifically linked to whole plant processes rather than to direct effects of low temperatures on the leaves per se (Strauss et al., 2007). This study showed that chilling effects on nodules and the transport of ureides from the roots to the leaves are crucial in the observed responses of photosynthesis to dark chilling (Strauss et al., 2007). In particular, the whole plant chilling treatment decreased PAN leaf ureide contents and enhanced the inhibition of CO2 assimilation leading to severe chlorosis, effects that were related to nitrogen limitation in the chilling-sensitive genotype (Strauss et al., 2007). The PAN shoot phenotype shown here (Fig. 1) following longer term exposures to dark chilling is consistent with an inability to sustain nitrogen fixation at low night temperatures. Exposure to chilling can also limit the transport of nitrogen metabolites leading to chlorosis (Castle et al., 2006). While the effects of nitrogen limitation such as chlorosis are often most evident in the oldest leaves, all of the leaves on plants grown under long-term nitrogen limitation exhibited a chlorotic phenotype (see figure 9 in Pellny et al., 2008). The effect of dark chilling on the shoot phenotype of another genotype of similar chilling sensitivity is virtually abolished when the plants are provided with a nitrogen source (Van Heerden et al., 2004), demonstrating that the primary target for the chilling-induced inhibition is SNF in the nodules. Taken together, the results of the previous studies (Van Heerden et al., 2004; Strauss et al., 2007) and this study emphasize the central role of SNF in chilling sensitivity of PAN and other chilling-sensitive genotypes and the importance of maintaining the nitrogen supply for repair of the photosynthetic machinery.
The data presented here show that dark chilling resulted in a rapid loss of nitrogenase activity even though the enzyme content was unaffected. In this study, we did not analyze the effect of chilling on nitrogenase subunit composition, and a changed nitrogenase protein composition that results in altered activity could remain undetected by the limited western-blot analysis performed here. Since chilling decreased the abundance of the measured transcripts encoding nitrogenase subunits, we cannot rule out the possibility that altered SNF capacity could, at least in part, result from altered nitrogenase subunit composition. Our current knowledge of the factors that contribute to stress-induced inhibition of nitrogenase activity in soybean is largely restricted to studies on drought (King and Purcell, 2005). Several mechanisms are involved in the inhibition of nitrogenase activity during drought, including (1) depletion of carbohydrates, (2) oxygen limitations, and (3) feedback regulation by nitrogen accumulation (Serraj et al., 1999). The decreased rates of respiration that occur in nodules exposed to low temperatures will also tend to limit nitrogenase function in vivo by decreasing the supply of ATP required to drive SNF. An O2-based limitation to nitrogenase activity has been shown to occur in soybean nodules even under ambient temperature conditions (Hunt et al., 1989).
The data presented here show that controlling the O2 diffusion barrier is also an important factor restricting SNF during dark chilling. The decline in nodule respiration rates during dark chilling suggests a high probability of elevated Oi, thereby predisposing nitrogenase to O2-induced inactivation. The morphometric analysis of intercellular air spaces revealed genotypic differences in the ability to control the oxygen diffusion barrier in the nodules. These changes during chilling stress are consistent with those observed previously in soybean nodules in which Oi was increased artificially by exposure of nodules to high atmospheric O2 concentrations (Parsons and Day, 1990; James et al., 1991; Serraj et al., 1995; Minchin, 1997). Oxygen availability, and thus diffusive resistance, regulates both nodule respiration and nitrogenase activity. While the precise mechanism of reversible diffusion resistance control remains to be identified, the relative volumes of infected and uninfected cells as well as their relative frequencies contribute to this regulation (Thumfort et al., 1999). The relative fractions of cytosol and bacteroids were found to have a major impact on oxygen diffusivity in infected cells. However, nodule permeability to oxygen is also influenced by many other factors, such as alterations in the distribution and size of intercellular air spaces, rapid water movements into or out of intercellular air spaces, and the formation of glycoprotein occlusions inside intercellular air spaces (Parsons and Day, 1990; James et al., 1991; Purcell and Sinclair, 1994; Serraj et al., 1995, 1999; Minchin, 1997).
Drought-induced decreases in nitrogenase activity have been linked to the loss of Suc synthase activity and hence the ability of the nodule to metabolize Suc (Gordon et al., 1997, 1999). However, the close relationship between the acetylene reduction capacity of soybean nodules and Suc synthase activity that has been reported previously (Anthon and Emerich, 1990) was not observed following exposure to chilling. The results presented here demonstrate that the model of stress-induced loss of Suc synthase function does not apply to dark chilling. Indeed, Suc synthase protein and activity were actually slightly increased in PAN nodules after three nights of chilling. The activities of other enzymes involved in nodule carbon metabolism, such as phosphoenolpyruvate carboxylase and invertase, which have not been determined in this study, may also be affected by the chilling treatment. However, the absence of parallel increases or decreases in nodule Glc and Fru during the chilling treatments would argue against a significant role for invertase in stress-induced nodule Suc breakdown.
The carbon assimilation pathway in the leaves is impaired as a result of dark chilling, and it is logical to assume that the long-distance transport of Suc from the leaves to the nodules might also be impeded by dark chilling. However, because Suc, Glc, and starch accumulated in chilled PAN and HT nodules, it is reasonable to conclude that the long-distance transport of Suc remains sufficiently active to allow the import and accumulation of the sugars that are required to drive nodule respiration. In contrast, the observed chilling-dependent inhibition of nitrogenase activity led to a rapid depletion of ureide contents, particularly in PAN nodules, depriving the plant of its major source of organic nitrogen (Smith and Atkins, 2002). The PAN leaves experience significantly lower ureide contents after three consecutive exposures to dark chilling (Strauss et al., 2007). Ureides, like amino acids produced from them, are considered to be important signals coordinating whole plant stress responses (Parsons et al., 1993; Marino et al., 2007). However, the simultaneous decreases in nitrogenase activity and ureide availability observed in PAN nodules as a result of dark chilling argue against the operation of a simple feedback mechanism in which nodule ureide content feeds back to inhibit nitrogenase activity.
In addition to nitrogenase activity and ureide production, dark chilling will influence a wide range of other metabolic pathways, including respiration (Smith and Atkins, 2002). Nodule but not root respiration was inhibited rapidly following transfer to chilling temperatures in both genotypes (Fig. 7). However, while nodule respiration was inhibited to the same extent in PAN and HT nodules during the first 4 h of the dark chilling period, respiratory rates had increased significantly in HT nodules by the end of the night chilling period, and they increased much more rapidly in the subsequent light period compared with PAN nodules, which retained low respiration rates in the first hours of the light period at optimal growth temperatures. Together, these results demonstrate that PAN nodules experience a persistent form of inhibition of respiration as a result of dark chilling that is largely absent from HT. These results suggest that HT nodules are able to sense and orchestrate an acclimatory response to the limitations on respiration imposed by low temperatures. This acclimation response in respiration clearly distinguishes the stress response in HT from that observed in PAN. Moreover, the acclimation response is specific to nodule respiration, because root respiration rates were unaffected by chilling in either genotype.
The results presented here are particularly interesting as it is generally accepted that the ability of plants to acclimate to low temperature plays an important role in determining geographic distribution because of adverse effects on plant performance. Reduced rates of respiration are commonly found in plants exposed to low temperatures, leading to a decrease in ATP supply for biosynthesis and/or cellular maintenance (Atkin and Tjoelker, 2003). The inhibition of nodule respiration observed in this study will also compound the problem of oxygen-mediated repression of nitrogenase activity by simultaneously limiting the availability of ATP to drive the enzyme-catalyzed reaction. Chilling-resistant species such as Arabidopsis (Arabidopsis thaliana) are able to reestablish growth during extended periods of exposure to low temperatures because they are able to induce acclimatory responses that lead to increased respiration rates, such as an increased abundance or size of mitochondria and/or enhanced abundance of proteins associated with respiratory ATP synthesis in cold-acclimated tissues (Kurimoto et al., 2004; Armstrong et al., 2006). We can only speculate that similar processes must occur in the recovery of HT nodule respiration during the chilling stress, allowing increased rates of ATP synthesis and hence recovery from the stress. However, the ability to sense the chilling-induced impairment of metabolism or the ability to trigger or orchestrate the required acclimatory response observed in HT must be absent or diminished in PAN.
A diminished capacity for respiration could inhibit nitrogenase activity through altered nodule oxygen homeostasis and/or decreased ATP and reducing power availability. Precise maintenance of nodule oxygen homeostasis is critical for SNF, because the presence of sufficient oxygen is essential to sustain the respiratory terminal oxidases, but low oxygen concentrations at the site of N2 fixation are also needed to prevent rapid inactivation of nitrogenase. Respiration is the major sink for oxygen in the nodule, since the respiratory cytochrome c oxidase has a very high affinity for oxygen. Chilling-induced inhibition of respiration will inhibit this process, therefore causing the nodule oxygen content to rise, breaking the diffusion barrier and inhibiting nitrogenase (Kuzma et al., 1995). However, our results provide new evidence that the HT soybean plants have the capacity to sense and respond to the chilling-induced impairment of respiration, perhaps via sensing of the nodule oxygen content. Exposure to dark chilling led to the formation of electron-dense occlusions that greatly decreased the size of the individual intercellular air spaces in all zones of HT nodules. These occlusions must increase resistance to O2 diffusion within the nodule, thereby protecting nitrogenase from O2-induced deactivation. The formation of similar occlusions in the intercellular air spaces was observed in soybean nodules exposed to high O2 (Parsons and Day, 1990; James et al., 1991; Serraj et al., 1995). However, our results demonstrate, to our knowledge for the first time, that genotypic differences exist in soybean with regard to the ability to induce these occlusions in response to stress. Nodule gene expression is considered to be exquisitely sensitive to oxygen (Soupene et al., 1995). Oxygen availability also regulates mitochondrial structure and composition; for example, the subunit composition of cytochrome c oxidase is regulated by O2 availability (Fukuda et al., 2007).
The presence of chilling-dependent formation of intercellular occlusions in HT nodules suggests an ability to control diffusion resistance. While the nature of the extracellular deposits observed in the light micrographs remains to be determined, they probably consist of glycoprotein in addition to other proteins or carbohydrates (Parsons and Day, 1990; James et al., 1991). A preliminary transcriptome analysis of the PAN and HT nodules using the Affymetrix GeneChip Soybean Genome Array, which detects approximately 37,500 transcripts, revealed that transcripts encoding cell wall enzymes were much more abundant in HT than in PAN nodules in the absence of stress (data not shown), perhaps reflecting the inherently greater ability of HT to alter apoplastic parameters. It is possible, therefore, that high Oi values resulting from stresses such as dark chilling readily trigger the formation of occlusions in the HT genotype. The function of the occlusions would be to constrict the intercellular air spaces and thus limit O2 diffusion within the nodule. In PAN, however, where transcripts encoding cell wall enzymes are inherently less abundant (data not shown), this process is not triggered and air space size is unchanged. Hence, it is probable that nitrogenase is less protected against O2-mediated deactivation in stress situations. The accumulation of sugars (Fig. 6, C and G) and depletion of ureides (Fig. 3E) in chilled PAN nodules would accentuate the negative effects of high Oi, as they will tend to favor a decrease in the water content of the intercellular air spaces, thereby increasing permeability to O2. The osmotic hypothesis for the regulation of oxygen permeability proposed by Purcell and Sinclair (1994) suggests that these conditions will favor water loss from the intercellular air spaces of soybean nodules. Hence, a higher sugar content in the symplast and a low ureide content in the apoplast would drive water loss from the apoplast/intercellular air space into the symplast. The sugar and ureide contents of the HT nodules were much less affected by chilling (Figs. 3F and 6, D and H), preventing O2-mediated deactivation of nitrogenase via these osmotic mechanisms.
CONCLUSION
The results presented here reveal several key factors that are important in the soybean stress response that protects nodule SNF and that are central to legume sustainability. First, genotypic variation exists in the chilling-dependent acclimation of respiration. Second, chilling can lead to a strong response in the cell walls/apoplast compartment of the cells, such that electron-dense material occludes the intercellular spaces in chilled HT nodules. Third, there is genotypic variation in the ability of nodules to produce occlusions in response to chilling. These structural changes are known to be associated with changes in the oxygen diffusion barrier that occur in response to varying external O2 concentrations or to exposure to environmental stresses such as nitrate stress or drought. Any inhibition of respiration by chilling temperatures, therefore, would be predicted to cause a transient increase in Oi prior to diffusion barrier closure, to reduce Oi to subinhibitory levels. Together, these findings have implications for agriculture in countries such as South Africa, which are dependent on “green fertilization” of soils. In this system, legume nodules are a major mechanism of soil fertilization; hence, dark chilling is a major limitation on crop productivity and yield. The data presented here provide evidence that mitochondrial respiration and oxygen-sensing mechanisms leading to the regulation of cell wall metabolism to modulate air space volume are potential targets for improving the sustainability of nodules to chilling stress. Therefore, associated mitochondrial markers could be useful in conventional breeding efforts aimed at the development of higher yielding soybean genotypes.
MATERIALS AND METHODS
Plant Growth and Dark Chilling Treatment
Seeds of two South African soybean (Glycine max) genotypes, PAN (chilling sensitive) and HT (chilling tolerant), were inoculated with the same Bradyrhizobium japonicum bacterial strain (WB 74; Soygro Biofertilizers) and grown in a greenhouse at 26°C/19°C day/night temperatures (Rothamsted Research and North-West University) in pots containing vermiculite. Plants received distilled water daily and nitrogen-free Hoagland nutrient solution twice per week. Four weeks after sowing, 12 plants of each genotype were transferred to a refrigerated chamber controlled at 7°C for one entire dark period. The rest of the plants were kept under normal conditions in the greenhouse and represented the control treatment. At the end of the dark period, the chilled plants were returned to the greenhouse containing the control plants for the subsequent light period. This temperature regime was repeated for three consecutive light/dark cycles on the same set of plants. Following the chilling treatment period, plants were allowed to recover for 3 d under normal day/night temperatures. All samples for nitrogenase activity, ureide, carbohydrate, protein, and transcript analysis were collected at midday (noon). In a separate experiment, plants were chilled under exactly the same conditions but for longer periods (up to 12 nights), to determine the effects of chilling on shoot phenotype.
CO2 Assimilation
Five hours after transfer back to the greenhouse (11:00 am) following the first and third nights of chilling treatment as well as during the recovery period, CO2 assimilation was measured with a portable photosynthesis system and leaf chamber with light, humidity, and temperature control (CIRAS-1; PP Systems). Measurements were conducted on the central leaflet of the youngest fully expanded trifoliate leaf of three plants from the control and dark chilling treatments. Measurements were conducted at a leaf temperature of 26°C, an irradiance of 800 μmol photons m−2 s−1, and a CO2 concentration of 360 μmol mol−1.
Diurnal Root and Nodule Respiration Rates
In a separate experiment, diurnal root and nodule respiration rates were measured at regular time intervals during actual nighttime exposure to chilling temperatures and also during the subsequent day periods. Respiration measurements were conducted with the CIRAS-1 IR gas analyzer connected to a modified conifer-type photosynthetic leaf chamber (PP Systems), capable of accommodating whole detached root systems. At each time point during the diurnal cycle, total (root + nodule) respiration rates were first measured in root systems with nodules attached. This was followed by measurements of root respiration rates in the same root systems but after the removal of all nodules. Three people working together undertook these measurements in order to ensure rapid removal of the nodules from the roots. The total time from start of the first measurement to completion of the second measurement was between 7 and 8 min. Root and nodule respiration rates were expressed on a root or nodule fresh weight basis. A high flow rate (600 mL min−1) was maintained in the IR gas analyzer in order to facilitate rapid volume changes in the chamber. All measurements were taken only after steady-state respiration rates were obtained.
Morphometric Analysis
Nodules from control and chilled plants were excised, cut into small pieces, and fixed for 12 h at 4°C in Todd's fixative (Todd, 1986). Material was postfixed for 1 h in 0.5% OsO4 in phosphate buffer, dehydrated in an ethanol series, and embedded in LR White (medium grade) resin. Semithin sections were cut with a Reichert Ultracut R ultramicrotome, and sections were stained in 0.05% aqueous toluidine blue followed by 0.05% aqueous neofuchsin. Sections were examined with a Nikon Eclipse 80e light microscope, and micrographs were captured digitally. The areas of 50 or more intercellular spaces were measured in the infected zone, inner cortex, and middle cortex of each treatment using Nikon NIS-Elements version 2.3 image-analysis software.
Nitrogenase Activity
Plants were removed from the pots, and the root systems were carefully rinsed to remove most of the vermiculite. Whole root systems with attached nodules were incubated for 10 min in 250-mL flasks in the presence of 1% (v/v) acetylene for the measurement of nitrogenase activity with the acetylene reduction assay (Turner and Gibson, 1980). Although nitrogenase may be inhibited to some extent by acetylene (Minchin et al., 1983), comparative measurements over a short assay time are acceptable and provide reliable data. In addition, we established that the presence of 1% acetylene only started to inhibit nitrogenase activity after incubation times exceeding 20 min under the assay conditions employed in these experiments. A 1% acetylene concentration was used because it resulted in much higher rates of nitrogenase activity than the commonly used concentration of 10% (Supplemental Table S1). Supplemental Table S1 shows that incubation with 1% acetylene resulted in higher nitrogenase activities than incubation with 10% acetylene and also, importantly, that the relative effect of dark chilling was the same. The ethylene peak areas increased linearly over time for at least 25 to 28 min after incubation of root systems in flasks containing 1% acetylene. Maximal activities were maintained for at least 20 min in the presence of acetylene in both genotypes. Hence, the use of the 10-fold lower acetylene concentration did not compromise the measurements, because the relative differences in nitrogenase activity between control and chilled plants were still maintained.
Nodule Ureide Content
Immediately after the acetylene reduction assay, the nodules were excised, weighed, and frozen in liquid nitrogen for the measurement of ureide (allantoin and allantoic acid) content. Ureides were extracted from nodules with 1 mL of 0.2 m NaOH followed by boiling for 20 min. After centrifugation (10 min at 10,000g), the ureide content of each supernatant was determined colorimetrically (525 nm) in the presence of HCl/phenylhydrazine and HCl/KFeCn according to the method of Young and Conway (1942). Ureide content was calculated from a standard curve ranging between 0 and 8 μg of allantoin.
Nodule Suc Synthase Activity
Extraction of host plant proteins from nodules for the measurement of Suc synthase activity was conducted according to the method described by Gordon et al. (1997). Suc synthase activity in aliquots (35 μL) of desalted supernatant was determined in the Suc synthesis direction in assay buffer (35 μL) containing 50 mm imidazole (pH 8.5), 5 mm MgCl2, 20 mm Fru, 80 mm Glc-6-P, and 20 mm UDPG. Reactions (10 min at 25°C) were terminated by the addition of 70 μL of 30% (w/v) KOH. Suc formation was determined with the resorcinol method (Huber and Israel, 1982).
Carbohydrates
About 100 mg of nodule material was ground in liquid nitrogen to a fine powder. Soluble carbohydrates were extracted in 1 mL of 80% (v/v) ethanol at 80°C for 30 min. The supernatant was used for enzymatic determination of sugar content (Jones et al., 1977). Glc, Fru, and Suc contents were measured by successive addition of 0.12 units of hexokinase, 0.04 units of phosphoglucoisomerase, and 4 units of invertase to the sample (10 μL of extract in 200 μL of 100 mm imidazole buffer [pH 6.9], 10 mm MgCl2, 1.1 mm ATP, 0.5 mm NADP+, and 0.7 units mL−1 Glc-6-P dehydrogenase). Proteins were extracted from the ethanol-insoluble fraction by the addition of 0.5 mL of 50 mm Tris-HCl buffer (pH 7.6). The soluble protein content was determined according to the method of Bradford (1976). The remaining pellet was washed twice with distilled water and used for the estimation of starch content. Full conversion of starch to Glc was achieved by incubation in 0.5 mL of reaction buffer (50 mm sodium acetate, pH 4.8, containing 2 units of α-amylase and 0.05 units of amyloglycosidase) at 37°C for 36 h. Glc content of the supernatant was measured as described above. Five independent samples were prepared for each treatment and measured in triplicate on microtiter plates.
Western-Blot Analysis
Soybean nodules were ground in liquid nitrogen, and proteins were extracted in 50 mm MOPS (pH 7.0) containing 4 mm MgCl2, 20 mm KCl, and 0.1 mm phenylmethylsulfonyl fluoride. Extracts were frozen and thawed three times and sonicated for 60 s to achieve disruption of bacterial membranes. After centrifugation at 20,000g for 10 min at 4°C, the supernatant was used for the determination of soluble protein content according to Bradford (1976). Equal amounts of protein were mixed with 2× sample buffer (125 mm Tris-HCl, pH 6.8, 10% [v/v] glycerol, 2% [w/v] SDS, 0.05% [w/v] bromphenol blue, and 10% [v/v] β-mercaptoethanol), boiled for 10 min, and loaded onto SDS-polyacrylamide gels (12% standard resolving gel and 7% stacking gel) for the detection of nitrogenase and Suc synthase. Tricine-SDS-PAGE was performed for the leghemoglobin samples according to the method of Schägger and von Jagow (1987). After electrophoresis at 150 V for 4 h, proteins were transferred to a nitrocellulose membrane (Hybond C-extra; Amersham Pharmacia Biotech). Protein detection was conducted using specific antibodies against nitrogenase, leghemoglobin, and Suc synthase. The nitrogenase antibody was a universal mixture against dinitrogenase reductase from Azotobacter vinelandii and Rhodospirillum rubrum (kindly provided by Dr. Luis M. Rubio, University of California-Berkeley). The leghemoglobin (Gordon and Kessler, 1990) and Suc synthase (Gordon et al., 1992) antibodies were kindly provided by Dr. Anthony J. Gordon (Institute of Grassland and Environmental Research, Plas Gogerddan, UK). Specific signals were detected using an anti-rabbit secondary antibody conjugated to horseradish peroxidase with hydrogen peroxide and 4-chloronaphthol as a substrate.
Gene Expression
Total RNA was extracted by grinding 100 mg of the harvested nodule material in liquid nitrogen and adding 2 mL of TRIzol reagent (Invitrogen) to the thawing paste. After 5 min at room temperature, the samples were centrifuged at 12,000g for 15 min. The samples were washed twice with equal amounts of chloroform, and RNA was precipitated by the addition of 0.5 volumes of isopropanol. After washing with 75% ethanol, the RNA was dried in a desiccator and resuspended in diethyl pyrocarbonate-treated water. Genomic DNA was removed from the samples by incubation of 2 μg of RNA with 2 μL of DNase I Amp grade (Invitrogen) for 15 min at room temperature. First-strand cDNA synthesis was performed via the SuperScript II system (Invitrogen) and used as template for PCR. The following primer pairs were used for PCR: for actin, 5′-GAGCTTCCTGATGGGCAAGTT-3′ and 5′-GCAACGGAATCTCTCAGCTCC-3′; for leghemoglobin, 5′-ATCCTAAGCTCACGGGCCAT-3′ and 5′-AGTCACGCACCAATGCAAAA-3′; for NifK, 5′-TCTGGCACATGCGTTCACTC-3′ and 5′-CAATCAGAAAATCGACCGGC-3′; and for NifH, 5′-ATGGCAATGTATGCCGCAA-3′ and 5′-CGCGTATTTCAGGATCCCCT-3′. PCR conditions were optimized for every primer pair. Expression levels in the two genotypes at the various time points were expressed relative to those obtained in HT control samples at the start of the experiment. Actin transcript was used for verification of equal template loading (data not shown).
Statistical Analysis
Significant differences between treatment means were determined using Student's t test.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. A comparison of soybean nodule nitrogenase activities measured in the presence of either 1% or 10% acetylene.
Supplementary Material
Acknowledgments
We thank Dr. Luis M. Rubio and Dr. Anthony J. Gordon for providing the antibodies directed against dinitrogenase reductase, leghemoglobin, and Suc synthase. We are grateful to Dr. Stephen Hunt for critical reading of the manuscript.
This work was supported by a joint grant (grant no. 2068793) from the Royal Society (United Kingdom) and the National Research Foundation (South Africa) as well as by funding from the Oil and Protein Seeds Development Trust (Rivonia, South Africa). Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christine H. Foyer (christine.foyer@ncl.ac.uk).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Anthon GE, Emerich DW (1990) Developmental regulation of enzymes of sucrose and hexose metabolism in effective and ineffective soybean nodules. Plant Physiol 92 346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A, Logan DC, Tobin AK, O'Toole P, Atkin OA (2006) Heterogeneity of plant mitochondrial responses underpinning respiratory acclimation to the cold in Arabidopsis thaliana leaves. Plant Cell Environ 29 940–949 [DOI] [PubMed] [Google Scholar]
- Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8 343–351 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–253 [DOI] [PubMed] [Google Scholar]
- Castle ML, Crush JR, Rowath JS (2006) The effect of root and shoot temperature of 8°C or 24°C on the uptake and distribution of nitrogen in white clover (Trifolium repens L.). Aust J Agric Res 57 577–581 [Google Scholar]
- Duke SH, Schrader LE, Henson CA, Servaites JC, Vogelzang RD, Pendleton JW (1979) Low root temperature effects on soybean nitrogen metabolism and photosynthesis. Plant Physiol 63 956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129 111–122 [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Kessler W (1990) Defoliation-induced stress in nodules of white clover. 2. Immunological and enzymatic measurements of key proteins. J Exp Bot 41 1255–1262 [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Minchin FR, Sköt L, James CL (1997) Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol 114 937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Thomas BJ, Reynolds PHS (1992) Localization of sucrose synthase in soybean root nodules. New Phytol 122 35–44 [DOI] [PubMed] [Google Scholar]
- Huber SC, Israel DW (1982) Biochemical basis for partitioning of photosynthetically fixed carbon between starch and sucrose in soybean (Glycine max Merr.) leaves. Plant Physiol 69 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DJ, Jackson AKH (1981) Frost tolerance in soybeans. Crop Sci 21 689–692 [Google Scholar]
- Hunt S, King BJ, Layzell DB (1989) Effects of gradual increases in O2 concentration on nodule activity in soybean. Plant Physiol 91 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James EK, Sprent JI, Minchin FR, Brewin NJ (1991) Intercellular location of glycoprotein in soybean nodules: effect of altered rhizosphere oxygen concentration. Plant Cell Environ 14 467–476 [Google Scholar]
- Jones M, Outlaw W Jr, Lowry O (1977) Enzymatic assay of 10−7 to 10−14 moles of sucrose in plant tissue. Plant Physiol 60 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser HH, Li F (1992) Potential for increasing biological nitrogen fixation in soybean. Plant Soil 141 119–135 [Google Scholar]
- King CA, Purcell LC (2005) Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol 137 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto K, Millar AH, Lambers H, Day DA, Noguchi K (2004) Maintenance of growth rate at low temperature in rice and wheat cultivars with a high degree of respiratory homeostasis is associated with a high efficiency of respiratory ATP production. Plant Cell Physiol 45 1015–1022 [DOI] [PubMed] [Google Scholar]
- Kuzma MM, Topunov AF, Layzell DB (1995) Effects of temperature on infected cell O2 concentration and adenylate levels in attached soybean nodules. Plant Physiol 107 1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn RJ, Hume DJ (1985) Response of tropical and temperate soybean genotypes to temperature during early reproductive growth. Crop Sci 25 137–142 [Google Scholar]
- Layzell DB, Rochman P, Canvin DT (1984) Low root temperatures and nitrogenase activity in soybean. Can J Bot 62 965–971 [Google Scholar]
- Lazár D (2006) The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biol 33 9–30 [DOI] [PubMed] [Google Scholar]
- Lazár D, Ilík P, Nauš J (1997) An appearance of K-peak in fluorescence induction depends on the acclimation of barley leaves to higher temperatures. J Lumin 72–74: 595–596 [Google Scholar]
- Legros T, Smith DL (1994) Root zone temperature sensitivity of nitrogen fixing and nitrate-supplied soybean [Glycine max (L.) Merr. cv Maple Arrow] and lupin (Lupinus albus L. cv Ultra) plants. Environ Exp Bot 34 117–127 [Google Scholar]
- Lynch DH, Smith DL (1994) The effects of low root-zone temperature stress on two soybean (Glycine max) genotypes when combined with Bradyrhizobium strains of varying geographic origin. Physiol Plant 90 105–113 [Google Scholar]
- Marino D, Frendo P, Ladrera R, Zabalza A, Puppo A, Arrese-Igor C, Gonzalez EM (2007) Nitrogen fixation control under drought stress: localized or systemic? Plant Physiol 143 1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Baird LM, Escuredo PR, Dalton DA, Minchin FR, Iturbe-Oramaetxe I, Rubio MC, Moran JF, Gordon AJ, Becana M (1999) Stress-induced legume root nodule senescence: physiological, biochemical and structural alterations. Plant Physiol 121 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin FR (1997) Regulation of oxygen diffusion in legume nodules. Soil Biol Biochem 29 881–888 [Google Scholar]
- Minchin FR, Sheehy JE, Witty JF (1986) Further errors in the acetylene reduction assay: effects of plant disturbance. J Exp Bot 37 1581–1591 [Google Scholar]
- Minchin FR, Witty JF, Sheehy JE, Muller M (1983) A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J Exp Bot 34 641–649 [Google Scholar]
- Musser RL, Thomas SA, Kramer PJ (1983) Short and long term effects of root and shoot chilling of Ransom soybean. Plant Physiol 73 778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RL, Thomas SA, Wise RR, Peeler TC (1984) Chloroplast ultrastructure, chlorophyll fluorescence, and pigment composition in chilling-stressed soybeans. Plant Physiol 74 749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R, Day DA (1990) Mechanism of soybean nodule adaptation to different oxygen pressures. Plant Cell Environ 13 501–512 [Google Scholar]
- Parsons R, Stanforth A, Raven JA, Sprent JI (1993) Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant Cell Environ 16 125–136 [Google Scholar]
- Pellny T, Van Aken O, Dutilleul C, Wolff T, Groten K, Bor M, De Paepe R, Reyss A, van Breusegem F, Noctor G, et al (2008) Mitochondrial respiratory pathways modulate nitrate sensing and nitrogen-dependent regulation of plant architecture in Nicotiana sylvestris. Plant J 54 976–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, de Felipe MR, Harrison J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165 683–701 [DOI] [PubMed] [Google Scholar]
- Purcell LC, Sinclair TR (1994) An osmotic hypothesis for the regulation of oxygen permeability in soybean nodules. Plant Cell Environ 17 837–843 [Google Scholar]
- Schägger H, von Jagow G (1987) Tricine-sodium dodecylsulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166 368–379 [DOI] [PubMed] [Google Scholar]
- Seddigh M, Jolliff GD, Orf JH (1989) Night temperature effects on soybean phenology. Crop Sci 29 400–406 [Google Scholar]
- Serraj R, Fleurat-Lessard P, Jaillard B, Drevon JJ (1995) Structural changes in the inner-cortex cells of soybean root nodules are induced by short-term exposure to high salt or oxygen concentrations. Plant Cell Environ 18 455–462 [Google Scholar]
- Serraj R, Sinclair TR (1996) Inhibition of nitrogenase activity and nodule oxygen permeability by water deficit. J Exp Bot 47 1067–1073 [Google Scholar]
- Serraj R, Sinclair TR, Purcell LC (1999) Symbiotic N2 fixation response to drought. J Exp Bot 50 143–155 [Google Scholar]
- Smith JMB (1994) Crop, Pasture and Timber Yield Index. Cedara Report, N/A/94/4. Natal Agricultural Research Institute, Cedara, South Africa
- Smith PMC, Atkins CA (2002) Purine biosynthesis: big in cell division—even bigger in nitrogen assimilation. Plant Physiol 128 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Foussard M, Boistard P, Truchet G, Batut J (1995) Oxygen as a key developmental regulator of Rhizobium meliloti N2-fixation gene expression within the alfalfa root nodule. Proc Natl Acad Sci USA 92 3759–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RJ, Srivastava A, Tsimilli-Michael M (2004) Analysis of the chlorophyll a fluorescence transient. In G Papageorgiou, Govindjee, eds, Advances in Photosynthesis and Respiration, Vol 19. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 321–362
- Strauss AJ, Krüger GHJ, Strasser RJ, Van Heerden PDR (2006) Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P. Environ Exp Bot 56 147–157 [Google Scholar]
- Strauss AJ, Krüger GHJ, Strasser RJ, Van Heerden PDR (2007) The role of low soil temperatures in the inhibition of growth and PSII function during dark chilling in soybean genotypes of contrasting tolerance. Physiol Plant 131 89–105 [DOI] [PubMed] [Google Scholar]
- Thumfort PP, Layzell DB, Atkins CA (1999) Diffusion and reaction of oxygen in the central tissue of ureide-producing legume nodules. Plant Cell Environ 22 1351–1363 [Google Scholar]
- Todd WJ (1986) Effects of specimen preparation on the apparent ultrastructure of micro-organisms. In HC Aldrich, WJ Todd, eds, Ultrastructure Techniques for Micro-Organisms. Plenum, New York, p 87
- Turner GL, Gibson AH (1980) Measurement of nitrogen fixation by indirect means. In FJ Bergersen, ed, Methods for Evaluating Biological Nitrogen Fixation. John Wiley & Sons, Chichester, UK, pp 315–335
- Van Heerden PDR, Krüger GHJ, Loveland JE, Parry MAJ, Foyer CH (2003) Dark chilling imposes metabolic restrictions on photosynthesis in soybean. Plant Cell Environ 26 323–337 [Google Scholar]
- Van Heerden PDR, Strasser RJ, Krüger GHJ (2004) Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol Plant 121 239–249 [DOI] [PubMed] [Google Scholar]
- Vessey KJ, Walsh KB, Layzell DB (1988) Oxygen limitation of N2 fixation in stem-girdled and nitrate-treated soybean. Physiol Plant 73 113–121 [Google Scholar]
- Walsh KB, Layzell DB (1986) Carbon and nitrogen assimilation and partitioning in soybeans exposed to low root temperatures. Plant Physiol 80 249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EG, Conway CF (1942) On the estimation of allantoin by the Rimini-Schryver reaction. J Biol Chem 142 839–853 [Google Scholar]
- Zhang H, Prithiviraj B, Charles TC, Driscoll BT, Smith DL (2003) Low temperature tolerant Bradyrhizobium japonicum strains allowing improved nodulation and nitrogen fixation of soybean in a short season (cool spring) area. Eur J Agron 19 205–213 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.