Abstract
The bioenergy feedstock grass Miscanthus × giganteus is exceptional among C4 species for its high productivity in cold climates. It can maintain photosynthetically active leaves at temperatures 6°C below the minimum for maize (Zea mays), which allows it a longer growing season in cool climates. Understanding the basis for this difference between these two closely related plants may be critical in adapting maize to colder weather. When M. × giganteus and maize grown at 25°C were transferred to 14°C, light-saturated CO2 assimilation and quantum yield of photosystem II declined by 30% and 40%, respectively, in the first 48 h in these two species. The decline continued in maize but arrested and then recovered partially in M. × giganteus. Within 24 h of the temperature transition, the pyruvate phosphate dikinase (PPDK) protein content per leaf area transiently declined in M. × giganteus but then steadily increased, such that after 7 d the enzyme content was significantly higher than in leaves growing in 25°C. By contrast it declined throughout the chilling period in maize leaves. Rubisco levels remained constant in M. × giganteus but declined in maize. Consistent with increased PPDK protein content, the extractable PPDK activity per unit leaf area (Vmax,ppdk) in cold-grown M. × giganteus leaves was higher than in warm-grown leaves, while Vmax,ppdk was lower in cold-grown than in warm-grown maize. The rate of light activation of PPDK was also slower in cold-grown maize than M. × giganteus. The energy of activation (Ea) of extracted PPDK was lower in cold-grown than warm-grown M. × giganteus but not in maize. The specific activities and Ea of purified recombinant PPDK from M. × giganteus and maize cloned into Escherichia coli were similar. The increase in PPDK protein in the M. × giganteus leaves corresponded to an increase in PPDK mRNA level. These results indicate that of the two enzymes known to limit C4 photosynthesis, increase of PPDK, not Rubisco content, corresponds to the recovery and maintenance of photosynthetic capacity. Functionally, increased enzyme concentration is shown to increase stability of M. × giganteus PPDK at low temperature. The results suggest that increases in either PPDK RNA transcription and/or the stability of this RNA are important for the increase in PPDK protein content and activity in M. × giganteus under chilling conditions relative to maize.
In C4 grasses, the mesophyll cells fix atmospheric CO2 via cytosolic phosphoenolpyruvate carboxylase (PEPc; E.C.4.1.1.31) using PEP regenerated via chloroplastic pyruvate phosphate dikinase (PPDK; E.C.2.7.9.1). The C4 product is transported to bundle sheath cells, where CO2 is released by decarboxylation of this product and refixed via Rubisco (E.C.4.1.1.39). The physiological significance of this process is to increase CO2 concentration relative to O2 around Rubisco such that the oxygenase activity of Rubisco and in turn photorespiration are in practice eliminated. As a result, C4 photosynthesis has a higher potential efficiency of light, water, and nitrogen use than C3 photosynthesis (Long, 1983, 1999; Sage, 1999). Although the process has a theoretical advantage, even in cool climates (Long, 1983, 1999), this is rarely realized. This is because few C4 plants can maintain photosynthetically competent leaves at chilling temperatures (<15°C). Although many C4 species can survive in cold conditions, Miscanthus × giganteus appears exceptional in being able to maintain high photosynthetic rates when transferred to chilling conditions (Beale et al., 1996). Even the productive cool-temperate grass Spartina anglica shows a loss of assimilatory capacity when transferred to cool conditions (Dunn et al., 1987), a loss that is not observed for M. × giganteus (Beale et al., 1996; Farage et al., 2006). Loss of photosynthetic capacity in chilling conditions, rather than chilling effects on the multitude of other physiological processes that could limit growth, has been shown to be the key limitation to extending the range of maize (Zea mays), the most cold-tolerant of the major C4 crops, into colder climates and to extending its growing season in the temperate zone (Miedema, 1982; Long, 1983; Long et al., 1994).
An exception among C4 grasses is the bioenergy crop Miscanthus × giganteus, a rhizomatous perennial of the Andropogoneae, which is highly productive in cool climates (Beale and Long, 1995; Bullard et al., 1995; Beale et al., 1996; Heaton et al., 2004). M. × giganteus is closely related to sugar cane (Saccharum officinarum), sorghum (Sorghum bicolor), and maize. Like these crop species, M. × giganteus uses the NADP-malic enzyme pathway of C4 photosynthesis, while sequences of its photosynthetic genes share >99% sequence identity with sugar cane (Naidu et al., 2003; Wang et al., 2008). When grown in the cool temperate climate of southern England (52°N), M. × giganteus produced 30 tons of dry matter per ha per year, one of the highest dry matter yields recorded for the United Kingdom. In marked contrast to maize grown at the same location, it showed no loss of maximum photosynthetic efficiency during cold spring weather (Baker et al., 1989; Beale and Long, 1995; Beale et al., 1996). Growing maize at 14°C results in a more than 90% reduction of maximum photosynthetic rate relative to leaves grown at 25°C, whereas M. × giganteus grown at 14°C or 10°C shows little loss of photosynthetic capacity (Nie et al., 1992; Naidu and Long, 2004; Farage et al., 2006). Understanding how this is achieved may be critical to adapting maize and other C4 crops to colder climates or in extending the growing season into cooler periods of the year so allowing greater use of the available solar radiation.
The molecular mechanism by which M. × giganteus is able to maintain high photosynthetic rates at low temperatures remains unclear. In saturating light and chilling temperatures (<15°C), CO2 assimilation for chilling-intolerant C4 species is severely reduced, and in turn utilization of observed excitation energy leads to photoinhibition and photooxidation (Long, 1983; Baker et al., 1989; Long et al., 1994). M. × giganteus avoids this damage both by maintaining a high rate of CO2 uptake, to increase utilization of absorbed light (Beale et al., 1996), and by increased nonphotochemical quenching, which correlates with a large increase in xanthophyll content and its de-epoxidation (Farage et al., 2006). Chilling-induced decreases in the leaf photosynthetic rate of CO2 uptake of C4 species have been correlated with decreases in: carboxylation efficiency via PEPc (Kingston-Smith et al., 1997; Chinthapalli et al., 2003), capacity for PEP regeneration via PPDK (Du et al., 1999a), Rubisco activity (Kingston-Smith et al., 1997; Du et al., 1999a; Pittermann and Sage, 2000; Pittermann and Sage, 2001; Chinthapalli et al., 2003), or by a combination of these. Analyses of flux control coefficients in transgenic plants of the C4 dicot Flaveria bidentis suggest that Rubisco and PPDK share control and limit light-saturated C4 photosynthesis (Furbank et al., 1997). This applies at saturating intercellular mesophyll CO2 concentration, a condition that is met in the current atmosphere in both cold- and warm-grown M. × giganteus (Naidu and Long, 2004).
In warm-grown F. bidentis, transformed with antisense Rubisco small subunit gene constructs, in vitro activities of Rubisco matched the in vivo photosynthetic rates at low temperature, which suggested that Rubisco content controlled the C4 photosynthetic rate at low temperature (Kubien et al., 2003). In addition, for each of the chilling-tolerant C4 species Bouteloua gracilis, Muhlenbergia glomerata, and Amaranthus retroflexus, in vivo photosynthetic rates below 20°C showed the same pattern of decline as maximum activities of Rubisco in crude extracts (Pittermann and Sage, 2000; Sage, 2002; Kubien and Sage, 2004). These findings led Sage and McKown (2006) to propose that because Kranz anatomy limits the enzyme to the bundle sheath, C4 plants are inherently more prone to Rubisco limitation as the activity of the enzyme declines with temperature. When grown at 14°C and measured at 10°C, M. × giganteus achieves about twice the photosynthetic rate of the cold-tolerant C4 species M. glomerata (Kubien and Sage, 2004; Naidu and Long, 2004). Yet in cold-grown M. × giganteus, Rubisco content remained unchanged relative to warm-grown plants, while it decreased in cold-grown maize (Naidu et al., 2003). Furthermore, the cold tolerance of C4 photosynthesis in M. × giganteus could not be attributed to the catalytic properties of Rubisco, which showed no differences between cold- and warm-grown M. × giganteus nor with warm-grown maize (Wang et al., 2008).
Other studies have inferred that PPDK is the key limiting factor for C4 photosynthesis at low temperature. Low extractable activities, often only just sufficient to support in vivo rates of photosynthesis, have been reported frequently (Long, 1983; Edwards et al., 1985). PPDK catalyzes the regeneration of PEP as the primary acceptor of atmospheric CO2 from pyruvate, ATP, and phosphate in C4 photosynthesis. PPDK undergoes diurnal dark/light regulation of activity, which is controlled by a bifunctional regulatory protein (Burnell and Hatch, 1984; Ashton et al., 1990; Chastain et al., 2008). The fully functional C4 isoform of PPDK is a tetramer. The subunits dissociate between 10°C and 15°C, causing an increase in the energy of activation (Ea) and a loss of catalytic rate with further decline in temperature (Shirahashi et al., 1978; Ohta et al., 1996; Du et al., 1999b). In addition, the maximum extractable PPDK activity correlated well with cold tolerance in three Saccharum species (Du et al., 1999a) and across maize cultivars (Sugiyama and Boku, 1976). In contrast to the effects of temperature on the PPDK enzyme, there appears to be no correlation between thermal properties of other C4 photosynthetic enzymes and differences in cold sensitivity of plant ecotypes (Du et al., 1999b; Hamel and Simon, 2000). Furthermore, an analysis of transgenic F. bidentis with antisense PPDK gene constructs showed that PPDK shares control of C4 photosynthetic rate with Rubisco under ambient CO2 and high light (Furbank et al., 1997). A key role for PPDK in low temperature tolerance is also inferred in two chilling-tolerant PEP carboxykinase type C4 species, which apparently maintain photosynthetic rates at low temperature by bypassing PPDK in PEP synthesis, via PEP carboxykinase (Smith and Woolhouse, 1983; Matsuba et al., 1997). Although analysis of the cDNA sequences for PPDK in M. × giganteus and maize have failed to reveal any differences in putative amino acids sequences that could confer cold tolerance, the amount of PPDK was reported to increase significantly in M. × giganteus when grown at low temperature. This was in sharp contrast to the PPDK decreases observed in maize (Naidu et al., 2003). If PPDK is a limitation at low temperature, then low temperature-adapted species might be expected either to up-regulate expression of the enzyme in response to chilling or possess an isoform with a higher specific activity at low temperatures.
Here, the hypothesis that an increase in PPDK content, but not kinetics properties of PPDK, corresponds to the ability of M. × giganteus to maintain photosynthetic capacity when transferred to low temperature is examined. CO2 assimilation rate and quantum yield of PSII (ΦPSII) were measured in parallel with protein and transcript levels of PPDK in the leaves of M. × giganteus and maize during a transition of growth conditions from warm to cold temperature. The catalytic rates, Ea, and light activation kinetics of PPDK in both crude leaf extracts and in purified recombinant PPDK were compared between M. × giganteus and maize, as was the effect of enzyme concentration on the cold stability of PPDK.
RESULTS
Influence of Chilling Temperature on Chlorophyll Content, CO2 Assimilation Rate, and ΦPSII
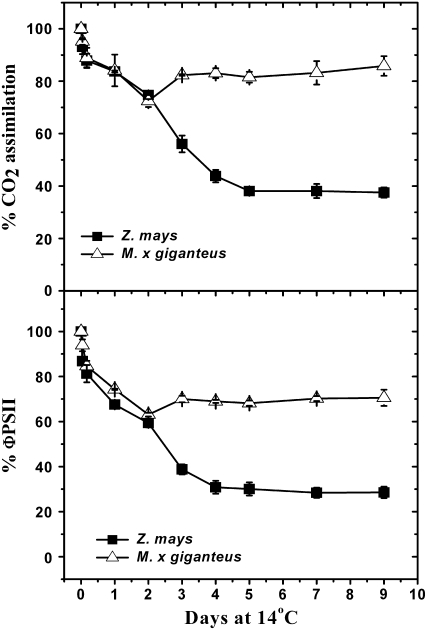
Following transition from a 25°C (warm) to a 14°C (chilling) growth temperature, CO2 assimilation rate (A) and ΦPSII measured on existing fully expanded maize leaves declined progressively over 9 d to about 38% and 30%, respectively, of prechilling levels (Fig. 1). A similar decline occurred in fully expanded M. × giganteus leaves for the first 2 d, but was followed by a recovery stabilizing at about 88% of the preexposure level for A and 73% for ΦPSII by day 9 (Fig. 1).
Figure 1.
Change in leaf CO2 uptake rate and ΦPSII following transfer of plants from 25°C to 14°C. Means (±1 se) for five plants are expressed as a percentage of rate observed immediately on transfer to 14°C, which were 14.8 ± 0.6 μmol m−2 s−1 and 0.12 ± 0.02 for maize and 16.2 ± 0.9 μmol m−2 s−1 and 0.14 ± 0.03 for M. × giganteus.
Chlorophyll contents of the existing leaves of both species were lower after being transferred to 14°C growth condition. Before transition to 14°C, the chlorophyll contents for fully expanded M. × giganteus and maize leaves were 562 ± 22 and 585 ± 26 μmol/m−2, respectively, while the chlorophyll contents declined to 69% in M. × giganteus and to 35% in maize after 14 d at 14°C (not shown).
Changes in Protein and Transcript Contents of PPDK in M. × giganteus and Maize
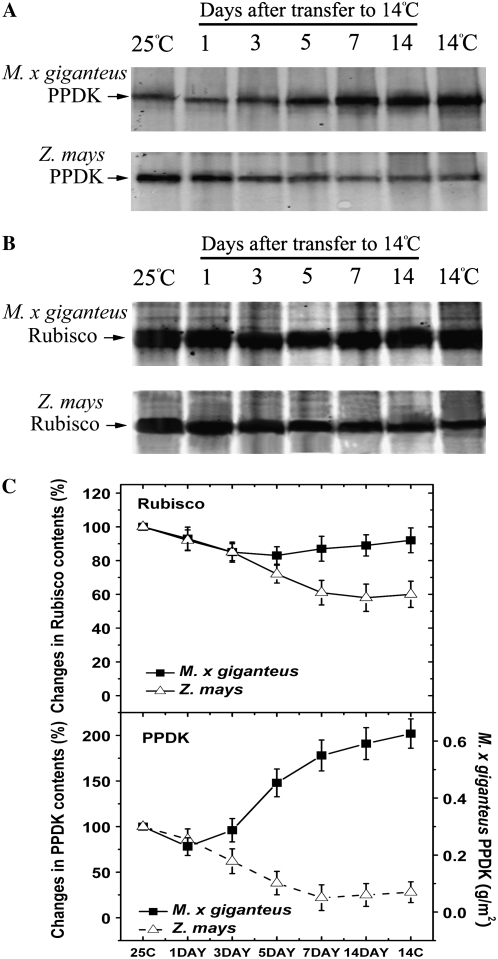
M. × giganteus leaves developed at 25°C and then transferred to 14°C after completion of expansion accumulated PPDK protein, while maize mature leaves given the same treatment lost PPDK (Fig. 2A). In parallel with the kinetics of the response of A to transfer to 14°C, PPDK protein declined slightly in M. × giganteus on the first day at low temperature, but then accumulated above the original level by day 3 and the amount was nearly doubled (calculated from band density) by day 7 (Fig. 2A). When quantified in absolute terms, PPDK content in 25°C grown M. × giganteus leaves was 0.30 g/m2 and increased to 0.56 g/m2 after 14 d at 14°C (Supplemental Fig. S1). However, for Rubisco, M. × giganteus mature leaves developed at 25°C and then transferred to 14°C showed no significant loss of Rubisco, in contrast to a marked decline in maize leaves (Fig. 2B).
Figure 2.
PPDK (A) and Rubisco (B) protein contents in M. × giganteus and maize for the leaves of Figure 1 following transfer from 25°C/20°C to 14°C/12°C (day/night). Prior to transfer, these plants had been grown continuously at 25°C/20°C. In addition, the lane marked “25C” shows protein contents immediately prior to the transfer. The lane marked “14C” is for leaves of the same developmental stage of plants that were grown and developed entirely at 14°C/12°C (day/night), this is included for comparison. For each lane, protein was extracted from 1.9 cm2 of leaf area into 500 μL of extraction buffer, of which 20 μL was loaded and separated by 10% SDS-PAGE. Western blots used polyclonal primary antibodies against maize PPDK and spinach Rubisco, respectively. The bands on Figure 2, A and B, were quantified in relative terms by densitometry (Odyssey V1.2 application software; C). Means (±1 se) for three plants' Rubisco and PPDK protein contents, expressed as a percentage of the amount present immediately prior to the transfer to 14°C/12°C; i.e. the first lane of A and B. The amounts of M. × giganteus PPDK were also quantified from a standard curve, generated from known quantities of recombinant PPDK (Supplemental Fig. S1) and shown via the right-hand axis of the lower segment.
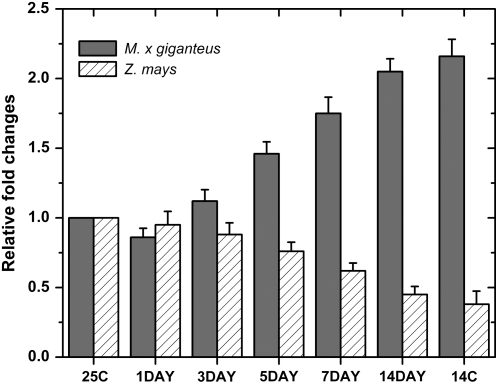
Quantitative real-time reverse transcription (RT)-PCR showed that the changes in PPDK protein upon transfer of plants to 14°C were paralleled by changes in the amounts of the mRNA coding for PPDK (Fig. 3). An obvious increase in M. × giganteus PPDK mRNA was observed after day 3 following the exposure to 14°C (Fig. 3). In contrast, maize PPDK mRNA declined continuously following transfer to 14°C (Fig. 3). By the end of 14 d of exposure to 14°C, the M. × giganteus PPDK mRNA was 2.1-fold higher than that grown continuously at 25°C, while for maize PPDK mRNA was 45% lower. For both species, the PPDK mRNA levels after 14-d chilling exposure were similar to those in leaves grown continuously at 14°C (Fig. 3).
Figure 3.
Quantitative real-time RT-PCR analysis of PPDK expression in leaves of M. × giganteus and maize. The leaf samples were collected 1, 3, 5, 7, and 14 d after transfer from 25°C to 14°C or collected from plants grown continuously at 25°C (25C) and 14°C (14C), respectively. The total RNAs were isolated and quantitative real-time RT-PCR in triplicate was conducted using a pair of primers specific for PPDK genes. The expression of PPDK from plants continuously grown at 25°C (25C) was used as control and set as 1.0. The changes in expression of PPDK were determined relative to the control. Bars are the means (+1 se) of three replicate plants, relative to the level immediately prior to transfer to 14°C.
Temperature Dependency and Ea of PPDK Activity in Crude Leaf Extracts
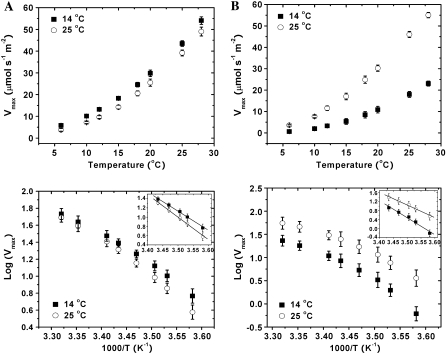
The extractable activity of PPDK 14 d after transfer to 14°C corresponded closely to A in these leaves, 5.3 versus 5.6 μmol m−2 s−1, respectively, for maize, and 18.3 versus 14.1 μmol m−2 s−1 for M. × giganteus (Table I; Fig. 1). The apparent Ea was calculated separately for a low (6°C −18°C; Ea,6–18) and a high (18°C–28°C; Ea,18–28) temperature range (Fig. 4, A and B). For the control plants grown at 25°C, the Ea,6–18 was about 1.5-fold of Ea,18–28 for PPDK from M. × giganteus, and was slightly and significantly greater at 1.8-fold for PPDK from maize (Table I). For plants exposed to 14°C for 14 d, the Ea,6–18 was about 1.4-fold of Ea,18–28 for PPDK from M. × giganteus and again higher at 2.0-fold for PPDK from maize (Table I). For M. × giganteus, the Ea,6–18 of PPDK extracted from leaves exposed to 14°C was 15% lower than that of the control plant, indicating some acclamatory effect, while no significant difference was observed for Ea,18–28 (Table I). In sharp contrast to M. × giganteus, the Ea,6–18 and Ea,18–28 of PPDK activity from maize leaves exposed to 14°C was 37% and 24% higher than that of the control plant, respectively (Table I).
Table I.
Vmax and Ea of PPDK M. × giganteus and maize from crude leaf extracts (n = 5 replicate plants) and from E. coli (n = 3 independent transformations) into which the genes from each species had been cloned
Values given are means (and ses) and their units are given at the end of the table. Leaf and recombinant protein extracts were made as described in “Materials and Methods.”
| Species | Growth Temperature |
Vmax,ppdka
|
Ea (kJ mol−1)b
|
||
|---|---|---|---|---|---|
| 25°C | 15°C | 6°C–18°C (6°C–15°C) | 18°C–28°C (18°C–28°C) | ||
| M. × giganteus crude leaf extract | 25°C | 39.2 (1.5) | 14.2 (0.9) | 93.5 (6.4) | 63.2 (3.8) |
| 14°C | 43.5 (1.3) | 18.3 (0.7) | 79.1 (5.2) | 57.3 (3.2) | |
| Maize crude leaf extract | 25°C | 46.1 (0.9) | 17.1 (1.6) | 106.2 (8.9) | 58.1 (2.9) |
| 14°C | 18.0 (1.2) | 5.3 (0.9) | 145.8 (9.5) | 71.9 (4.2) | |
| M. × giganteus recombinant PPDK | – | 7.3 (0.5) | 3.3 (0.4) | 115.7 (6.2) | 51.6 (2.9) |
| Maize recombinant PPDK | – | 7.6 (0.4) | 3.1 (0.3) | 114.3 (5.8) | 52.1 (3.6) |
The units for Vmax,ppdk of PPDK in crude leaf extract are μmol s−1 m−2 and for recombinant PPDK μmol min−1 mg−1.
Ea for PPDK in crude leaf extract was calculated from slopes of Arrhenius plots at low (6°C–18°C) and high (18°C–28°C) temperature ranges; for recombinant PPDK at low (6°C–15°C) and high (18°C–28°C) temperature ranges.
Figure 4.
Maximum rates of PPDK-catalyzed PEP synthesis expressed on a leaf area basis versus assay temperature, transformed to provide Arrhenius plots for M. × giganteus (A) and maize (B) grown at 25°C (○) and transferred to 14°C (▪) for 14 d. PPDK was extracted from leaves of M. × giganteus and maize. Values are the means (±1 se) of five plants. The inset shows the linear regression fitted to the Arrhenius plots for low (6°C–18°C) measuring temperatures.
The Maximum Enzyme Rates and Ea of Purified Recombinant PPDK
To test if the lower Ea of PPDK in crude extracts from M. × giganteus at low temperature is due to any inherent difference in the protein and related gene sequence differences, C4 PPDK genes from both species were cloned and expressed in Escherichia coli so that the two enzymes could be synthesized and extracted from a common background. The specific activities of purified His-tagged recombinant PPDK of M. × giganteus and maize increased with temperature from 6°C to 28°C but showed no significant difference across this range (Fig. 5A). For example, the specific activities of PPDK of M. × giganteus and maize at 25°C were 7.3 ± 0.5 and 7.6 ± 0.4 μmol min−1 mg−1, respectively, and 3.3 ± 0.4 and 3.1 ± 0.3 μmol min−1 mg−1, respectively, at 15°C. In contrast to crude extracts, the Arrhenius plots of Vmax,ppdk showed no significant difference between M. × giganteus and maize across all measuring temperatures (Fig. 5B; Table I). An apparent break-point in the Arrhenius plot was observed at 15°C for both M. × giganteus and maize PPDK (Fig. 5B). A shift in the slopes of the fitted lines at high (18°C–28°C) and low (6°C–15°C) temperature was observed (Fig. 5B). Therefore, the apparent Ea of the PPDK was calculated separately for these two regions of the plot. The Ea at low measuring temperatures was 2.2-fold of that above 15°C for PPDK activity from both M. × giganteus and maize (Table I). No significant difference in the Ea in either temperature range was observed between recombinant PPDK from two species. The lack of difference between the two recombinant PPDKs suggests that differences observed in leaf extracts are a result of posttranslational differences or differences within the stromal environments and not differences between the gene sequences coding for the two polypeptides.
Figure 5.
Maximum rates of PPDK-catalyzed PEP synthesis per unit protein versus assay temperatures (A) and transformed to provide Arrhenius plots (B) for purified recombinant His-tagged PPDK of M. × giganteus (▪) and maize (○) cloned in E. coli. The linear regressions in the Arrhenius plot are for low (6°C–15°C) and high (18°C–28°C) measuring temperatures. Values are the means (±1 se) of three different transformation events. The arrow indicates the apparent break point in Arrhenius plot for both M × giganteus and maize PPDKs at 15°C.
Cold Inactivation of PPDK at Varying Protein Concentrations
To examine the relationship between cold inactivation of PPDK activity and enzyme concentration, the specific PPDK activity at varying protein concentrations (0.1–2 mg/mL) was measured following 10, 20, 40, and 60 min of incubation at 0°C (Fig. 6). The initial PPDK activities immediately before 0°C incubation (at time 0) were very similar and about 6.9 μmol mg−1 min−1 at all enzyme concentrations. The PPDK activities decreased during the cold treatment at all PPDK concentrations, but the decreases were much slower at higher enzyme concentration. The half-time when PPDK activity falls to 50% of the initial value (t1/2) at a protein concentration of 0.1 mg/mL was 9.2 min, while t1/2 at a concentration of 2 mg/mL was 22.6 min (Fig. 6). By the end of 10 min incubation at 0°C, the PPDK activity at the concentration of 0.1 mg/mL was only 36% of the control, while the PPDK activity at the concentration of 2 mg/mL was 73% of the control (Fig. 6).
Figure 6.
Cold inactivation of M. × giganteus recombinant PPDK at varying enzyme concentrations. The purified recombinant M. × giganteus PPDKs were diluted to varying protein concentrations and incubated at 0°C for the time indicated. The specific PPDK activities immediately before the cold treatment at time 0 were assigned as 100%. The data were fitted to a first-order exponential decay (Origin-Pro 7.5, OriginLab). The t1/2 (the time points at which PPDK activity fell to 50% of the initial value) at the different protein concentrations are shown.
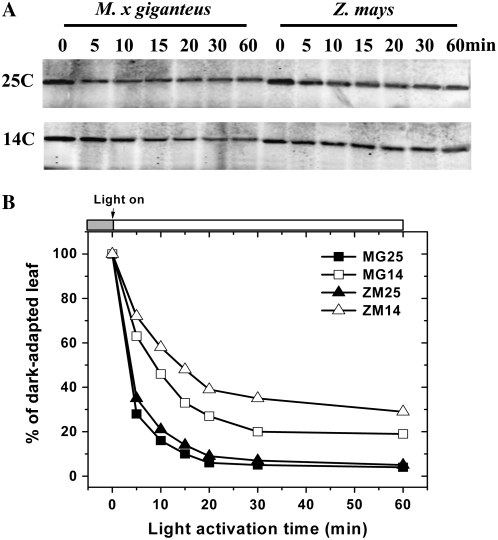
Influence of Chilling Temperature on Light Activation of PPDK
Activity of the C4 PPDK is known to be tightly regulated by the light/dark cycle. To examine the influence of chilling, the activation kinetics of PPDK from warm-grown and chilling-exposed M. × giganteus and maize were compared by measuring maximum activities of PPDK during the dark to light transition. The PPDK polypeptide contents showed no significant changes within 1 h following the dark to light transition in either species or in either temperature (Supplemental Fig. S2). However, acclimation to 14°C reduced the rate of activation of PPDK in both species, but to a much greater extent in maize (Fig. 7; Table II). The time required to reach 50% of the maximum PPDK activity from chilling-acclimated plants increased to 2.6-fold (M. × giganteus) and 4.7-fold (maize) in comparison to that for warm-grown plants (Fig. 7; Table II). However, on completion of activation, the activity in cold-grown leaves of M. × giganteus was about 20% higher than in warm-grown leaves, in sharp contrast to maize (Table II).
Figure 7.
Activation kinetics of PPDK in leaf crude extract from M. × giganteus (MG) and maize (ZM) grown continuously at 25°C (25) or grown at 25°C and then at 14°C (14) for 14 d. The leaves were dark adapted for 10 h in their respective growth environments. The leaf discs were collected into liquid N2 immediately before illumination and then at time points following illumination time points. The maximum activities of PPDK expressed on a leaf area basis were measured at 25°C as in Figure 4. Values are the means (±1 se) of five plants.
Table II.
Activation kinetics of PPDK from warm-grown and chilling-adapted M. × giganteus (MG) and maize (ZM) grown continuously at 25°C (25) or grown at 25°C and then transferred to 14°C (14) for 14 d
Because light activation of PPDK is tightly associated with dephosphorylation, regulated by the bifunctional PPDK regulatory protein, the phosphorylation level of PPDK from warm-grown (25°C) and chilling-acclimated (14°C) M. × giganteus and maize was also examined using PPDK Thr-P-specific antibodies during the dark to light transition, in parallel with enzyme activity assays. The antibodies used here were previously shown to be specific to phosphorylated PPDK with a very low background for nonphosphorylated PPDK, such as the recombinant form expressed in E. coli (Chastain et al., 1996; Chastain et al., 2000). The phosphorylation level of PPDK declined progressively following illumination in both species (Fig. 8, A and B). As observed in the enzyme assays, acclimation at 14°C reduced the rate of PPDK activation in both species, as indicated by a significantly greater t1/2 for dephosphorylation of PPDK (Table II). The t1/2 for dephosphorylation of PPDK from chilling-acclimated M. × giganteus leaves increased to 2.3-fold compared to warm-grown leaves, while it increased 3.1-fold in maize (Table II). By the end of 1-h illumination, the phosphorylation level of PPDK was 15% higher in chilling-adapted M. × giganteus leaves than that in warm-grown leaves, while it was 25% higher for maize (Fig. 8, A and B).
Figure 8.
Changes in phosphorylation level of PPDK upon illumination from leaves of M. × giganteus (MG) and maize (ZM) grown continuously at 25°C (25) or grown at 25°C and then at 14°C (14) for 14 d. The leaf samples were collected as described in Figure 7. The phospho-PPDK was probed with an affinity-purified rabbit polyclonal antibodies raised against a synthetic phosphopeptide conjugate corresponding to the Thr-phosphorylation domain of maize C4 PPDK. A representative immunoblot is shown (A). The band density shown in Figure 8A was quantified by densitometry (Odyssey V1.2 application software). The changes in phosphorylation level of PPDK at illumination times were expressed as percentage of that from dark-adapted leaves at time 0 (B).
DISCUSSION
Exposure to chilling temperatures is a key limitation to the production of maize both in the early and late growing season, particularly near the high-latitude limits of current cultivation (Miedema, 1982; Allen and Ort, 2001). Chilling events can occur after planting or before completion of grain fill. These result in a loss of photosynthetic capacity, which is exacerbated by photoinhibition and photooxidation (Long et al., 1983, 1994; Baker et al., 1989). The chilling tolerance of M. × giganteus is shown by comparison here to an inbred maize line selected for the corn belt. This inbred line also parallels responses observed previously for hybrids selected for use at the cold temperature limit of maize cultivation in Europe (Miedema, 1982; Long et al., 1983; Nie et al., 1992). While M. × giganteus retains a high photosynthetic rate in leaves transferred to 14°C (18.3 μmol m−2 s−1), the rate in maize declined to almost one-quarter of that value (5.3 μmol m−2 s−1), even though these leaves had very similar rates of CO2 uptake to those of M. × giganteus prior to being transferred to 14°C.
Both Rubisco and PPDK have been suggested as the potential control points limiting C4 photosynthesis at chilling temperatures (Furbank et al., 1997; Kubien et al., 2003). Upon transfer of the chilling-tolerant NADP-malic enzyme C4 grass M. × giganteus grown at 25°C to 14°C, mature leaves show a small transient loss of photosynthetic capacity. Their recovery and maintenance of a high photosynthetic rate under these cool conditions correlated with a marked increase in PPDK protein and mRNA. By contrast, Rubisco content remained constant during this treatment. Similarly, Naidu et al. (2003) showed that leaves of M. × giganteus developed at 14°C accumulated higher amounts of PPDK protein than leaves grown at 25°C but had similar levels of Rubisco as those grown at 25°C, in contrast to maize, where both PPDK and Rubisco declined. The dramatic increase of PPDK protein concentration in M. × giganteus leaves may be the mechanism by which PPDK activity in vivo is maintained in cool temperatures. Alteration of the stromal environment by concentrating this protein may enhance the level of active tetramer association (Hatch, 1979). This is supported by observations of increased cold stability of PPDK as protein concentrations are increased, as shown both here and in previous studies (Shirahashi et al., 1978; Salahas et al., 1990). Is there any evidence that increased expression of PPDK can increase photosynthetic rate in C4 plants at chilling temperatures? Ohta et al. (2006) recently overexpressed PPDK from the moderately cold-tolerant C4 species Flaveria brownii in maize and found that the transgenic plants could maintain a photosynthetic rate 23% greater than the untransformed line down to a leaf temperature of 8°C. This is consistent with the inference from the present study, where the ability to maintain photosynthetic rate during chilling correlates with increases in the amount of PPDK. In addition, a stress-induced (drought-induced and cold-induced) increase in PPDK expression has been shown in previous studies of other C4 species (Michalowski et al., 1989; Moons et al., 1998; Nogueira et al., 2003). For example, a RNA profile of cold-responsive genes using filter arrays showed that PPDK transcripts significantly increased on transfer of a sugar cane hybrid (Saccharum sp. ‘SP80-3280’) from 26°C to 4°C (Nogueira et al., 2003). In summary, these previous reports coupled with our findings suggest an important role for PPDK in acclimation to chilling in C4 species.
Not only is PPDK expression increased, but the apparent kinetics and activation of the enzyme are altered. In crude extracts, PPDK from M. × giganteus shows lower activation energy at low temperature and so shows less loss of activity as temperature is decreased. However, this does not appear to result from differences in the primary polypeptide, because when PPDK was cloned and expressed in E. coli, the recombinant-expressed protein from M. × giganteus showed no difference in activation energy to that from maize. This is consistent with the observation that there are few amino acid changes that distinguish C4 PPDK sequences among M. × giganteus, maize, and cold-intolerant sugar cane (Naidu et al., 2003). Previous analyses of sugar cane lines with different cold sensitivity (Du et al., 1999a) and Echinochloa crus-galli ecotypes from contrasting climates (Simon, 1996) also concluded that cold tolerance in those species is not due to the intrinsic properties of PPDK. The difference in crude extracts is therefore the result of either posttranslational modification or the presence of activators or inhibitors that affect the activity of the enzyme in leaf extracts. Divalent cations (Mn2+, Mg2+, and Ca2+), PEP, polyols (glycerol and sorbitol), and free amino acids (Pro) or their derivatives have been shown to protect PPDK against cold inactivation, possibly by preventing the dissociation of tetrameric active enzyme (Krall et al., 1989; Salahas et al., 2002).
Low temperature decreased the activation of PPDK in both M. × giganteus and maize, which is consistent with previous reports on maize and E. crus-galli (Edwards et al., 1980; Simon and Hatch, 1994). Early analyses showed that activation of PPDK from the C4 grass E. crus-galli subjected to cold treatment was faster in ecotypes from cool climates compared to those native to warmer climates (Simon and Hatch, 1994). Activation is also faster in M. × giganteus than maize. This may be critical in protecting the leaf against photoinhibition. When carbon metabolism is inhibited, utilization of absorbed light energy is decreased, which increases the potential for photoinhibition and photooxidative damage (Long et al., 1983; Powles and Bjorkman, 1983). Maize is particularly vulnerable to such damage on cool mornings with clear skies (Baker et al., 1989; Long et al., 1994). Slow induction of PPDK at low temperature could exacerbate this damage.
The findings presented here, coupled with those from the PPDK overexpressor transformants of maize (Ohta et al., 2006), provide strong evidence that this enzyme is critical to maintaining C4 photosynthesis at low temperature. Rubisco has previously been suggested as the most likely candidate for limiting C4 photosynthesis at low temperatures (Sage, 2002; Kubien et al., 2003). However, Rubisco levels remained constant in M. × giganteus leaves at low temperature, whereas PPDK increased, which is consistent with the suggestion by Sage (2002) that C4 leaves cannot physically accommodate more Rubisco. But restriction at Rubisco cannot explain the observed decline and recovery in leaf photosynthetic rates for M. × giganteus upon transfer to low temperature nor the increased photosynthetic rate measured at low temperature in transgenic maize that overexpresses PPDK.
One major difference in the C4 photosynthetic pathway between M × giganteus and chilling-sensitive maize appears to be higher steady-state PPDK mRNA accumulation, which may result from either increased transcriptional activity of the C4 PPDK gene or greater stability of its mRNA. The basis of this increase is unknownbut may reflect a pattern of acclimation to other stresses where one or more transcription factors are up-regulated (Thomashow et al., 2001). Discovering the regulatory network underlying increased C4 PPDK expression will be critical to gaining a more complete picture of the mechanistic basis for successful acclimation of C4 photosynthesis to low temperature, as realized in M. × giganteus.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Miscanthus × giganteus and maize (Zea mays) FR1064 (a commercial inbred line, Illinois Foundation Seeds) was grown in soil-less potting media (Sunshine Mix LC12; Sun Gro Horticulture) following the procedure of Naidu and Long (2004). Plants were grown at 14°C/12°C (cold) or 25°C/20°C (warm) day/night temperature and 14-h-day/10-h-night cycle under 500 μmol photons m−2 s−1 in controlled-environment chambers equipped with a mixture of high-pressure mercury and sodium lamps (E-15H; Conviron). To examine the ability of leaves to maintain photosynthetic capacity on chilling, plants originally grown in a controlled-environment chamber with 25°C/20°C (warm) day/night temperature were transferred to an identical chamber but with 14°C/12°C (cold) day/night temperature, and all other environmental conditions were unchanged. Leaves that had just completed expansion, as judged by ligule emergence, were collected after 3 h of illumination for assays based on crude extracts or time specified for light activation assays. The leaf discs were cut from fully expanded leaves and immediately frozen into and then stored in liquid nitrogen. Light-saturated leaf CO2 assimilation (ACO2) and ΦPSII were measured on fully expanded leaves that had been illuminated for 3 to 4 h.
RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA from leaf samples was isolated using RNeasy kit (Qiagen) according to the manufacturer's instructions. First-strand cDNAs were synthesized from DNaseI-treated total RNA using RT (Superscript II RNase H−; Invitrogen). For real-time quantitative PCR, reaction mixes of 25 μL were prepared including 12.5 μL of the SYBR Green PCR MasterMix (Applied Biosystems), 0.4 pmol of a primer pair, and 1 μL of 10-fold diluted first-strand cDNA. PCR amplifications were carried out on a Cepheid Smart Cycler system. The reactions were started with a step of 50°C for 2 min and 95°C for 10 min to activate the polymerase, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Dissociation kinetics analysis of the amplification products were performed to ensure specific amplification. The PCR for ubiquitin gene was used as the internal control. The primer pairs for PPDK genes are: for M. × giganteus PPDK (accession no. AY262272), forward primer 5′-GCGCCGATTGCGACGAAAAAGAG-3′ and reverse primer 5′-CCCAGCAGTTCCTTCATGCTCTTGT-3′; and for maize PPDK (accession no. J03901), forward primer 5′-CGCCGATACAGACGACCAAAAAGAGG-3′ and reverse primer 5′-CCCAGCAGTTCCTTCATGGTCTTGT-3′. The primers of each pair contained the sequences of the ends of two contiguous exons to avoid amplification of genomic DNA. The primer pairs for the ubiquitin gene are: for M. × giganteus ubiquitin, forward primer 5′- CCTCTGACACCATCGACAATGTGAA-3′ and reverse primer 5′- GCTGCTTGCCGGCGAAGATG-3′; and for maize ubiquitin, forward primer 5′- GCTCTGACACCATCGACAACGTGAA-3′ and reverse primer 5′- GCTGCTTGCCGGCGAAGATC-3′, respectively.
Expression and Purification of Recombinant His-Tagged PPDK
The cDNA of MgPPDK13 (Naidu et al., 2003) was cloned into BamHI and EcoRI sites of pRSETa (Invitrogen) with a pair of primers: forward primer TTGGATCCTCCGACGCCGGCGCGGGACGG and reverse primer ATGAATTCTCAGACAAGCACCTGAGCTGCAGC with the restriction sites BamHI and EcoRI underlined, respectively. The plasmid construction was confirmed by sequencing. The plasmid construction for maize PPDK cDNA (zmPPDK) was a gift from Dr. Chastain (Minnesota State University). The plasmids were transformed into BL21 (DE3) competent strains of Escherichia coli. The overexpression and purification of recombinant His-tagged PPDK were performed as described previously (Chastain et al., 1996).
Chlorophyll Contents, Chlorophyll Fluorescence, and Gas Exchange
Chlorophyll contents were measured and calculated according to Porra et al. (1989). The light-saturated CO2 uptake and transpiration were measured simultaneously with modulated chlorophyll fluorescence on fully expanded attached leaves using a LI-COR 6400 portable photosynthesis system as described previously (Naidu and Long, 2004; Wang and Portis, 2007). All measurements were conducted in ambient air under 1,000 μmol m−2 s−1 of light illumination. Five replicate plants were measured for each measurement temperature and treatment combination. Actinic light was supplied by light-emitting diodes (90% red light, 630 nm; 10% blue light, 470 nm) to record the steady-state chlorophyll fluorescence level (Fs). A pulse-modulated measuring light (630 nm; 1 μmol photons m−2 s−1) was used to determine the minimum chlorophyll fluorescence at the open PSII center. An 800-ms saturating pulse of about 5,000 μmol m−2 s−1 was applied to measure the maximum chlorophyll fluorescence at the closed PSII center in the dark (Fm) or during actinic light illumination (Fm′). The ΦPSII of illuminated leaves was calculated as (Fm′ − Fs)/Fm′ (Genty et al., 1989).
PPDK Extraction and Activity Assays
Extraction of PPDK followed the procedure of Crafts-Brandner and Salvucci (2002) with the following modifications. The extraction buffer contained 50 mm HEPES-NaOH, pH 8.0, 10 mm MgCl2, 5 mm dithiothreitol, 1 mm EDTA, 1% (w/v) casein, 1% (w/v) polyvinylpyrrolidone, 0.05% (v/v) Triton X-100, 20 mm NaF (for phospho-PPDK immunoassays only), 2 μm orthovanadate (for phospho-PPDK immunoassays only), and one protease inhibitor cocktail tablet (EDTA-free) per 10 mL of extraction buffer (Roche Applied Science). Two leaf discs (1.1-cm diameter) were ground rapidly in 500 μL of extraction buffer using a Tenbroeck tissue homogenizer at room temperature. The extract was then centrifuged for 30 s at 15,000g.
The activity of PPDK was measured by coupling the production of PEP to NADH oxidation via PEPc and malate dehydrogenase modified from Ashton et al. (1990). Except where noted below, all enzymes and chemicals were obtained from Sigma-Aldrich. NADH oxidation was monitored for 1 min in a dual-beam UV/VIS spectrophotometer at a wavelength of 340 nm in temperature-controlled cuvettes (Cary I and Temperature Control Accessory; Varian). The measurement temperatures were 6°C, 8°C, 10°C, 12°C, 15°C, 18°C, 20°C, 25°C, and 28°C. A 20-μL aliquot of crude extract supernatant was added to the cuvette (pre-equilibrated at assay temperature in the spectrophotometer) containing 1 mL of assay buffer and was incubated for 5 min at each measuring temperature. The assay buffer consisted of 100 mm HEPES-NaOH, pH 8.0, 15 mm MgCl2, 0.15 mm EDTA, 5 mm NaHCO3, 0.3 mm NADH, 5 mm NH4Cl, 2.5 mm K2HPO4, 5 mm dithiothreitol, 1 mm Glc-6-P, 1.5 mm ATP, and 10.5 units malate dehydrogenase. The reaction was initiated by the addition of pyruvate to 1.25 mm final concentration and 3 units of purified maize PEPCase (Bio-Research Products).
Immunoblot Analysis
Total soluble proteins on a leaf area basis were separated by 10% Tris-Gly SDS-PAGE and blotted onto the polyvinylidene difluoride membrane. PPDK and Rubisco proteins were detected with rabbit polyclonal antibodies raised against recombinant maize PPDK (Budde and Chollet, 1986) and native spinach (Spinacia oleracea) Rubisco. The phospho-PPDK was probed with the affinity-purified rabbit polyclonal antibodies raised against a synthetic phosphopeptide conjugate corresponding to the Thr-phosphorylation domain of maize C4 PPDK (Chastain et al., 2000). The blots were incubated with fluorescent-conjugated rabbit secondary antibodies (IRdye; LI-COR). The polyvinylidene difluoride membranes were scanned by using an Odyssey Infrared Imaging system (LI-COR), and relative protein expression levels were quantified with the Odyssey V1.2 application software as described previously (Wang and Portis, 2006).
Calculations
PPDK activity was calculated from the change in absorbance and the extinction coefficient of NADH (6,221 μL μmol−1 cm−1), accounting for the stoichiometry of the reactions linking each enzyme from NADH to CO2: i.e. 1 mol of PEP is consumed for each mol of NADH and 1 mol of bicarbonate for each of PEP. The maximum enzyme activity was reported as Vmax for PEP formation. To calculate the Ea, Log Vmax versus the inverse of the measuring temperature (K) times 1,000 was plotted (Arrhenius plot), and the slope of the line determined by linear regression (Origin 7.0). The slopes were determined separately at high (15°C–28°C or 18°C–28°C) and low (6°C–15°C or 6°C–15°C) temperature, because a break point, reflecting a shift in Ea for PPDK, was apparent at 15°C. Fitting two lines on either side of the break at 15°C accounted for significantly more residual variation than use of a single line (F, statistic). Ea was calculated as the product of the slope, 2.3 (to convert from log10 to ln), and the ideal gas constant (R= 8.3143 J/mol K) to yield Ea in units of kJ/mol.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Quantification of PPDK in crude leaf extract of M. × giganteus grown continuously at 25°C (25) and or grown at 25°C and then transferred to 14°C (14) for 14 d, before sampling.
Supplemental Figure S2. Changes in PPDK protein contents in leaves of M. × giganteus (MG) and maize (ZM) grown continuously at 25°C (25) and then at 14°C (14) for 14 d upon illumination.
Supplementary Material
Acknowledgments
We thank Dr. Shawna L. Naidu for development of PPDK activity assay, Bosola Oladeinde and Ayodele Gomih for technical assistance with plasmid construction of His-tagged M. × giganteus PPDK, and Dr. Chris Chastain for providing plasmid DNA constructs for His-tagged maize PPDK and antibodies for PPDK and phospho-PPDK.
This work was supported by the National Science Foundation (grant no. 0446018).
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Stephen P. Long (stevel@life.uiuc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6 36–42 [DOI] [PubMed] [Google Scholar]
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD (1990) Enzymes of C4 photosynthesis. In PJ Lea, ed, Methods in Plant Biochemistry, Vol 3. Academic Press, New York, pp 39–72
- Baker NR, Bradbury M, Farage PK, Ireland CR, Long SP (1989) Measurements of the quantum yield of carbon assimilation and chlorophyll fluorescence for assessment of photosynthetic performance of crops in the field. Philos Trans R Soc Lond B Biol Sci 323 295–308 [Google Scholar]
- Beale CV, Bint DA, Long SP (1996) Leaf photosynthesis in the C4-grass Miscanthus x giganteus, growing in the cool temperate climate of southern England. J Exp Bot 47 267–273 [Google Scholar]
- Beale CV, Long SP (1995) Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates? Plant Cell Environ 18 641–650 [Google Scholar]
- Budde RJA, Chollet R (1986) In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol 82 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard MJ, Heath MC, Nixon PMI (1995) Shoot growth, radiation interception and dry matter production and partitioning during the establishment phase of Miscanthus sinensis ‘Giganteus’ grown at two densities in the U. K. Ann Appl Biol 126 365–378 [Google Scholar]
- Burnell JN, Hatch MD (1984) Dark-light regulation of pyruvate, Pi dikinase in C4 plants: evidence that the same protein catalyses activation and inactivation. Biochem Biophys Res Commun 111 288–293 [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Botschner M, Harrington GE, Thompson BJ, Mills SE, Sarath G, Chollet R (2000) Further analysis of maize C4 pyruvate, orthophosphate dikinase phosphorylation by its bifunctional regulatory protein using selective substitutions of the regulatory Thr-456 and catalytic His-458 residues. Arch Biochem Biophys 375 165–170 [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Thompson BJ, Chollet R (1996) Maize recombinant C4-pyruvate, orthophosphate dikinase: expression in Escherichia coli, partial purification, and characterization of the phosphorylatable protein. Photosynth Res 49 83–89 [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Xu W, Parsley K, Sarath G, Hibberd JM, Chollet R (2008) The pyruvate, orthophosphate dikinase regulatory proteins of Arabidopsis possess a novel, unprecedented Ser/Thr protein kinase primary structure. Plant J 53 854–863 [DOI] [PubMed] [Google Scholar]
- Chinthapalli B, Murmu J, Raghavendra AS (2003) Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. J Exp Bot 54 707–714 [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129 1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YC, Nose A, Wasano K (1999. a) Effects of chilling temperature on photosynthetic rates, photosynthetic enzyme activities and metabolite levels in leaves of three sugarcane species. Plant Cell Environ 22 317–324 [Google Scholar]
- Du YC, Nose A, Wasano K (1999. b) Thermal characteristics of C4 photosynthetic enzymes from leaves of three sugarcane species differing in cold sensitivity. Plant Cell Physiol 40 298–304 [Google Scholar]
- Dunn R, Thomas SM, Keyss AJ, Long SP (1987) A comparison of the growth of the C4 grass spartina anglica with the C3 grass lolium perenne at different temperatures. J Exp Bot 38 433–441 [Google Scholar]
- Edwards G, Ujihira M, Sugiyama T (1980) Light and temperature dependence of the rate and degree of activation of pyruvate, Pi dikinase in vivo in maize. Photosynth Res 1 199–207 [DOI] [PubMed] [Google Scholar]
- Edwards GE, Nakamoto H, Burnell JN, Hatch MD (1985) Pyruvate, Pi dikinase and NADP-malate dehydrogenase in C4 photosytnthesis: properties and mechanism of light/dark regulation. Annu Rev Plant Physiol 36 255–286 [Google Scholar]
- Farage PK, Blowers DA, Long SP, Baker NR (2006) Low growth temperatures modify the efficiency of light use by photosystem II for CO2 assimilation in leaves of two chilling-tolerant C4 species, Cyperus longus L. and Miscanthus x giganteus. Plant Cell Environ 29 720–728 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, von Caemmerer S, Ashton AR (1997) Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Aust J Plant Physiol 24 477–485 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- Hamel N, Simon JP (2000) Molecular forms and kinetic properties of phosphoenolpyruvate carboxylase from barnyard grass (Echinochloa crus-galli (L.) Beauv.: Poaceae). Can J Bot 78 619–628 [Google Scholar]
- Hatch MD (1979) Regulation of C4 photosynthesis: factors affecting cold-mediated inactivation and reactivation of pyruvate, Pi-dikinase. Aust J Plant Physiol 6 607–619 [Google Scholar]
- Heaton E, Clifton-Brown JC, Voigt T, Jones M, Long SP (2004) Miscanthus for renewable energy generation: European Union esperience and projections for Illinois. Mitig Adapt Strategies Glob Change 9 433–451 [Google Scholar]
- Kingston-Smith AH, Harbinson J, Williams J, Foyer CH (1997) Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol 114 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall JP, Edwards GE, Andreo CS (1989) Protection of pyruvate,Pi dikinase from maize against cold lability by compatible solutes. Plant Physiol 89 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubien DS, Sage RF (2004) Low-temperature photosynthetic performance of a C4 grass and a co-occurring C3 grass native to high latitudes. Plant Cell Environ 27 907–916 [Google Scholar]
- Kubien DS, von Caemmerer S, Furbank RT, Sage R (2003) C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of Rubisco. Plant Physiol 132 1577–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP (1983) C4 photosynthesis at low temperatures. Plant Cell Environ 6 345–363 [Google Scholar]
- Long SP (1999) Environmental responses. In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 215–249
- Long SP, East TM, Baker NR (1983) Chilling damage to photosynthesis in young Zea mays. 1. Effects of light and temperature-variation on photosynthetic CO2 assimilation. J Exp Bot 34 177–188 [Google Scholar]
- Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45 633–662 [Google Scholar]
- Matsuba K, Imaizumi N, Kaneko S, Samejima M, Oshugi R (1997) Photosynthetic responses to temperature of phosphoenolpyruvate carboxykinase type C4 species differing in cold sensitivity. Plant Cell Environ 20 268–274 [Google Scholar]
- Michalowski CB, Olson SW, Piepenbrock M, Schmitt JM, Bohnert HJ (1989) Time course of mRNA induction elicited by salt stress in the common ice plant (Mesembryanthemum crystallinum). Plant Physiol 89 811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema P (1982) The effects of low temperature on Zea mays. Adv Agron 35 93–128 [Google Scholar]
- Moons A, Valcke R, Van Montagu M (1998) Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant J 15 89–98 [DOI] [PubMed] [Google Scholar]
- Naidu SL, Long SP (2004) Potential mechanisms of low-temperature tolerance of C4 photosynthesis in Miscanthus x giganteus: an in vivo analysis. Planta 220 145–155 [DOI] [PubMed] [Google Scholar]
- Naidu SL, Moose SP, Al-Shoaibi AK, Raines CA, Long SP (2003) Cold tolerance of C4 photosynthesis in Miscanthus x giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiol 132 1688–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Long SP, Baker NR (1992) The effects of development at suboptimal growth temperatures on photosynthetic capacity and susceptibility to chilling-dependent photoinhibition in Zea mays. Physiol Plant 85 554–560 [Google Scholar]
- Nogueira FTS, De Rosa VE Jr, Menossi M, Ulian EC, Arruda P (2003) RNA expression profiles and data mining of sugarcane response to low temperature. Plant Physiol 132 1811–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Ishida Y, Usami S (2006) High-level expression of cold-tolerant pyruvate, orthophosphate dikinase from a genomic clone with site-directed mutations in transgenic maize. Mol Breed 18 29–38 [Google Scholar]
- Ohta S, Usami S, Ueki J, Kumashiro T, Komari T, Burnell JN (1996) Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii pyruvate, orthophosphate dikinase. FEBS Lett 396 152–156 [DOI] [PubMed] [Google Scholar]
- Pittermann J, Sage R (2001) The response of the high altitude C4 grass Muhlenbergia montana (Nutt.) A. S. Hitchc. to long- and short-term chilling. J Exp Bot 52 829–838 [DOI] [PubMed] [Google Scholar]
- Pittermann J, Sage RF (2000) Photosynthetic performance at low temperature of Bouteloua gracilis Lag., a high-altitude C4 grass from the Rocky Mountains, USA. Plant Cell Environ 23 811–823 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Powles SBBJ, Bjorkman O (1983) Interaction between light and chilling temperature on the inhibition of photosynthesis chilling-sensitive plants. Plant Cell Environ 6 117–123 [Google Scholar]
- Sage RF (1999) Why C4 photosynthesis? In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 3–16
- Sage RF (2002) Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53 609–620 [DOI] [PubMed] [Google Scholar]
- Sage RF, McKown AD (2006) Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? J Exp Bot 57 303–317 [DOI] [PubMed] [Google Scholar]
- Salahas G, Cormas E, Zervoudakis G (2002) Cold inactivation of phosphoenolpyruvate carboxylase and pyruvate orthophosphate dikinase from the C4 perennial plant Atriplex halimus. Russ J Plant Physiol 49 211–215 [Google Scholar]
- Salahas G, Manetas Y, Gavalas NA (1990) Effects Of glycerol on the in-vitro stability and regulatory activation-inactivation of pyruvate orthophosphate dikinase of Zea-mays L. Photosynth Res 26 9–18 [DOI] [PubMed] [Google Scholar]
- Shirahashi K, Hayakawa S, Sugiyama T (1978) Cold lability of pyruvate, orthophosphate dikinase in the maize leaf. Plant Physiol 62 826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JP (1996) Molecular forms and kinetic properties of pyruvate, Pi dikinase from two populations of barnyard grass (Eichinochloa crus-galli) from sites of contrasting climates. Aust J Plant Physiol 23 191–199 [Google Scholar]
- Simon JP, Hatch MD (1994) Temperature effects on the activation and inactivation of pyruvate, Pi dikinase in two populations of the C4 weed Echinochloa crus-galli (barnyard grass) from sites of contrasting Climates. Aust J Plant Physiol 21 463–473 [Google Scholar]
- Smith AM, Woolhouse HW (1983) Metabolism of phosophoenolpyruvate in the C4 cycle during photosynthesis in the phosphoenolpyruvate-carboxykinase C4 grass Spartina anglica Hubb. Planta 159 175–189 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Boku K (1976) Differing sensitivity of pyruvate orthophosphate dikinase to low temperature in maize cultivars. Plant Cell Physiol 17 851–854 [Google Scholar]
- Thomashow MF, Gilmour SJ, Stockinger EJ, Jaglo-Ottosen KR, Zarka DG (2001) Role of the Arabidopsis CBF transcriptional activators in cold acclimation. Physiol Plant 112 171–175 [Google Scholar]
- Wang D, Naidu SL, Portis AR Jr, Moose SP, Long SP (2008) Can the cold tolerance of C4 photosynthesis in Miscanthus x giganteus relative to Zea mays be explained by differences in activities and thermal properties of Rubisco? J Exp Bot 59 1779–1787 [DOI] [PubMed] [Google Scholar]
- Wang D, Portis AR Jr (2006) Increased sensitivity of oxidized large isoform of ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) activase to adp inhibition is due to an interaction between its carboxyl extension and nucleotide-binding pocket. J Biol Chem 281 25241–25249 [DOI] [PubMed] [Google Scholar]
- Wang D, Portis AR Jr (2007) A novel nucleus-encoded chloroplast protein, PIFI, is involved in NAD(P) H dehydrogenase complex-mediated chlororespiratory electron transport in Arabidopsis. Plant Physiol 144 1742–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.