Abstract
CDC48/p97 is a conserved homohexameric AAA-ATPase chaperone required for a variety of cellular processes but whose role in the development of a multicellular model system has not been examined. Here, we have used reverse genetics, visualization of a functional Arabidopsis (Arabidopsis thaliana) CDC48 fluorescent fusion protein, and morphological analysis to examine the subcellular distribution and requirements for AtCDC48A in planta. Homozygous Atcdc48A T-DNA insertion mutants arrest during seedling development, exhibiting decreased cell expansion and displaying pleiotropic defects in pollen and embryo development. Atcdc48A insertion alleles show significantly reduced male transmission efficiency due to defects in pollen tube growth. Yellow fluorescent protein-AtCDC48A, a fusion protein that functionally complements the insertion mutant defects, localizes in the nucleus and cytoplasm and is recruited to the division mid-zone during cytokinesis. The pattern of nuclear localization differs according to the stage of the cell cycle and differentiation state. Inducible expression of an Atcdc48A Walker A ATPase mutant in planta results in cytokinesis abnormalities, aberrant cell divisions, and root trichoblast differentiation defects apparent in excessive root hair emergence. At the biochemical level, our data suggest that the endogenous steady-state protein level of AtCDC48A is dependent upon the presence of ATPase-active AtCDC48A. These results demonstrate that CDC48A/p97 is critical for cytokinesis, cell expansion, and differentiation in plants.
Members of the AAA (ATPase associated with different cellular activities)-ATPase protein family are characterized by either one (type I) or two (type II) 220 to 250 amino acid ATPase domains containing both conserved Walker A and B motifs per protomer (Beyer, 1997; Neuwald et al., 1999). The ATPase domains elicit protein conformational changes upon the nucleotide binding, hydrolysis, and product release that is believed to be required for the function of the mechano-chemical enzyme (Rouiller et al., 2000, 2002; Zhang et al., 2000; Beuron et al., 2003, 2006; DeLaBarre and Brunger, 2003, 2005; Huyton et al., 2003; Wang et al., 2003; Davies et al., 2005). The conservation and widespread use of the AAA domain suggests that AAA-ATPase proteins may use common mechanisms that utilize their ATPase activity to carry out a wide range of cellular functions (Lupas and Martin, 2002).
CDC48/p97 is a highly abundant type II AAA-ATPase (Peters et al., 1990) involved in cell cycle control (Moir et al., 1982) and cell proliferation (Egerton and Samelson, 1994). Gene disruption of CDC48/p97 in budding and fission yeasts (Saccharomyces cerevisiae and Schizosaccharomyces pombe; Fröhlich et al., 1991; Ikai and Yanagida, 2006), trypanosomes (TbVCP; Lamb et al., 2001), mouse (Muller et al., 2007), and Drosophila (ter94; Leon and McKearin, 1999) demonstrates that CDC48/p97 is essential in unicellular and multicellullar organisms. At the molecular level, CDC48/p97 is involved in many distinct cellular processes (Woodman, 2003; Dreveny et al., 2004; Meyer, 2005; Jentsch and Rumpf, 2007). Current models indicate that the targeting of CDC48/p97 family members to cellular pathways is accomplished via recruitment by adapter proteins or cofactors. For example, in mammalian cells, CDC48/p97 requires the cofactor p47 to mediate endoplasmic reticulum (ER) and Golgi membrane assembly (Kondo et al., 1997; Roy et al., 2000; Yuan et al., 2001) and nuclear envelope reformation (Hetzer et al., 2001). An additional cofactor complex, Ufd1-Npl4, is required for p97-mediated formation of the chromatin-associated nuclear envelope network, consolidation of the nuclear envelope (Hetzer et al., 2001), mitotic spindle dynamics (Cao et al., 2003; Cao and Zheng, 2004; Cheeseman and Desai, 2004), and ER-associated protein degradation (Alzayady et al., 2005; Schuberth and Buchberger, 2005; Römisch, 2006).
Mutations in CDC48/p97-interacting proteins have provided insight into the physiological and developmental functions of CDC48/p97-dependent biochemical pathways. eyes closed, a loss-of-function allele of the Drosophila p47 adapter protein ortholog (Sang and Ready, 2002), shows nuclear envelope assembly defects in early zygotic divisions in Drosophila. In Arabidopsis (Arabidopsis thaliana), loss of PUX1, a negative regulator of CDC48/p97 function (Rancour et al., 2004; Park et al., 2007), results in accelerated plant growth due to increased cell division and expansion (Rancour et al., 2004).
Plant morphological development requires the coordination of cell division, expansion, and differentiation (Meijer and Murray, 2001; Beemster et al., 2003; Fleming, 2006). Our previous work has suggested that AtCDC48 may be directly involved in cell division and expansion (Rancour et al., 2002, 2004). The Arabidopsis genome encodes three CDC48 isoforms: AtCDC48A (At3g09840), AtCDC48B (At3g53230), and AtCDC48C (At5g03340). These isoforms are predicted to share 91% (AtCDC48B) and 95% (AtCDC48C) amino acid identity to AtCDC48A (Rancour et al., 2002). Expression of AtCDC48A mRNA is highest in proliferating cells of the vegetative shoot, root, and flowers in rapidly growing plants (Feiler et al., 1995; Zimmermann et al., 2004, 2005).
At the subcellular level, AtCDC48A has been shown by immunofluorescence microscopy to be localized to the cytoplasm, nucleus, and to the phragmoplast mid-zone during cytokinesis (Feiler et al., 1995; Rancour et al., 2002). In addition, overexpression studies in plant protoplasts of fluorescent fusion protein-tagged AtCDC48A have suggested that the chaperone is associated with the ER and plasma membrane (Aker et al., 2006, 2007).
The role of the CDC48/p97 protein family during growth and development has not been examined to date in planta. Here, we show, through live-cell imaging and the expression and analysis of loss-of-function and inducible dominant-negative ATPase-defective mutants, that AtCDC48A is essential for plant growth and development at various stages.
These results provide evidence for CDC48/p97 function in plant cytokinesis, cell expansion, and differentiation. In addition, our data support a role for AtCDC48A ATPase function in maintenance of steady-state AtCDC48A protein levels, thus suggesting a mode of protein turnover autoregulation.
RESULTS
Molecular Characterization and Phenotypic Analysis of Atcdc48A T-DNA Insertion Mutants
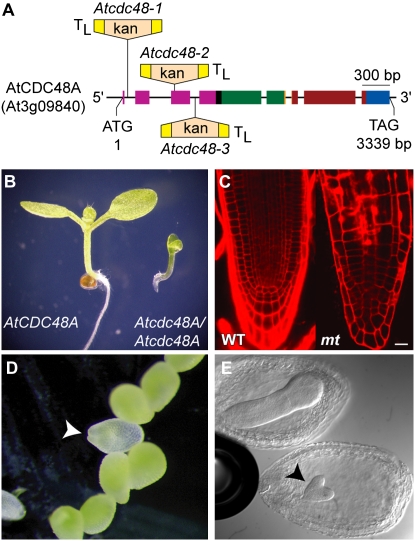
The gene encoding AtCDC48A is 3.3 kb in length and composed of eight exons (Fig. 1A). To characterize the biological function of AtCDC48A, we identified three independent Atcdc48A T-DNA insertion lines (Atcdc48AT-DNA-1, Atcdc48AT-DNA-2, and Atcdc48AT-DNA-3). All three Atcdc48A alleles exhibited identical phenotypes. The T-DNA insertion site in each of the alleles was verified by PCR amplification and DNA sequence analysis. The T-DNAs in Atcdc48AT-DNA-1, Atcdc48AT-DNA-2, and Atcdc48AT-DNA-3 were inserted in the first intron, third exon, and third intron, respectively, of AtCDC48A (Fig. 1A). All three T-DNA insertion sites are upstream of the sequences encoding the two ATPase domains. No viable soil-grown homozygous plants for any of the Atcdc48AT-DNA insertion alleles were identified from progeny of self-fertilized heterozygous parent plants. To verify that the mutant alleles are recessive, progeny from self-fertilized heterozygous Atcdc48AT-DNA mutants were grown on solid Murashige and Skoog media (Murashige and Skoog, 1962) and were monitored for growth. Approximately 4% of the germinated seedlings (14 seedlings from 346 seeds plated) on solid Murashige and Skoog media were homozygous for the T-DNA insertion. Homozygous Atcdc48AT-DNA seedlings arrested 1 d after germination, and the mutants exhibited severely disorganized root morphology (Fig. 1B). As shown in Figure 1B, roots of homozygous Atcdc48AT-DNA mutants were less than 0.5 mm in length with a root tip that was narrower than wild-type (Fig. 1C). Morphologically, Atcdc48AT-DNA mutant roots displayed abnormal cell files, with no apparent elongation or meristematic regions and a disorganized root cap (Fig. 1C). These results indicated that AtCDC48A is critical for seedling development and growth.
Figure 1.
Schematic representation of Atcdc48AT-DNA mutant alleles and the phenotypes of Atcdc48AT-DNA seedlings, seeds, and embryos. A, The exon/intron gene structure of AtCDC48A is shown to scale with broad-colored boxes representing exons and black inter-exon thin lines representing introns. The colors of the exons correspond to the DNA-coding regions encoding the protein domains of AtCDC48A: pink, N terminus; black, linker 1; green, D1 ATPase; orange, linker 2; red, D2 ATPase; blue, C terminus. The position and directions of T-DNA inserts with left border sequences are indicated (TL). T-DNAs are not drawn to scale. kan, T-DNA neomycin phosphotransferase selectable gene marker. B, Stereo micrograph of 5-d-old wild-type AtCDC48A and homozygous Atcdc48AT-DNA mutant seedlings. Homozygous Atcdc48AT-DNA plants die soon after this stage of development. C, Roots of 5-d-old wild-type AtCDC48A (WT) and homozygous Atcdc48AT-DNA mutant (mt) plants stained with propidium iodide and imaged by LSCM. The mutant root image comprises the entire root length (as shown in B), while the left image corresponds to only tip of the wild type. Scale bar = 10 μm. D, Stereo micrograph of a portion of an immature silique from a self-fertilized heterozygous Atcdc48AT-DNA plant approximately 10 d after fertilization. A pale green Atcdc48A homozygous seed is indicated by an arrowhead. E, Sibling embryos of an immature silique (approximately 5 d after fertilization) from a self-fertilized heterozygous Atcdc48AT-DNA plant. Mutant embryo development is shown arrested at the heart stage (arrowhead).
The allelic ratio of F1 progeny from a self-fertilized heterozygous Atcdc48AT-DNA/+ plant did not follow the predicted frequency of segregation for a typical recessive mutation, suggesting earlier defects in development. Therefore, 10 d after flowering, immature siliques from two generations of self-fertilized heterozygous plants (approximately 40 siliques of each generation) were examined. In a visual survey of seeds from heterozygous Atcdc48AT-DNA siliques, pale-green seeds, suggesting embryo development defects (Meinke, 1994; http://www.seedgenes.org/Tutorial.html), were observed at a frequency of five pale seeds out of 60 total (approximately 8%; Fig. 1D). PCR-based genotypic analysis of embryos from the pale-green seeds indicated they were homozygous for the Atcdc48AT-DNA insertions (Supplemental Fig. S1). To determine at which stage in embryo development homozygous Atcdc48AT-DNA mutants arrested, 5-d-postfertilization siliques from wild-type and heterozygous Atcdc48AT-DNA/+ mutant plants were cleared with Hoyers solution (Liu and Meinke, 1998) and examined by light microscopy using differential interference contrast (DIC) optics. Atcdc48AT-DNA mutant embryo growth was arrested at the early heart stage of development (Fig. 1E), supporting a role for AtCDC48A in plant embryogenesis.
PCR-based genotype analysis of 10-d-old soil-grown surviving progeny from self-fertilized heterozygous Atcdc48AT-DNA/+ plants showed an altered segregation ratio (78 wild type:114 heterozygote [41%:59%, respectively]; n = 192) in contrast to the predicted frequency for a recessive embryo/seedling lethal mutation (1 wild type:2 heterozygote [33%:67%, respectively]). The altered segregation ratio suggests impairment of mutant allele transmission through the gametes. To confirm this, male and female transmission efficiencies of the Atcdc48AT-DNA alleles were determined by performing reciprocal crosses between heterozygous mutants and wild-type plants. Pollen grains from the wild-type plants were able to fertilize Atcdc48AT-DNA/+ plants to yield progeny that segregated in the ratio of 1:0.73 (124 wild type:91 heterozygote [58%:42%, respectively]; n = 215). Using Atcdc48AT-DNA/+ pollen to fertilize wild-type stigmas resulted in a highly skewed segregation of progeny genotype (235 wild-type:13 heterozygote [95%:5%, respectively]; n = 248). Atcdc48AT-DNA alleles show significantly reduced male transmission efficiency and modest female transmission efficiency defects. These results suggest a critical role for AtCDC48A in pollen development and/or function.
Analysis of Pollen Development in Atcdc48A Mutants
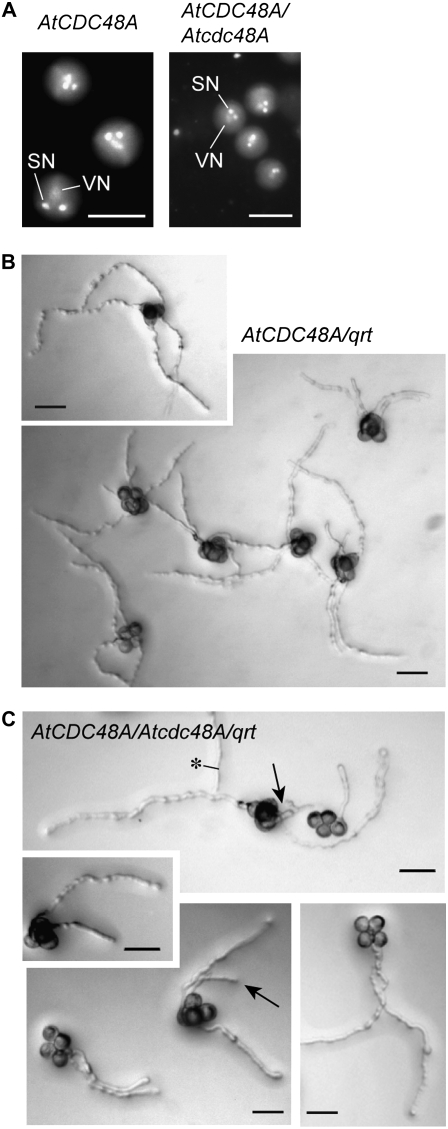
To determine at which developmental stage Atcdc48AT-DNA pollen was defective, the development, maturation, and germination of pollen grains from wild-type and heterozygous Atcdc48AT-DNA/+ plants were analyzed by DIC and epifluorescence microscopy. Staining of nuclear DNA with 4,6-diamidino-2-phenylindole (DAPI) was used to analyze pollen developmental progression. No apparent defects in late microsporogenesis, including tetrad formation and microspore release, were observed compared to wild type. After microspore release and pollen mitosis I and II, all the pollen grains from wild-type and Atcdc48AT-DNA/+ plants contained two generative and one vegetative nucleus (Fig. 2A) and thus appeared normal. To investigate whether Atcdc48AT-DNA mutant pollen arrest at an early developmental stage prior to late microsporogenesis (meiosis II) or, alternatively, during stages after pollen mitosis II, the Atcdc48AT-DNA-1 and quartet (qrt) mutant alleles were introgressed by cross-pollination between heterozygous Atcdc48AT-DNA-1/+ and qrt mutant plants. The qrt mutation results in pollen grains that remain physically associated in a tetrad and undergo synchronous development due to a defect in the pollen mother cell wall that results in microspore dissociation failure after meiosis II (Preuss et al., 1994; Rhee and Somerville, 1998). Pollen from wild-type qrt (AtCDC48A;qrt) and heterozygous Atcdc48AT-DNA-1/+;qrt lines was monitored by bright-field microscopy (Fig. 2, B and C). As shown, four pollen grains of Atcdc48AT-DNA-1/+;qrt remained associated, indicating that the Atcdc48AT-DNA-1 mutation does not disrupt meiotic processes in pollen development.
Figure 2.
Analysis of development and tube germination of pollen from AtCDC48A and heterozygous Atcdc48A plants. A, Pollen grains from wild-type (left) and heterozygous Atcdc48AT-DNA (right) plants were examined at onset of desiccation by epifluorescence microscopy after DAPI staining. SN, Sperm nuclei; VN, vegetative nucleus. Scale bars = 15 μm. B and C, Pollen grains from qrt (AtCDC48A;qrt; B) and heterozygous Atcdc48AT-DNA;qrt (Atcdc48A/+;qrt; C) plants were germinated on pollen germination media at 28°C for 24 h and then imaged with DIC optics. Arrows indicate Atcdc48A mutant pollen tubes. Asterisk indicates a pollen tube from outside the field of view. Scale bars = 50 μm.
To test whether pollen germination was affected by the Atcdc48AT-DNA-1 insertion mutation, in vitro pollen germination assays were performed. Pollen tubes were observed protruding from all four germinating wild-type AtCDC48A;qrt pollen grains (Fig. 2B). In contrast, only two pollen grains germinated from Atcdc48AT-DNA-1/+;qrt pollen (Fig. 2C), with approximately 5% showing delayed growth of a third pollen tube (Fig. 2C, arrows). These data indicate a critical role for AtCDC48A in pollen germination and tube elongation that manifests in significant transmission inhibition of the Atcdc48AT-DNA insertion alleles.
Characterization and Localization of Native Promoter∷YFP-AtCDC48A in Homozygous Atcdc48AT-DNA Seedlings
To examine the tissue and subcellular localization of AtCDC48A, transgenic Arabidopsis plants expressing an N-terminal tagged yellow fluorescent protein (YFP; Heim and Tsien, 1996) AtCDC48A fusion protein (YFP-AtCDC48A) under control of the native AtCDC48A promoter were generated. This construct was introduced into heterozygous Atcdc48AT-DNA/+ plants, and the T2 population was analyzed by both YFP screening and PCR genotyping (Supplemental Fig. S2) to assess complementation. The YFP-AtCDC48A construct rescued all the phenotypic defects associated with the Atcdc48AT-DNA mutants described above. Immunoblot analysis of protein extracts prepared from wild-type and Atcdc48A;YFP-AtCDC48A seedlings using anti-AtCDC48A and -GFP antibodies confirmed that the 118-kD YFP-AtCDC48A fusion protein was intact (Supplemental Fig. S3).
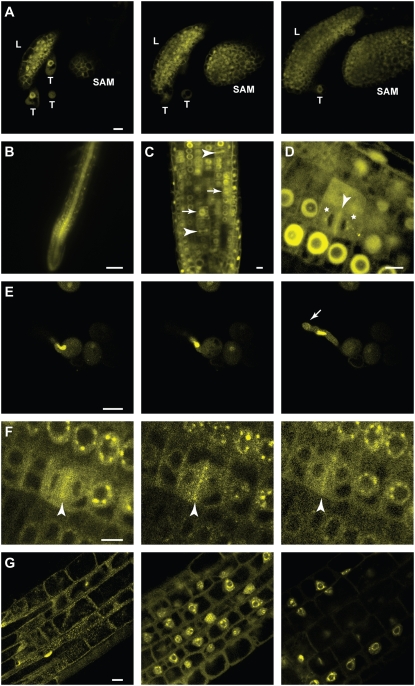
Expression of YFP-AtCDC48A was detected throughout developing Atcdc48AT-DNA/Atcdc48AT-DNA;YFP-AtCDC48A transgenic plants (Fig. 3; Supplemental Movies S1–S4) and is consistent with publicly available gene expression data (Supplemental Fig. S4). YFP-AtCDC48A fusion protein localizes to the nucleus and cytoplasm of all cells throughout all tissues of the seedling, including leaves, the shoot apical meristem (Fig. 3A; Supplemental Movie S1), and the root (Fig. 3, B–D; Supplemental Movies S3 and S4). In addition, YFP-AtCDC48A fusion protein was observed in developing ovules, pollen sacs, and pollen in mature plants. In germinating pollen, YFP-AtCDC48A was detected in the vegetative nucleus and surrounding the nuclear periphery but was not detected in the two sperm nuclei. Additionally, YFP-AtCDC48A was localized to the growing tip of the pollen tubes (Fig. 3E; Supplemental Movie S2).
Figure 3.
Localization of YFP-AtCDC48A in transgenic homozygous Atcdc48AT-DNA seedlings. Transgenic T3 seedlings were examined 3 d after germination. A, Representative LSCM images of YFP-AtCDC48A expressed in leaves (L), trichomes (T), and the shoot apical meristem (SAM). Scale bar = 10 μm. The three segments correspond to the first (left), middle, and last (right) images of a serial Z-stack set taken down the vertical axis of the plant. See Supplemental Movie S1 for complete Z-stack series of images. B, Expression of YFP-AtCDC48A in the root visualized by wide-field fluorescence microscopy. Scale bar = 100 μm. C and D, YFP-AtCDC48A expression in the root division zone visualized by LSCM. Scale bar = 10 μm. Division plane localization is indicated by arrowheads. Spindle localization is indicted by arrows. Asterisks indicate vesicle-like structures located neighboring developing nuclear membranes. E, The vegetative nucleus during pollen tube elongation. The three segments correspond to the first (left), middle, and last (right) images of a time series taken during pollen tube elongation in vitro. See Supplemental Movie S2 for complete time series. Arrow indicates the tip of the pollen tube. Scale bar = 10 μm. F, YFP-AtCDC48A localization during karyokinesis in dividing root cells. The three segments correspond to the first (left), middle, and last (right) images of a time series taken during root cell division. See Supplemental Movie S3 for complete time series. Arrowhead indicates a dividing cell division plane. Scale bar = 10 μm. G, YFP-AtCDC48A nucleus localization in the primary root elongation zone. The three segments correspond to the first (left), middle, and last (right) images of a serial Z-stack set taken perpendicular to the root radial axis. See Supplemental Movie S4 for complete Z-stacks. Scale bar = 10 μm.
Upon closer examination in root cells, YFP-AtCDC48A subcellular localization was cell cycle dependent. During mitosis, YFP-AtCDC48A was associated with the mitotic spindle (Fig. 3C, arrow) and subsequently with the phragmoplast mid-zone during cytokinesis (Fig. 3, C and D, arrowhead). During karyokinesis, YFP-AtCDC48A containing vesicle-like structures began to localize and coalesce around the chromosomal material (Fig. 3, D and F; Supplemental Movie S3). Following cell division, YFP-AtCDC48A was associated with the nuclear envelope, nucleoplasm, and nucleolar cavity (Fig. 3, C, D, F, and G; Supplemental Movie S3).
Phenotypic Analysis of Transgenic Atcdc48A Dominant-Negative Mutant Plants
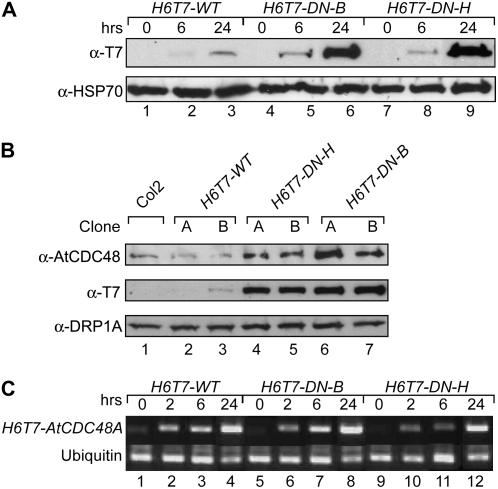
Loss-of-function insertion alleles of Atcdc48T-DNA-1, Atcdc48T-DNA-2, and Atcdc48T-DNA-3 display early pleiotropic developmental phenotypes. To examine the role of AtCDC48A in later stages of plant development, transgenic plants expressing dominant-negative mutant Atcdc48A proteins (Atcdc48ADN) under the control of an ethanol-inducible promoter system from Aspergillus nidulans (Caddick et al., 1998; Roslan et al., 2001) were generated. Using this system, the function of endogenous AtCDC48A was temporally inactivated during the seedling and later stages of plant development, thus circumventing the gametophyte and seedling lethal phenotypes encountered with the Atcdc48AT-DNA loss-of-function insertion alleles. Previous studies have demonstrated that the incorporation of cdc48/p97 D1 and D2 ATP binding and hydrolysis mutant subunits into endogenous CDC48/p97 hexameric complexes disrupts their function in vivo and in vitro (Lamb et al., 2001; Dalal et al., 2004; Park et al., 2007), and thus function as dominant-negative proteins (subunits). Ethanol-inducible AtCDC48A (H6T7-WT), Atcdc48ADN-H (H6T7-DN-H; ATP hydrolysis [E308Q/E581Q]), and Atcdc48ADN-B (H6T7-DN-B; ATP binding [K254A/K572A]) plant expression constructs contained an N-terminal six His and T7 (H6T7) tandem epitope tag to facilitate immunological detection of the transgene products. In the absence of ethanol, H6T7-tagged wild-type and Atcdc48ADN-H, -B mutant proteins were not detected by immunoblot analysis (Fig. 4A, lanes 1, 4, and 7). Upon the addition of (2%) ethanol to the growth medium, expression of H6T7-tagged wild-type AtCDC48A and Atcdc48ADN-H, -B proteins were readily detected after 6 h (Fig. 4A, lanes 2, 5, and 8), and after approximately 24 h the levels of H6T7-DN mutant protein (Fig. 4A, lanes 3, 6, and 9) were approximately 10-fold higher than H6T7-WT based on densitometric immunoblot analysis. Induction of H6T7-tagged wild-type and Atcdc48ADN-H, -B mutant proteins did not vary over an ethanol application range of 0.5% to 2% (v/v). In addition, untransformed seedling growth was not affected under any of the induction conditions used.
Figure 4.
Expression of conditional dominant-negative Atcdc48A mutants. A, Time course of transgenic ethanol-induced H6T7-Atcdc48 mutant protein expression. Expression of H6T7-AtCDC48A (H6T7-WT; lanes 1–3), H6T7-Atcdc48DN-B (H6T7-DN-B; lanes 4–6), and H6T7-Atcdc48DN-H (H6T7-DN-H; lanes 7–9) proteins was monitored by SDS-PAGE and immunoblot analysis using an anti-T7 antibody (top) and an anti-HSP70 antibody as a protein load control (bottom). Seedling samples were prepared prior to ethanol treatment (lanes 1, 4, and 7) or after 6 h (lanes 2, 5, and 8) or 24 h (lanes 3, 6, and 9) post-ethanol treatment. B, Immunoblot analysis of endogenous AtCDC48 and H6T7-AtCDC48A protein expression levels from untransformed (Col2, lane 1) and independent transgenic ethanol-induced wild-type H6T7-AtCDC48A (H6T7-WT; lanes 2 and 3), H6T7-Atcdc48ADN-H (H6T7-DN-H; lanes 4 and 5), and H6T7-Atcdc48ADN-B (H6T7-DN-B; lanes 6 and 7). Segments correspond to probing of samples with anti-AtCDC48A (top), anti-T7 (middle), and anti-DRP1A (bottom, load control). Samples were processed and analyzed at 48 h postinduction. C, Reverse transcription-PCR analysis of H6T7-AtCDC48A and H6T7-Atcdc48ADN gene expression. cDNA was synthesized from total RNA from H6T7-AtCDC48A (H6T7-WT; lanes 1–4), H6T7-Atcdc48DN-B (H6T7-DN-B; lanes 5–8), and H6T7-Atcdc48DN-H (H6T7-DN-H; lanes 9–12) isolated prior to ethanol treatment (lanes 1, 5, and 9) or after 2 h (lanes 2, 6, and 10), 6 h (lanes 3, 7, and 11), or 24 h (lanes 4, 8, and 12) post-ethanol treatment. cDNA fragments were PCR amplified using primers specific to the transgene (top) or the ubiquitin control (bottom).
After 48 h of post-ethanol treatment, steady-state levels of total AtCDC48A protein (Fig. 4B, top) are highly elevated in lines expressing H6T7-DN protein (Fig. 4B, lanes 4–7). Parallel ethanol treatment of H6T7-WT protein-expressing lines (Fig. 4B, lanes 2 and 3) shows that total AtCDC48A protein levels are similar to wild-type plants (Fig. 4B, lane 1). In lines expressing H6T7-DN proteins, the levels of endogenous AtCDC48 and transgene H6T7-AtCDC48-DN protein products was greatly increased relative to H6T7-WT (Fig. 4B, middle anti-T7, compare lanes 2 and 3–7). DRP1A protein detection was used as a loading control (Fig. 4B, bottom).
To determine if the elevated levels of H6T7-DN proteins, relative to H6T7-WT, were due to altered transcript levels for the transgenes, reverse transcription-PCR analysis was performed on total RNA from ethanol-treated transgenic seedlings (Fig. 4C). Expression of transgenic Atcdc48 mRNA was detected 2 h after ethanol induction (Fig. 4C, lanes 2, 6, and 10) and increased steadily over the 24-h time course (Fig. 4C, lanes 3 and 4, 7 and 8, and 11 and 12). mRNA expression levels were comparable between wild-type and mutant transgenes.
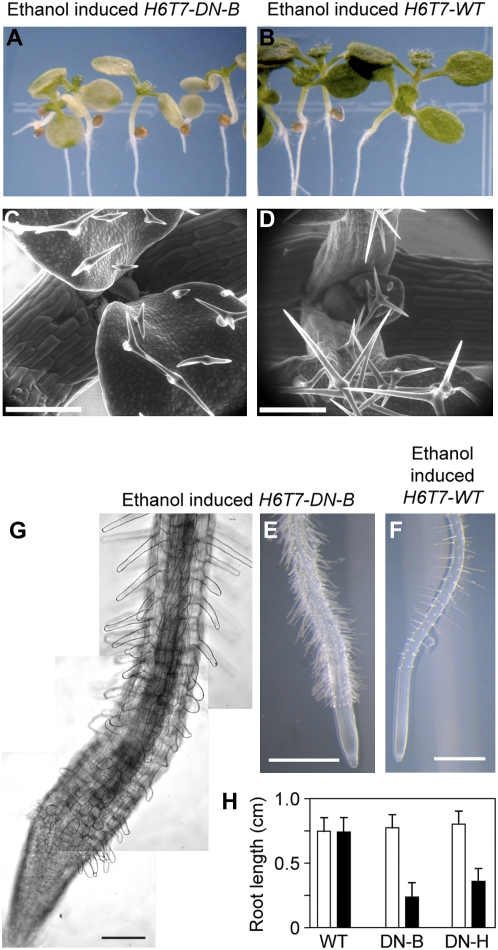
Transgenic plants expressing wild-type AtCDC48A (H6T7-WT) exhibited no abnormal phenotypes (Fig. 5, B, D and F; Supplemental Fig. S5). In contrast, transgenic plants expressing either Atcdc48ADN-B or Atcdc48ADN-H exhibited aberrant morphological phenotypes in both the aerial and root regions of the plant (Fig. 5, A, C, E, and G; Supplemental Fig. S5). As shown in Figure 5A, the cotyledons of seedlings expressing H6T7-DN-B stopped expanding and became chlorotic as compared to plants expressing H6T7-WT, the wild-type control (Fig. 5B; Supplemental Fig. S5). H6T7-DN-B trichomes were primarily bi-branched and smaller when compared to those from AtCDC48A plants (Fig. 5, C and D, respectively). Roots of H6T7-DN-B plants displayed altered root hair morphology, frequency, and distribution (Fig. 5, E, compared to wild-type right segment, F). Seedling roots expressing H6T7-DN-B protein showed an enhanced number of epidermal cell root hairs (Fig. 5, E and G) relative to root expressing H6T7-WT (Fig. 5F). In addition, root growth arrested upon induction of H6T7-DN-B and H6T7-DN-H dominant-negative protein expression relative to H6T7-WT (Fig. 5H). H6T7-DN-H plants exhibited less severe phenotypes overall than H6T7-DN-B plants, including root length (Fig. 5H), excessive root hair emergence, and aerial yellowing as observed in the H6T7-DN-B plants (Supplemental Fig. S5).
Figure 5.
Phenotypic analysis of conditional Atcdc48ADN mutant plants. Five-day-old H6T7-Atcdc48ADN-B (H6T7-DN-B; A, C, E, and G) and H6T7-AtCDC48A (H6T7-WT; B, D, and F) seedlings were treated with 2% (v/v) ethanol and imaged after 4 d. A and B, Stereo micrographs of the aerial portion of ethanol-treated H6T7-DN-B (A) and H6T7-WT (B) plants. C and D, SEM of primary leaves and trichomes from ethanol-treated H6T7-DN-B (C) and H6T7-WT (D) plants. Scale bar = 200 μm. E and F, Stereo micrographs of roots from ethanol-treated H6T7-DN-B (E) and H6T7-WT (F) plants. Scale bar = 0.5 mm. G, Higher magnification DIC image composite of an ethanol-treated H6T7-DN-B seedling root. Scale bar = 50 μm. H, Total seedling root growth for H6T7-WT (WT) and H6T7-Atcdc48ADN mutants (ATP binding: DN-B, and ATP hydrolysis: DN-H) was measured after 4 d post-ethanol (black bars) or mock (white bars) treatment. The data represent a minimum sample size of 25 plants for each construct. The sd is represented as error bars for each group.
Analysis of the Effects of Atcdc48DN-B Expression on Root Cell Development
To further investigate the morphology of seedlings expressing Atcdc48ADN-B, root cross sections from plants before and after ethanol induction were examined via microscopic analysis. Transverse sections of seedling roots from plants expressing H6T7-WT and H6T7-DN-B were analyzed from the root tip to the differentiation zone (Fig. 6). Root cell file organization and differentiation differ according to their longitudinal position (Supplemental Fig. S6). Root cell division occurs predominantly within the zone proximal to the root tip (approximately 100 μm). Within the expansion zone, approximately 300 μm from the root tip, the root cap cells can be seen in the outermost cell layer, and cells start to expand longitudinally. Distal to the region of cell expansion (approximately 1 mm relative to the root tip), root hairs start to emerge from root epidermal cells (trichoblasts) positioned over the junction of two underlying cortex cells (Dolan et al., 1993; Schiefelbein et al., 1997).
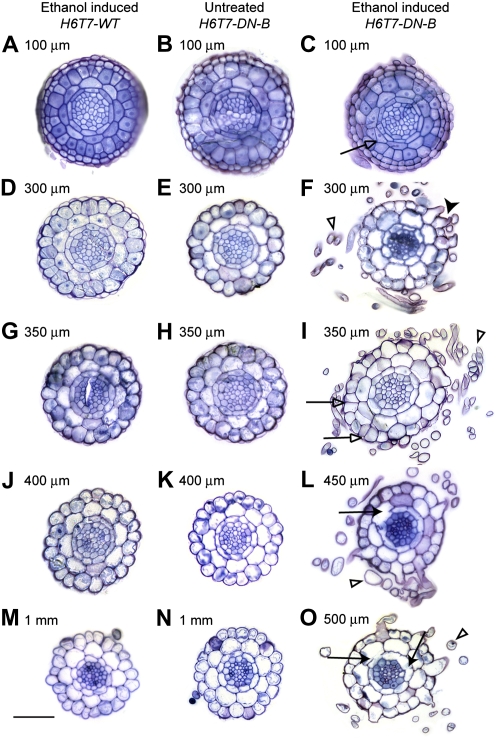
Figure 6.
Cell division and trichoblast differentiation are affected in conditional Atcdc48ADN-B mutant H6T7-AtCDC48A (H6T7-WT; column 1) and H6T7-Atcdc48ADN-B (H6T7-DN-B; columns 2 and 3) seedlings were analyzed 4 d after 2% (v/v) ethanol (columns 1 and 3) or mock treatment (column 2). Sections were stained with toluidine blue O and imaged by bright-field microscopy. Figure rows correspond to similar distances of tissue sections from the root tip. A root guide is available in Supplemental Figure S6. A to C, Approximately 100 μm above the root tip. D to F, Approximately 300 μm above the root tip corresponding to the division zone. G to I, Approximately 350 μm above the root tip corresponding to the division-expansion transition zone. J to L, Approximately 400 to 450 μm above the root tip corresponding to the expansion zone. M to O, Approximately 500 to 1,000 μm from the root tip corresponding to the expansion-differentiation transition zone. Black arrows highlight apparent cytokinesis defects. White arrows highlight aberrant cell divisions. Black arrowheads indicate root hairs emerging from inappropriate epidermal cells. White arrowheads highlight root hair projections. Scale bars = 50 μm.
Prior to the addition of ethanol, H6T7-DN-B serial transverse root section analysis indicated no variation in the number of cell files or layers throughout the root length when compared to ethanol-induced H6T7-WT wild-type roots (Fig. 6, columns 1 and 2; Dolan et al., 1993). Root hairs were observed to emerge from a root trichoblast cell, as presented previously (Supplemental Fig. S6), in both ethanol-induced H6T7-WT and uninduced H6T7-DN-B mutant roots (Fig. 6, M and N). In contrast, root hairs of the ethanol-treated H6T7-DN-B mutant seedlings were observed immediately above the root cap and emerged from epidermal cells positioned relative to either one or two underlying cortex cells (Figs. 5G and 6F, black arrowhead), thus suggesting cell fate determination defects in the presence of H6T7-DN-B. In addition to root hair abnormalities, cell division defects were observed in endodermal and cortex cells (Fig. 6, C, I, L, and O) of ethanol-treated H6T7-DN-B plants. The observed cell division defects manifest as either (1) apparent cytokinesis defect, including cell wall stubs (incomplete divisional cell walls), resulting in inappropriate cellular continuity between neighboring endodermal and/or cortical cell files (Fig. 6, L and O, black arrows), or (2) inappropriate cell divisions, including spurious periclinal divisions in the cortex (Fig. 6C, white arrows) and extra anticlinal divisions in the epidermis (Fig. 6I, white arrows). These data demonstrate a role for CDC48/p97 in plant cell division, cytokinesis, and cell fate determination.
DISCUSSION
AtCDC48A Has Multiple Roles during Development
The AAA-ATPase CDC48/p97 in non-plant systems has been shown to function in various pathways, including organelle biogenesis and protein degradation (Peters et al., 1992; Acharya et al., 1995; Rabouille et al., 1995). Localization studies and other studies in Arabidopsis implicate a role for AtCDC48A in plant cytokinesis and cell expansion (Feiler et al., 1995; Rancour et al., 2002, 2004).
To determine if CDC48/p97 function is required for plant cell division, three independent Arabidopsis T-DNA insertion alleles of Atcdc48A were identified and characterized. All three Atcdc48AT-DNA alleles were recessive and displayed the same phenotypes, including defects in pollen tube germination and expansion, embryo developmental arrest, and seedling lethality (Figs. 1 and 2). The nature of the variable expressivity of the mutant phenotypes remains to be determined.
AtCDC48A is a highly abundant protein in Arabidopsis (Rancour et al., 2004). One possible explanation for the variable expressivity of the T-DNA mutant phenotypes is that sufficient levels of paternal and/or maternal levels of AtCDC48A are present during embryogenesis to grant some homozygous embryos the capacity to proceed to the next major AtCDC48A-dependent developmental stage. Another possible explanation is that expression of AtCDC48B or AtCDC48C may compensate for the loss of AtCDC48A in early stages of development, as AtCDC48B and AtCDC48C share 91% and 95% amino acid sequence identity, respectively, with full-length AtCDC48A (Rancour et al., 2002). Nevertheless, analysis of homozygous Atcdc48AT-DNA mutants demonstrated that AtCDC48A is absolutely required for seedling development.
Morphological analysis of Atcdc48AT-DNA seedlings has implicated AtCDC48A involvement in multiple seedling developmental processes. Root cells in homozygous Atcdc48A seedlings were disorganized and morphologically abnormal suggesting roles for AtCDC48A in cell proliferation and expansion (Fig. 1C) and plasma membrane integrity. CDC48 has been shown to be associated with the plasma membrane in soybean (Glycine max; Shi et al., 1995), and Arabidopsis SERK1, a plasma membrane-localized Leu-rich repeat receptor-like Ser Thr kinase, interacts with AtCDC48A (Shah et al., 2002; Rienties et al., 2005). These results indicate that AtCDC48A may have a role in plasma membrane biogenesis and signaling.
To circumvent the range of early plant developmental defects observed in the Atcdc48AT-DNA mutants, we took an alternative approach, utilizing inducible dominant-negative Atcdc48ADN-B, -H gene expression, to examine the in vivo role of AtCDC48A in plant growth and development. The presence and accumulation of Atcdc48ADN-B protein correlated with phenotypes observed throughout the plant, including the arrest of leaf growth and expansion as well as early leaf senescence, alterations in the frequency and distribution of root hairs, and arrest of root growth with defects in cytokinesis and cell expansion (Figs. 5 and 6). The presence of ectopic root hairs in plants expressing Atcdc48ADN-B suggests that the chaperone may modulate signal transduction pathways and/or transcription factor levels essential for trichoblast/atrichoblast cell fate determination. More importantly, these in vivo dominant-negative mutant studies provided direct evidence that AtCDC48A is required for plant cytokinesis. Consistent with stereotypical plant cytokinesis defects (Lauber et al., 1997; Waizenegger et al., 2000; Strompen et al., 2002; Falbel et al., 2003; Kang et al., 2003), incomplete cell walls and cell wall stubs were observed in induced transgenic Atcdc48ADN-B root endodermal and cortical cells (Fig. 6, L and O). An intriguing feature of these cytokinesis defects is their prevalence in the endodermal and cortical cell layers and along their interface. The endodermis and cortex arise from a specific longitudinal division of a progenitor cell to give rise to two distinct cell type fates (Dolan et al., 1993). Based on our data, AtCDC48A may have a role in the completion of that longitudinal cell division. Future work is needed to determine the cell fate identity of the resulting cell types after ethanol treatment in the ethanol-inducible dominant-negative mutant plants.
YFP-AtCDC48A Localization
The presence of YFP-AtCDC48A signal in regions of cell proliferation and expansion are consistent with our hypothesis that AtCDC48A plays a role in cell division and expansion. In yeast and Xenopus, Cdc48p/p97 have been shown to be associated and to regulate mitotic spindle dynamics (Fröhlich et al., 1991; Cao et al., 2003; Cao and Zheng, 2004; Cheeseman and Desai, 2004). The mitotic spindle localization of AtCDC48A (Feiler et al., 1995) and YFP-AtCDC48A (this study) in plant cells provides additional support that the chaperone may function in plant mitosis.
Following mitosis, AtCDC48A is relocated to the phragmoplast mid-zone and during late telophase, with the reforming nuclear envelope. Our current hypothesis is that AtCDC48A is required for secretory and nuclear membrane dynamics to complete cell division. Consistent with this, CDC48/p97 have been shown to be required for secretory and nuclear membrane fusion and assembly in yeast and mammalian cells (Moir et al., 1982; Fröhlich et al., 1991; Hetzer et al., 2001; Sang and Ready, 2002).
In interphase cells, we observed that YFP-AtCDC48A was predominantly associated with the nucleus and, to a lesser extent, cytoplasm. The various specific patterns of nuclear-associated YFP-AtCDC48A localization observed within plant cell roots have not been reported in any other system. YFP-AtCDC48A was observed in discrete foci within the nucleoplasm and within the nucleolar cavity. The nucleoplasmic structures resemble nuclear bodies, such as speckles, which could implicate a function for AtCDC48A in mRNA processing and export (Pendle et al., 2005). The nucleolar cavity has been suggested to be the site of cleavage and modification of pre-ribosomal RNAs (Beven et al., 1995, 1996; Shaw and Brown, 2004). However, no detectable changes in 18S and 25S RNA processing were observed in Atcdc48A mutants line, indicating that the localization of AtCDC48A within the nucleolar cavity is likely unrelated to ribosomal RNA processing. The function of the AtCDC48A containing subnuclear structures remains to be determined.
AtCDC48A Protein Abundance Levels Are Self-Modulated
In addition to its roles in plant growth and development, dominant-negative expression of Atcdc48ADN-B, -H suggested that AtCDC48A may regulate its own steady-state protein level (Fig. 4). Our previous work demonstrates mutant Atcdc48ADN-B, -H protomers are incorporated into hexamers and are essentially ATPase inactive (Park et al., 2007). We found that the UBX-containing protein PUX1 functions to regulate the hexameric structure and ATPase activity of AtCDC48A (Rancour et al., 2004). However, PUX1 was unable to disassemble nucleotide binding or hydrolysis mutants of AtCDC48A complexes (Park et al., 2007). One model to explain the altered Atcdc48A mutant protein accumulation in planta is the inhibition of ATPase-defective hexamers to be disassembled (Park et al., 2007), a likely prerequisite for degradation. Yeast Cdc48p is polyubiquitylated (Mayor et al., 2005), suggesting that disassembly of the CDC48/p97 hexamer into individual subunits is a step in the pathway to its proteasome-dependent degradation. Taken together, these data support a model whereby a certain level or range of AtCDC48A activity is required for cell function and that AtCDC48A protein activity levels are self-maintained to maintain this homeostasis. Future experiments into how the active pool of AtCDC48A activity is sensed and subsequently balanced remain to be studied.
ATP Binding Is More Important Than ATP Hydrolysis for AtCDC48A Function
One subtle but reaffirmed observation was that plants expressing inducible Atcdc48ADN-B had a more severe phenotype than Atcdc48ADN-H during seedling growth and development. Given that expression levels of the mutant protein forms were comparable, this would suggest that there are functions for nucleotide substrate-bound form of the enzyme that are distinct from those requiring hydrolysis of that nucleotide substrate. A significant conformational change, including rearrangement of the N-terminal domains, occurs upon nucleotide binding to the CDC48/p97 hexamer (Rouiller et al., 2000). It has been hypothesized that the critical conformational change in the mammalian homolog of AtCDC48A, p97, is a large rearrangement upon nucleotide binding rather than the smaller changes associated with nucleotide hydrolysis. The location and number of molecules of the adaptor protein p47 molecules bound to p97 varies significantly in the nucleotide-free and ADP-bound states, suggesting that p47 requires ATP to form a tight complex with p97 (Dalal and Hanson, 2001). High affinity binding of substrates in the ATP-bound state has been proposed to be a common feature of AAA proteins (Vale, 2000). Our in vivo data is consistent with this proposal.
We conclude that AtCDC48A is required for many cellular processes necessary for plant growth and development. Our data provide new evidence for AtCDC48A function in plant cytokinesis and cell expansion and the ability of the enzyme to self-modulate its steady-state protein levels.
MATERIALS AND METHODS
Oligonucleotides Used in This Study
Oligonucleotide sequences shown in Table I were synthesized by Integrated DNA Technologies. Capitalized sequences represent those complementary to the AtCDC48A locus. Underlined lowercase letters in the oligonucleotide sequences indicate added restriction enzyme sites used for cloning.
Table I.
Oligonucleotides used in the study
| Name | Sequence (5′–3′) | Cloning/Screening |
|---|---|---|
| SB371 | tcagtatcagcggccgcAGTAATCCAAAAGTAGAG | 3′ H6T7-AtCDC48A cloning; NotI |
| SB372 | CAAACCAGCGTGGACCGCTTGCTGCAACT | Salk T-DNA left border |
| SB400 | ATTTGGGAGAAGTAGTTGG | 5′ AtCDC48A T-DNA insertion screening |
| SB401 | GTAGAACCACATAAGAATCC | 5′ AtCDC48A T-DNA insertion screening |
| SB439 | ggaattccatatgCATCATCATCATCATCAC | 5′ H6T7-AtCDC48A cloning; NdeI |
| SB504 | agagtcgacCATGGAGATTCCGAAGGTTGAAG | 5′ AtCDC48A promoter; SalI |
| SB513 | TGGATTCTTATGTGGTTCTAC | 5′ AtCDC48A T-DNA insertion screening |
| SB514 | CATTCAATAAAATTACACTCA | 3′ AtCDC48A T-DNA insertion screening |
| SB656 | catatgGATTGAGTTTGAGATTAACGAAAGAG | 3′ AtCDC48A promoter; NdeI |
| SB723 | gcggccgcCCATGGAGTCAAAGATTCAAATAG | 5′ Ethanol-inducible cassette cloning; NotI |
| SB737 | gcggccgcTCCTTAGCTCCTGAAAATCTCGAC | 3′ Ethanol-inducible cassette cloning; NotI |
Isolation of Atcdc48A T-DNA Insertion Mutants
Three independent Atcdc48A mutant lines containing T-DNA insertions (Atcdc48AT-DNA), SALK_064573, SALK_064893, and SALK_116074 (Alonso et al., 2003), were identified using T-DNA Express search algorithms from the Salk Institute Genomic Analysis Laboratory (SIGnAL, http://signal.salk.edu/cgi-bin/tdnaexpress), and seeds were obtained from the Arabidopsis Biological Resource Center (The Ohio State University, Columbus, OH). Atcdc48AT-DNA insertion lines were grown on solidified (0.6% phytoagar) Murashige and Skoog (Murashige and Skoog, 1962) medium (from either Gibco BRL or Caisson Laboratories) and transplanted into soil (either Superfine Germination Mix, Con-rad Fafard; or Metro Mix-360, Sun Gro Horticulture) treated with Adept (Uniroyal Chemical Company) and grown under fluorescent light illumination cycles of 16 h light and 8 h dark at 22°C. The mutant lines were screened for Atcdc48AT-DNA using oligonucleotide primers SB372, SB400, SB401, SB513, and SB514 (Table I). DNA sequencing of the PCR-amplified products determined the T-DNA insertion positions within AtCDC48A. The genotype of segregating plants was confirmed by PCR using allele-specific primer pairs. For analysis of AtCDC48AT-DNA mutant developing embryos, siliques were cleared in Hoyers solution (0.25 g [v/w] gum arabic, 3.3 g [v/w] chloral hydrate, 0.15 g [v/v] glycerol in deionized water; Meinke, 1994; Liu and Meinke, 1998) and observed with the bright-field light microscopy. To visualize cell organization in seedling roots, tissue was stained with 0.1 mg/mL propidium iodide for 30 s, washed four times with deionized water, and observed using laser scanning confocal microscopy (LSCM; Nikon Eclipse TE 2000-U, Japan). Captured images were processed using Image J 1.32 (Wayne Rasband, National Institutes of Health) and Adobe Photoshop and Illustrator (Adobe Systems) imaging software on Macintosh computers (Apple Computer).
In Vitro Pollen Germination
Pollen viability and germination was monitored as described (Li et al., 1999). Heterozygote Atcdc48A;qrt flowers were harvested and allowed to dehydrate at room temperature for 90 min at 22°C. Pollen grains were transferred to pollen germination media [18% (w/v) Suc, 0.6% (w/v) phytagar, 0.01% (w/v) boric acid, 1 mm MgSO4, 2.5 mm CaCl2, 2.5 mm Ca(NO2)2, pH 7.0] by tapping mature anthers on the surface of the pollen germination medium. Pollen grains were germinated at 28°C for 6 to 24 h and imaged with a Zeiss Axioskop (Carl Zeiss) equipped with DIC filter sets and a cooled charged-coupled device digital camera containing a 1,317- × 1,035-pixel array (Micromax, Princeton Instruments). Pollen grains were examined also by epifluorescence microscopy after staining with DAPI (100 mm sodium phosphate, pH 7.0, 1 mm EDTA, 0.1% [v/v] TX-100, 0.4 μg mL−1 DAPI) in both the epifluorescence (355-nm emission and 420-nm excitation filter set) and the DIC optics modes. Images were captured using IPLab Spectrum (Signal Analytics) and processed using Image J 1.32 and Adobe Photoshop/Illustrator imaging software.
Plant Transformation Vector Construction
A H6T7 epitope tag was generated as described (Rancour et al., 2004). The cDNA encoding H6T7-tagged AtCDC48A in pPZP211 was PCR amplified using primers SB439 and SB371, TA-cloned into pGEMT-easy (Promega), and verified by DNA sequence analysis. The AtCDC48A native promoter (754 bp upstream of the AtCDC48A start codon) was amplified by PCR with SB504 and SB656, restriction digested with SalI and NdeI, and subcloned into a SalI- and NdeI-digested H6T7-AtCDC48A-containing pGEMT-easy vector. The AtCDC48A promoter sequence was verified by DNA sequence analysis. The entire construct was subcloned as a SalI and KpnI restriction fragment into pPZP211 (Hajdukiewicz et al., 1994). To generate AtCDC48A promoter-EYFP-AtCDC48A fusion vector, EYFP was amplified with SB695 and SB683 from pCAM-35S-EYFP-C1 (Preuss et al., 2004) to replace the existing H6T7 tag of AtCDC48 in pGEMT-easy. This cassette was then subcloned into the SalI and KpnI sites of pPZP211. Images of plants expressing YFP-fused AtCDC48 were taken from an LSC microscope (Nikon) equipped with 40× and plan oil immersion 60× and 90× objectives using a 488-nm argon laser and 565 emission filter with 70-nm bandpass. Images were processed using Image J 1.32 (Wayne Rasband, National Institutes of Health) and Adobe Photoshop/Illustrator (Adobe Systems) imaging software on Macintosh computers (Apple Computer).
Generation of Ethanol-Inducible Dominant-Negative Mutants
The ethanol-inducible transcription factor and promoter from the alc cassettes of pSRN1 and pACN1 (pSRNACN_bin; Caddick et al., 1998) were amplified with SB723 and SB737. The amplified fragment was subcloned into the Bsp1201 site of the modified pPZP211 (Bsp1201, compatible with NotI, was engineered into the HindIII site of pPZP211; Scott Michaels, Indiana University) 5′ to H6T7-AtCDC48A. The double ATP hydrolysis and binding dominant-negative Atcdc48A (Atcdc48ADN) mutants were generated as described (Park et al., 2007). The constructs were introduced into wild-type plants (Arabidopsis [Arabidopsis thaliana] Columbia-2 ecotype) by a floral dip transformation method (Clough and Bent, 1998). Transgenic plants were selected on solidified (0.6% [w/v] phytoagar) Murashige and Skoog (Murashige and Skoog, 1962) medium containing 100 μg/mL kanamycin. Vertically grown T2 generations of ethanol-inducible wild-type and dominant-negative Atcdc48A seedlings (60 seedlings per 100- × 100- × 15-mm plate) were treated with 1 mL of 0.5%, 1%, and 2% ethanol at the bottom of plates 4 d after germination. Ethanol-treated plates were placed horizontally for 20 min and repositioned to vertical at 22°C. Root length was measured at 1, 2, and 4 d after ethanol treatment. Average and ses were calculated for groups of 24 seedlings. Images of seedling primary leaves and roots were obtained with a stereo microscope (Leica MZ6) equipped with a Leica DFC 480 digital camera (Leica Microsystems Imaging Solutions) 2 h, 6 h, 1 d, 2 d, and 4 d after ethanol treatment. Four days post-ethanol treatment, images of the first true leaves were taken using a Quanta 200 Environmental scanning electron microscope (SEM; FEI) under a 3.87-Torr vacuum using a 20-kV electron beam. Roots were observed by bright-field microscopy with a Zeiss Axioskop microscope, and digital micrograph images were captured with a cooled CCD digital camera and processed as above.
Analysis of Inducible H6T7-Atcdc48A Protein, YFP-AtCDC48A, and mRNA Expression
Total protein extracts were prepared from ethanol-inducible transgenic seedlings either mock treated or following the specified time post-ethanol treatment and from transgenic lines expressing YFP and YFP-AtCDC48A. Four seedlings were homogenized in 100 μL of 2× SDS-PAGE sample buffer (Laemmli, 1970) and incubated at 65°C for 15 min. The samples were cleared of insoluble debris by centrifugation at 16,000g for 5 min at room temperature, and 15 μL of the supernatant was resolved on a 12.5% (w/v) SDS-polyacrylamide minigel and analyzed by immunoblotting with anti-AtCDC48A (Rancour et al., 2002), anti-T7 (Novagen), and anti-YFP antibodies (Rockland; see Supplemental Fig. S1). Densitometric analysis of immunoblot film exposures was performed using a flatbed digital scanner and Image J 1.32 software.
Total RNA was isolated from mock-treated or, at specified times post-ethanol treatment, from whole ethanol-inducible, wild-type, and mutant Atcdc48ADN seedlings using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (2 μg) was treated with RQ DNAse (Promega) to eliminate genomic DNA. First-strand cDNA was generated using 1 μg of RQ DNAse-treated total RNA as template, oligo(dT), and Moloney murine leukemia virus reverse transcriptase (Promega) in a 20-μL reaction mixture. A 1-μL aliquot of a 20-fold dilution of the cDNA was subsequently PCR amplified (30 cycles) with the oligonucleotide primer pair for AtCDC48A (SB439 and SB36) and ubiquitin (SB747 and SB748) as a control.
Cross-Section Analysis of Wild-Type and Atcdc48DN Mutant Roots
The ethanol-inducible wild-type and mutant Atcdc48ADN seedlings, before and 4 d after 2% ethanol treatment, were vacuum infiltrated and fixed overnight at 4°C with 4% (v/v) gluteraldehyde in 50 mm potassium phosphate buffer, pH 7.0. Tissue was subsequently rinsed with 50 mm potassium phosphate, pH 7.0, buffer and dehydrated through a graded ethanol series (30%–100%). Tissue was embedded in LR White (EMS). Transverse sections (5 μm) were cut with a Reichert-Jung Ultracut model E microtome (Vienna) and stained with toluidine blue O as described previously (Kang et al., 2001).
Embryo DNA Isolation
Embryos were isolated from developing seed from siliques of self-fertilized Atcdc48AT-DNA/+ plants. DNA from individual embryos was isolated according to the following procedure. Isolated embryos were homogenized in 40 μL 250 mm NaOH followed by heating to 100°C for 30 s. To the sample, 20 μL of buffer (500 mm Tris-HCl, pH 8.0, 0.25% [v/v] NP-40) followed by 40 μL of 250 mm HCl were added, and the sample was mixed and incubated at 100°C for 2 min. Samples were cooled to ambient temperature and centrifuged at ambient temperature for 10 min at 16,000g. The supernatant was used directly for PCR analysis using primer sets for the gene (SB513 and SB514) and the T-DNA insertion (SB514 and SB372).
Analysis of Complementation of Atcdc48T-DNA Mutants with Native Promoter∷YFP-AtCDC48A cDNA
Complementation constructs were originally transformed into Atcdc48T-DNA/+ plants. DNA from T2 plants initially selected for the YFP-AtCDC48A complementation construct by fluorescence microscopic analysis was tested by PCR with primers specific for the uninterrupted endogenous gene (gene; primers SB513 and SB514) and the original T-DNA insertion into the AtCDC48A locus (T-DNA; primers SB514 and SB372). Those plants that tested initially as homozygous for the AtCDC48A locus T-DNA insertion were verified by PCR and the latter data are presented (Supplemental Fig. S2).
Immunoblot Analysis of the Expression of YFP-AtCDC48A in Wild-Type and Atcdc48AT-DNA Mutant Plants
Immunoblot analysis of total protein prepared from 4-d-old seedlings of transgenic lines expressing YFP alone in wild-type (AtCDC48A) plants, YFP-AtCDC48A in complemented Atcdc48T-DNA/Atcdc48T-DNA;YFP-AtCDC48A mutant plants, and untransformed wild-type AtCDC48A plants was performed. The immunoblots were probed using anti-GFP (Rockland Immunochemicals) and anti-AtCDC48 (Rancour et al., 2002) antibodies and detected via horseradish peroxidase-conjugates and chemiluminescence exposure to film.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. PCR genotyping of embryos from pale (P) and green (G) developing seed from self-fertilized Atcdc48T-DNA/+ plants.
Supplemental Figure S2. PCR verification of native promoter-driven YFP-AtCDC48A cDNA complementation of Atcdc48T-DNA mutant.
Supplemental Figure S3. Analysis of the expression of YFP-AtCDC48A in wild-type and Atcdc48A mutant plants
Supplemental Figure S4. AtCDC48 gene expression.
Supplemental Figure S5. Comparative phenotype of inducible ethanol-treated H6T7-WT, H6T7-DN-H, and H6T7-DN-B seedlings.
Supplemental Figure S6. Schematic of a wild-type Arabidopsis root.
Supplemental Movie S1. Native promoter∷YFP-AtCDC48A expression and localization in trichomes, leaves, and the shoot apical meristem of homozygous Atcdc48AT-DNA seedlings.
Supplemental Movie S2. The vegetative nucleus moves toward the growing tip of the pollen tub.
Supplemental Movie S3. YFP-AtCDC48A localizes to the nucleus membrane during karyokinesis.
Supplemental Movie S4. YFP-AtCDC48A localization in the nucleus.
Supplementary Material
Acknowledgments
We thank Adam Steinberg and Laura Vanderploeg for graphic arts assistance in preparation of the manuscript figures and movies, and members of our laboratory for discussion. We thank Dr. Sara Peterson for training in embedding and sectioning root tissues. SEM imaging was performed in the UW-Madison Plant Imaging Facility.
This work was supported by the National Science Foundation (grant nos. 0542034 [for Arabidopsis Biological Resource Center material distribution] and DBI–0421266), and by the Department of Energy, Division of Energy Biosciences (grant no. DE–FG02–ER20332 to S.Y.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sebastian York Bednarek (sybednar@wisc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Acharya U, Jacobs R, Peters JM, Watson N, Farquhar MG, Malhotra V (1995) The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell 82 895–904 [DOI] [PubMed] [Google Scholar]
- Aker J, Borst JW, Karlova R, de Vries S (2006) The Arabidopsis thaliana AAA protein CDC48A interacts in vivo with the somatic embryogenesis receptor-like kinase 1 receptor at the plasma membrane. J Struct Biol 156 62–71 [DOI] [PubMed] [Google Scholar]
- Aker J, Hesselink R, Engel R, Karlova R, Borst JW, Visser AJ, de Vries SC (2007) In vivo hexamerization and characterization of the Arabidopsis AAA ATPase CDC48A complex using forster resonance energy transfer-fluorescence lifetime imaging microscopy and fluorescence correlation spectroscopy. Plant Physiol 145 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Alzayady KJ, Panning MM, Kelley GG, Wojcikiewicz RJ (2005) Involvement of the p97-Ufd1-Npl4 complex in the regulated endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors. J Biol Chem 280 34530–34537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, Fiorani F, Inze D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8 154–158 [DOI] [PubMed] [Google Scholar]
- Beuron F, Dreveny I, Yuan X, Pye VE, McKeown C, Briggs LC, Cliff MJ, Kaneko Y, Wallis R, Isaacson RL, et al (2006) Conformational changes in the AAA ATPase p97-p47 adaptor complex. EMBO J 25 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuron F, Flynn TC, Ma J, Kondo H, Zhang X, Freemont PS (2003) Motions and negative cooperativity between p97 domains revealed by cryo-electron microscopy and quantised elastic deformational model. J Mol Biol 327 619–629 [DOI] [PubMed] [Google Scholar]
- Beven AF, Lee R, Razaz M, Leader DJ, Brown JW, Shaw PJ (1996) The organization of ribosomal RNA processing correlates with the distribution of nucleolar snRNAs. J Cell Sci 109 1241–1251 [DOI] [PubMed] [Google Scholar]
- Beven AF, Simpson GG, Brown JW, Shaw PJ (1995) The organization of spliceosomal components in the nuclei of higher plants. J Cell Sci 108 509–518 [DOI] [PubMed] [Google Scholar]
- Beyer A (1997) Sequence analysis of the AAA protein family. Protein Sci 6 2043–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick MX, Greenland AJ, Jepson I, Krause KP, Qu N, Riddell KV, Salter MG, Schuch W, Sonnewald U, Tomsett AB (1998) An ethanol inducible gene switch for plants used to manipulate carbon metabolism. Nat Biotechnol 16 177–180 [DOI] [PubMed] [Google Scholar]
- Cao K, Nakajima R, Meyer HH, Zheng Y (2003) The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell 115 355–367 [DOI] [PubMed] [Google Scholar]
- Cao K, Zheng Y (2004) The Cdc48/p97-Ufd1-Npl4 complex: its potential role in coordinating cellular morphogenesis during the M-G1 transition. Cell Cycle 3 422–424 [PubMed] [Google Scholar]
- Cheeseman IM, Desai A (2004) Cell division: AAAtacking the mitotic spindle. Curr Biol 14 R70–72 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dalal S, Hanson PI (2001) Membrane traffic: What drives the AAA motor? Cell 104 5–8 [DOI] [PubMed] [Google Scholar]
- Dalal S, Rosser MF, Cyr DM, Hanson PI (2004) Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol Biol Cell 15 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JM, Tsuruta H, May AP, Weis WI (2005) Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-Ray scattering. Structure 13 183–195 [DOI] [PubMed] [Google Scholar]
- DeLaBarre B, Brunger AT (2003) Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat Struct Biol 10 856–863 [DOI] [PubMed] [Google Scholar]
- DeLaBarre B, Brunger AT (2005) Nucleotide dependent motion and mechanism of action of p97/VCP. J Mol Biol 347 437–452 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119 71–84 [DOI] [PubMed] [Google Scholar]
- Dreveny I, Pye VE, Beuron F, Briggs LC, Isaacson RL, Matthews SJ, McKeown C, Yuan X, Zhang X, Freemont PS (2004) p97 and close encounters of every kind: a brief review. Biochem Soc Trans 32 715–720 [DOI] [PubMed] [Google Scholar]
- Egerton M, Samelson LE (1994) Biochemical characterization of valosin-containing protein, a protein tyrosine kinase substrate in hematopoietic cells. J Biol Chem 269 11435–11441 [PubMed] [Google Scholar]
- Falbel TG, Koch LM, Nadeau JA, Sack FD, Bednarek SY (2003) SCD1 is required for stomatal cell cytokinesis and polarized cell expansion in Arabidopsis. Development 130 4011–4024 [DOI] [PubMed] [Google Scholar]
- Feiler HS, Desprez T, Santoni V, Kronenberger J, Caboche M, Traas J (1995) The higher plant Arabidopsis thaliana encodes a functional CDC48 homologue which is highly expressed in dividing and expanding cells. EMBO J 14 5626–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AJ (2006) The co-ordination of cell division, differentiation and morphogenesis in the shoot apical meristem: a perspective. J Exp Bot 57 25–32 [DOI] [PubMed] [Google Scholar]
- Fröhlich KU, Fries HW, Rudiger M, Erdmann R, Botstein D, Mecke D (1991) Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J Cell Biol 114 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25 989–994 [DOI] [PubMed] [Google Scholar]
- Heim R, Tsien RY (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol 6 178–182 [DOI] [PubMed] [Google Scholar]
- Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW (2001) Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol 3 1086–1091 [DOI] [PubMed] [Google Scholar]
- Huyton T, Pye VE, Briggs LC, Flynn TC, Beuron F, Kondo H, Ma J, Zhang X, Freemont PS (2003) The crystal structure of murine p97/VCP at 3.6A. J Struct Biol 144 337–348 [DOI] [PubMed] [Google Scholar]
- Ikai N, Yanagida M (2006) Cdc48 is required for the stability of Cut1/separase in mitotic anaphase. J Struct Biol 156 50–61 [DOI] [PubMed] [Google Scholar]
- Jentsch S, Rumpf S (2007) Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem Sci 32 6–11 [DOI] [PubMed] [Google Scholar]
- Kang BH, Busse JS, Bednarek SY (2003) Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell 15 899–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Busse JS, Dickey C, Rancour DM, Bednarek SY (2001) The Arabidopsis cell plate-associated dynamin-like protein, ADL1a, is required for multiple stages of plant growth and development. Plant Physiol 126 47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature 388 75–78 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Lamb JR, Fu V, Wirtz E, Bangs JD (2001) Functional analysis of the trypanosomal AAA protein TbVCP with trans-dominant ATP hydrolysis mutants. J Biol Chem 276 21512–21520 [DOI] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jürgens G (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 139 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon A, McKearin D (1999) Identification of TER94, an AAA ATPase protein, as a Bam-dependent component of the Drosophila fusome. Mol Biol Cell 10 3825–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z (1999) Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Meinke DW (1998) The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J 16 21–31 [DOI] [PubMed] [Google Scholar]
- Lupas AN, Martin J (2002) AAA proteins. Curr Opin Struct Biol 12 746–753 [DOI] [PubMed] [Google Scholar]
- Mayor T, Lipford JR, Graumann J, Smith GT, Deshaies RJ (2005) Analysis of polyubiquitin conjugates reveals that the Rpn10 substrate receptor contributes to the turnover of multiple proteasome targets. Mol Cell Proteomics 4 741–751 [DOI] [PubMed] [Google Scholar]
- Meijer M, Murray JA (2001) Cell cycle controls and the development of plant form. Curr Opin Plant Biol 4 44–49 [DOI] [PubMed] [Google Scholar]
- Meinke DW (1994) Seed Development in Arabidopsis thaliana. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Meyer HH (2005) Golgi reassembly after mitosis: the AAA family meets the ubiquitin family. Biochim Biophys Acta 1744 108–119 [DOI] [PubMed] [Google Scholar]
- Moir D, Stewart SE, Osmond BC, Botstein D (1982) Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100 547–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Deinhardt K, Rosewell I, Warren G, Shima DT (2007) Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem Biophys Res Commun 354 459–465 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9 27–43 [PubMed] [Google Scholar]
- Park S, Rancour DM, Bednarek SY (2007) Protein domain-domain interactions and requirements for the negative regulation of Arabidopsis CDC48/p97 by the plant ubiquitin regulatory X (UBX) domain-containing protein, PUX1. J Biol Chem 282 5217–5224 [DOI] [PubMed] [Google Scholar]
- Pendle AF, Clark GP, Boon R, Lewandowska D, Lam YW, Andersen J, Mann M, Lamond AI, Brown JW, Shaw PJ (2005) Proteomic analysis of the Arabidopsis nucleolus suggests novel nucleolar functions. Mol Biol Cell 16 260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Harris JR, Lustig A, Muller S, Engel A, Volker S, Franke WW (1992) Ubiquitous soluble Mg(2+)-ATPase complex. A structural study. J Mol Biol 223 557–571 [DOI] [PubMed] [Google Scholar]
- Peters JM, Walsh MJ, Franke WW (1990) An abundant and ubiquitous homo-oligomeric ring-shaped ATPase particle related to the putative vesicle fusion proteins Sec18p and NSF. EMBO J 9 1757–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264 1458–1460 [DOI] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters JM, Warren G (1995) An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell 82 905–914 [DOI] [PubMed] [Google Scholar]
- Rancour DM, Dickey CE, Park S, Bednarek SY (2002) Characterization of AtCDC48. Evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol 130 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancour DM, Park S, Knight SD, Bednarek SY (2004) Plant UBX domain-containing protein 1, PUX1, regulates the oligomeric structure and activity of Arabidopsis CDC48. J Biol Chem 279 54264–54274 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Somerville CR (1998) Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J 15 79–88 [DOI] [PubMed] [Google Scholar]
- Rienties IM, Vink J, Borst JW, Russinova E, de Vries SC (2005) The Arabidopsis SERK1 protein interacts with the AAA-ATPase AtCDC48, the 14-3-3 protein GF14lambda and the PP2C phosphatase KAPP. Planta 221 394–405 [DOI] [PubMed] [Google Scholar]
- Römisch K (2006) Cdc48p is UBX-linked to ER ubiquitin ligases. Trends Biochem Sci 31 24–25 [DOI] [PubMed] [Google Scholar]
- Roslan HA, Salter MG, Wood CD, White MR, Croft KP, Robson F, Coupland G, Doonan J, Laufs P, Tomsett AB, et al (2001) Characterization of the ethanol-inducible alc gene-expression system in Arabidopsis thaliana. Plant J 28 225–235 [DOI] [PubMed] [Google Scholar]
- Rouiller I, Butel VM, Latterich M, Milligan RA, Wilson-Kubalek EM (2000) A major conformational change in p97 AAA ATPase upon ATP binding. Mol Cell 6 1485–1490 [DOI] [PubMed] [Google Scholar]
- Rouiller I, DeLaBarre B, May AP, Weis WI, Brunger AT, Milligan RA, Wilson-Kubalek EM (2002) Conformational changes of the multifunction p97 AAA ATPase during its ATPase cycle. Nat Struct Biol 9 950–957 [DOI] [PubMed] [Google Scholar]
- Roy L, Bergeron JJ, Lavoie C, Hendriks R, Gushue J, Fazel A, Pelletier A, Morre DJ, Subramaniam VN, Hong W, et al (2000) Role of p97 and syntaxin 5 in the assembly of transitional endoplasmic reticulum. Mol Biol Cell 11 2529–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang TK, Ready DF (2002) Eyes closed, a Drosophila p47 homolog, is essential for photoreceptor morphogenesis. Development 129 143–154 [DOI] [PubMed] [Google Scholar]
- Schiefelbein JW, Masucci JD, Wang H (1997) Building a root: the control of patterning and morphogenesis during root development. Plant Cell 9 1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuberth C, Buchberger A (2005) Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat Cell Biol 7 999–1006 [DOI] [PubMed] [Google Scholar]
- Shah K, Russinova E, Gadella TW Jr, Willemse J, De Vries SC (2002) The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes Dev 16 1707–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Brown JW (2004) Plant nuclear bodies. Curr Opin Plant Biol 7 614–620 [DOI] [PubMed] [Google Scholar]
- Shi J, Dixon RA, Gonzales RA, Kjellbom P, Bhattacharyya MK (1995) Identification of cDNA clones encoding valosin-containing protein and other plant plasma membrane-associated proteins by a general immunoscreening strategy. Proc Natl Acad Sci USA 92 4457–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strompen G, El Kasmi F, Richter S, Lukowitz W, Assaad FF, Jürgens G, Mayer U (2002) The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle-dependent manner. Curr Biol 12 153–158 [DOI] [PubMed] [Google Scholar]
- Vale RD (2000) AAA proteins. Lords of the ring. J Cell Biol 150 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger I, Lukowitz W, Assaad F, Schwarz H, Jürgens G, Mayer U (2000) The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr Biol 10 1371–1374 [DOI] [PubMed] [Google Scholar]
- Wang Q, Song C, Yang X, Li CC (2003) D1 ring is stable and nucleotide-independent, whereas D2 ring undergoes major conformational changes during the ATPase cycle of p97-VCP. J Biol Chem 278 32784–32793 [DOI] [PubMed] [Google Scholar]
- Woodman PG (2003) p97, a protein coping with multiple identities. J Cell Sci 116 4283–4290 [DOI] [PubMed] [Google Scholar]
- Yuan X, Shaw A, Zhang X, Kondo H, Lally J, Freemont PS, Matthews S (2001) Solution structure and interaction surface of the C-terminal domain from p47: a major p97-cofactor involved in SNARE disassembly. J Mol Biol 311 255–263 [DOI] [PubMed] [Google Scholar]
- Zhang X, Shaw A, Bates PA, Newman RH, Gowen B, Orlova E, Gorman MA, Kondo H, Dokurno P, Lally J, et al (2000) Structure of the AAA ATPase p97. Mol Cell 6 1473–1484 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hennig L, Gruissem W (2005) Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci 10 407–409 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.