Abstract
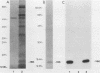
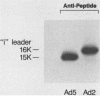
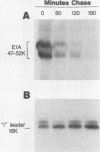
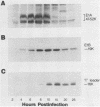
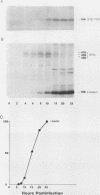
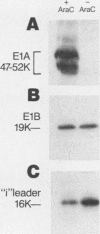
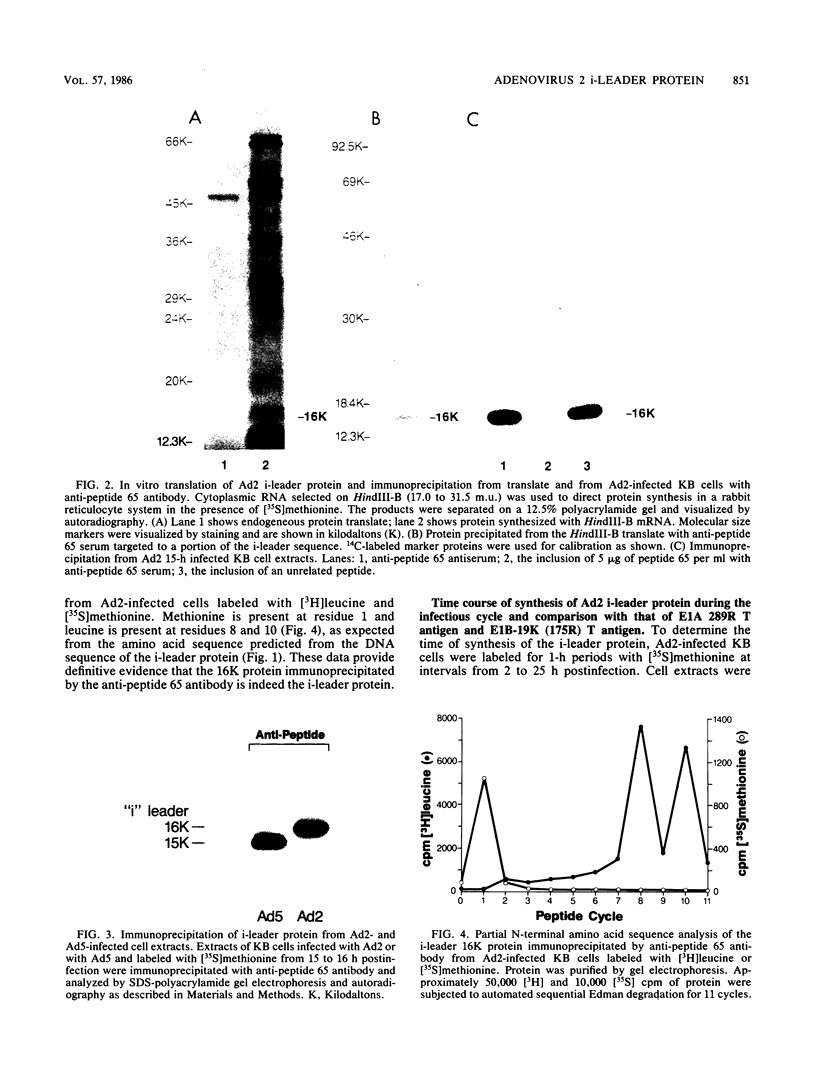
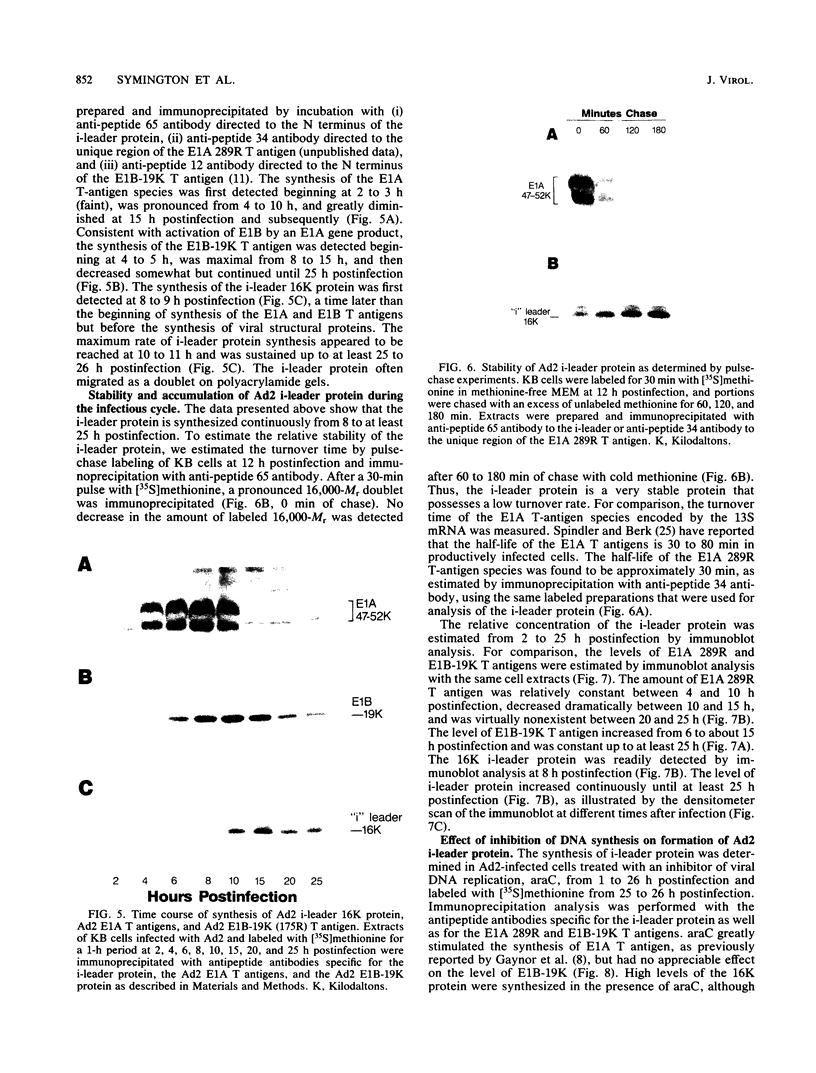
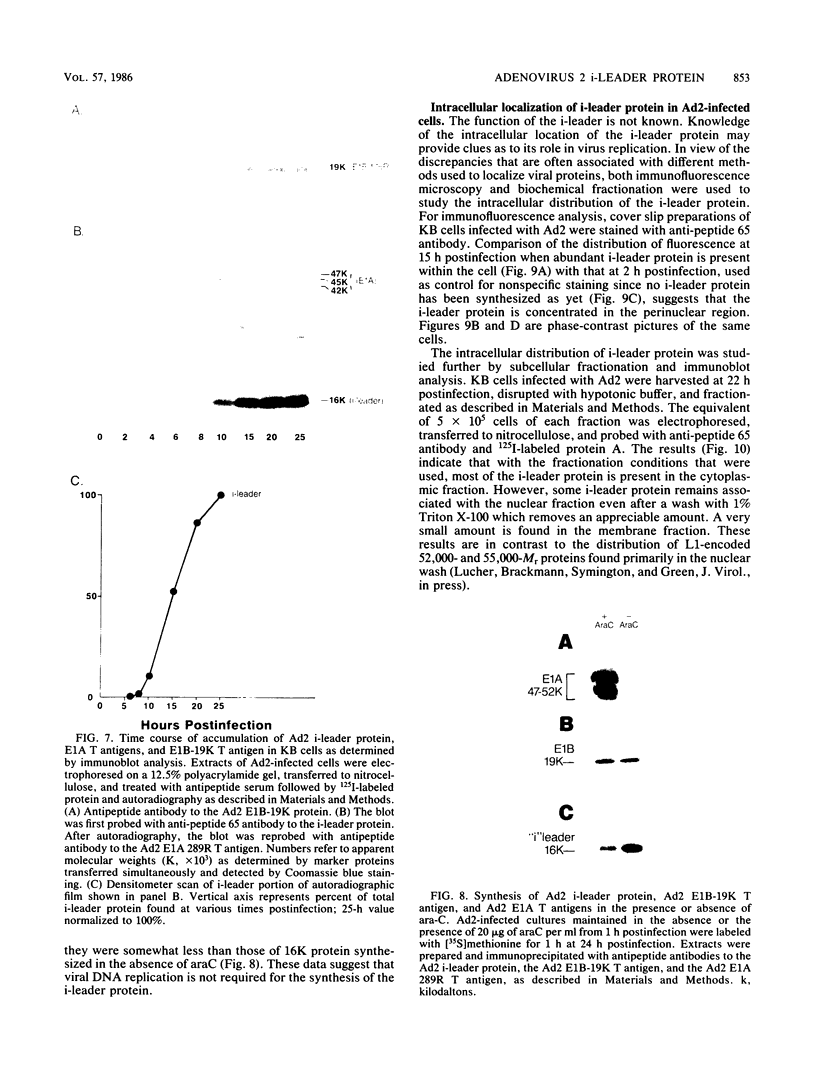
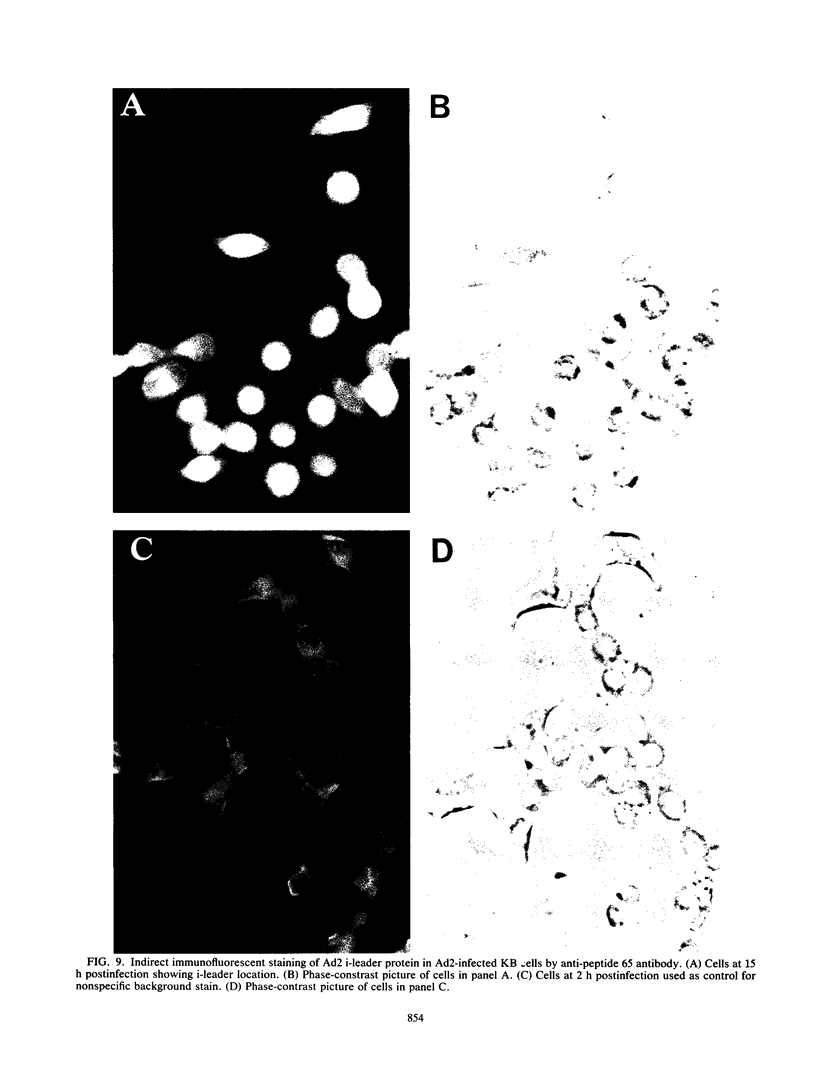
The i-leader is a 440-base-pair sequence located between 21.8 and 23.0 map units on the adenovirus type 2 genome and is spliced between the second and third segments of the major tripartite leader in certain viral mRNA molecules. The i-leader contains an open translational reading frame for a hypothetical protein of Mr about 16,600, and a 16,000-Mr polypeptide (16K protein) has been translated in vitro on mRNA selected with DNA containing the i-leader (A. Virtanen, P. Aleström, H. Persson, M. G. Katze, and U. Pettersson, Nucleic Acids Res. 10:2539-2548, 1982). To determine whether the i-leader protein is synthesized during productive infection and to provide an immunological reagent to study the properties and functions of the i-leader protein, we prepared antipeptide antibodies directed to a 16-amino acid synthetic peptide which is encoded near the N terminus of the hypothetical i-leader protein and contains a high acidic amino acid and proline content. Antipeptide antibodies immunoprecipitated from extracts of adenovirus type 2-infected cells a major 16K protein that comigrated with a 16K protein translated in vitro. Partial N-terminal amino acid sequence analysis by Edman degradation of radiolabeled 16K antigen showed that methionine is present at residue 1 and leucine is present at residues 8 and 10, as predicted from the DNA sequence, establishing that the 16K protein precipitated by this antibody is indeed the i-leader protein. Thus, the i-leader protein is a prominent species that is synthesized during productive infection. The i-leader protein is often seen as a doublet on polyacrylamide gels, suggesting that either two related forms of i-leader protein are synthesized in infected cells or that a posttranslational modification occurs. Time course studies using immunoprecipitation analysis with antipeptide antibodies revealed that the E1A 289R T antigen and the E1B-19K (175R) T antigen are synthesized beginning at 2 to 3 and 4 to 5 h postinfection, respectively, whereas the i-leader protein is synthesized starting at about 8 h postinfection and continues unabated until at least 25 h postinfection. The i-leader protein is very stable, as determined by pulse-chase labeling experiments, and accumulates continuously from 8 to 25 h postinfection, as shown by immunoblot analysis. The synthesis of i-leader protein does not depend upon viral DNA replication. Thus, the i-leader protein is a viral gene product of unknown function and high stability that is made in large quantities at intermediate times of productive infection.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Lewis J. B., Broker T. R. RNA transcription and splicing at early and intermediate times after adenovirus-2 infection. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):401–414. doi: 10.1101/sqb.1980.044.01.044. [DOI] [PubMed] [Google Scholar]
- Dekker B. M., van Ormondt H. The nucleotide sequence of fragment HindIII-C of human adenovirus type 5 DNA (map positions 17.1-31.7). Gene. 1984 Jan;27(1):115–120. doi: 10.1016/0378-1119(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Structure of a large segment of the genome of simian virus 40 that does not encode known proteins. Proc Natl Acad Sci U S A. 1977 Mar;74(3):827–831. doi: 10.1073/pnas.74.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey E., Ziff E. Sequence arrangement and protein coding capacity of the adenovirus type 2 "i" leader. J Virol. 1983 Jan;45(1):185–191. doi: 10.1128/jvi.45.1.185-191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Tsukamoto A., Montell C., Berk A. J. Enhanced expression of adenovirus transforming proteins. J Virol. 1982 Oct;44(1):276–285. doi: 10.1128/jvi.44.1.276-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Lucher L. A., Symington J. S., Kramer T. A. Human adenovirus 2 E1B-19K and E1B-53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J Virol. 1983 Dec;48(3):604–615. doi: 10.1128/jvi.48.3.604-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S., Brackmann K. H., Cartas M. A. Identification of families of overlapping polypeptides coded by early "transforming" gene region 1 of human adenovirus type 2. Virology. 1979 Sep;97(2):275–286. doi: 10.1016/0042-6822(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Jay G., Jay F. T., Friedman R. M., Levine A. S. Simian virus 40-specific ribosome-binding proteins induced by a nondefective adenovirus 2-simian virus 40 hybrid. J Virol. 1977 Sep;23(3):692–699. doi: 10.1128/jvi.23.3.692-699.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W. Proteins encoded near the adenovirus late messenger RNA leader segments. Virology. 1983 May;127(1):112–123. doi: 10.1016/0042-6822(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Fahnestock M. L., Hardy M. M., Anderson C. W. Presence in infected cells of nonvirion proteins encoded by adenovirus messenger RNAs of the major late transcription regions L0 and L1. Virology. 1985 Jun;143(2):452–466. doi: 10.1016/0042-6822(85)90385-x. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Kimelman D., Symington J. S., Brackmann K. H., Cartas M. A., Thornton H., Green M. Identification of adenovirus 12-encoded E1A tumor antigens synthesized in infected and transformed mammalian cells and in Escherichia coli. J Virol. 1984 Oct;52(1):136–144. doi: 10.1128/jvi.52.1.136-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. C., Mertz J. E., Sanden-Will S., Bina M. Simian virus 40 maturation in cells harboring mutants deleted in the agnogene. J Biol Chem. 1985 Jan 25;260(2):1127–1132. [PubMed] [Google Scholar]
- Nomura S., Khoury G., Jay G. Subcellular localization of the simian virus 40 agnoprotein. J Virol. 1983 Jan;45(1):428–433. doi: 10.1128/jvi.45.1.428-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Berk A. J. Rapid intracellular turnover of adenovirus 5 early region 1A proteins. J Virol. 1984 Nov;52(2):706–710. doi: 10.1128/jvi.52.2.706-710.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen A., Aleström P., Persson H., Katze M. G., Pettersson U. An adenovirus agnogene. Nucleic Acids Res. 1982 Apr 24;10(8):2539–2548. doi: 10.1093/nar/10.8.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]