Abstract
Metastatic melanoma cells express a number of protein tyrosine kinases (PTKs) that are considered to be targets for imatinib. We conducted a phase II trial of imatinib in patients with metastatic melanoma expressing at least one of these PTKs. Twenty-one patients whose tumours expressed at least one PTK (c-kit, platelet-derived growth factor receptors, c-abl, or abl-related gene) were treated with 400 mg of imatinib twice daily. One patient with metastatic acral lentiginous melanoma, containing the highest c-kit expression among all patients, had dramatic improvement on positron emission tomographic scan at 6 weeks and had a partial response lasting 12.8 months. The responder had a substantial increase in tumour and endothelial cell apoptosis at 2 weeks of treatment. Imatinib was fairly well tolerated: no patient required treatment discontinuation because of toxicity. Fatigue and oedema were the only grade 3 or 4 toxicities that occurred in more than 10% of the patients. Imatinib at the studied dose had minimal clinical efficacy as a single-agent therapy for metastatic melanoma. However, based on the characteristics of the responding tumour in our study, clinical activity of imatinib, specifically in patients with melanoma with certain c-kit aberrations, should be examined.
Keywords: phase II, imatinib, metastatic melanoma, protein tyrosine kinases, antiangiogenesis
Imatinib (STI-571, Gleevec; Novartis Pharmaceuticals, Basel, Switzerland) is a small-molecule inhibitor of protein tyrosine kinases (PTKs), including the Bcr–Abl fusion protein, c-abl, abl-related gene (ARG), platelet-derived growth factor receptor (PDGFR)-α, PDGFR-β, and the c-kit tyrosine kinase receptor (Druker and Lydon, 2000; Schindler et al, 2000; Okuda et al, 2001). In cell-based assays, imatinib selectively inhibits PDGF- and stem cell factor-mediated cellular signalling, including ligand-stimulated receptor autophosphorylation, inositol phosphate formation, and mitogen-activated protein kinase activation and proliferation (Buchdunger et al, 1995, 1996). These properties suggest that in addition to treating chronic myelogenous leukaemia and gastrointestinal stromal tumours, imatinib may be useful in the treatment of diseases that involve abnormal activation of c-kit, PDGFRs, or other PTKs.
We previously demonstrated differential expression of PTKs in paraffin-embedded human metastatic melanoma specimens (Shen et al, 2003). Out of 31 specimens examined by immunohistochemistry (IHC), 17 (55%) expressed c-kit, 30 (97%) expressed PDGFR-α, 21 (68%) expressed PDGFR-β, 23 (74%) expressed ARG, and 27 (87%) expressed c-abl. These findings indicate that a subset of patients with metastatic melanoma may have tumours that express imatinib's target PTKs. To evaluate the clinical efficacy of imatinib in patients with metastatic melanoma that expressed these PTKs, we conducted a phase II study of imatinib. We performed tumour biopsies before and during treatment to examine genetic mutations and PTK expression and to assess the effects of imatinib on apoptosis of tumour and tumour-associated endothelial cells.
Materials and methods
Patient selection
This was an open-label, phase II clinical trial. The protocol for this study was approved by the Institutional Review Board of The University of Texas M.D. Anderson Cancer Center. All patients gave written informed consent before enrollment.
Eligible patients were required to have histologically confirmed melanoma with measurable metastases and no more than one prior systemic treatment, not counting adjuvant interferon-α. A pretreatment tumour biopsy must have had at least 25% of the tumour cells stain positive by IHC for PDGFR-α, PDGFR-β, c-kit (CD117), c-abl, or ARG for patients to enroll in this trial. Patients in whom the brain was the only site of metastasis or patients who had symptomatic central nervous system metastases were excluded. However, patients with small asymptomatic brain metastases were eligible.
Treatment plan
Imatinib was administered orally at a dose of 400 mg twice a day until there was evidence of disease progression or unacceptable toxicity or until the patient refused to continue treatment. Because imatinib can irritate the gastrointestinal tract, patients were instructed to take the drug with meals. One course was defined as 6 weeks of treatment. All patients underwent tumour biopsies before treatment to assess target PTK expression by IHC. Patients were also asked to undergo a second biopsy during the second week of treatment for correlative studies.
Response evaluation
In addition to routine radiological assessment including computed tomography at intervals of 6 weeks, patients' disease was also evaluated by positron emission tomography (PET) at baseline and after 6 weeks of treatment. Clinical responses were evaluated using the international criteria proposed by the Response Evaluation Criteria in Solid Tumors Committee (Therasse et al, 2000). Responses were evaluated after every 6-week course. Overall clinical response included both complete and partial responses. Time to progression, duration of response, and overall survival were measured from the start of treatment.
Toxicity evaluation
Toxicities were evaluated and recorded according to the National Cancer Institute's Common Toxicity Criteria version 3.0. Patients were required to keep a daily record of adverse events and changes in concomitant medications. Complete blood counts and serum chemistry panels were obtained every 2 weeks.
Receptor kinase expression
Immunohistochemical staining protocols were used to detect c-kit, c-abl, ARG, PDGFR-α, and PDGFR-β in paraffin-embedded tumour specimens (Shen et al, 2003). A rabbit antihuman antibody against c-kit (dilution, 1 ng ml−1) was purchased from DAKO (Carpinteria, CA, USA); the rabbit (PDGFR-α, PDGFR-β) and goat (c-abl) anti-human antibodies against PDGFR-α, PDGFR-β (dilutions, 1 ng ml−1), c-abl (dilutions 1 : 200), and ARG (dilutions 1 : 200) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody staining was optimised using tissue sections before antibodies were applied to tissue microarray sections. PDGFR-α and PDGFR-β were retrieved by treatment with pepsin at 37°C for 10 min, c-kit was retrieved by microwave treatment for 5 min, and no antigen retrieval was performed for c-abl or ARG. All primary antibodies were applied to the slides and incubated at 4°C overnight. Diaminobenzidine was used as a chromogen. Both the percentage of positive cells and the intensity of staining were graded by two dermatopathologists. The scoring system for IHC staining for the PTKs was assigned in the following way. For the percentage of cells staining positively for PTK: 0, 0–5%; 1, 6–25%; 2, 26–75%; 3, >75%; for the intensity of staining: 1, weak; 2, moderate; 3, strong.
Tumour and endothelial cell apoptosis
Frozen tumour specimens were serially sectioned and stained for vessel count with an immunofluorescent antibody against the endothelial marker CD31. Apoptosis of tumour and endothelial cells was assessed by counting the number of cells (out of a total of 500) that stained positively with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL). Sections were superimposed on one another by computer imaging to determine the degree of endothelial cell apoptosis at the invasive edges of the tumours.
DNA sequencing
DNA was isolated from frozen tissue or from tissue stored in RNAlater (Ambion, Austin, TX, USA) using a QIAamp DNA minikit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RNA was extracted by standard methods using an RNeasy minicolumn (Qiagen) according to the manufacturer's instructions. cDNA was prepared using the two-step TaqMan reverse transcription reagent (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions, except that a primer specific for c-kit RNA (KIT 2961R, 5′-TTCCTGGAGGGGTGACCCAAACACT-3′) was used instead of random primers. The cDNA was then amplified by polymerase chain reaction. Nucleotide sequences were analysed with a 3730 × 1 DNA analyser (Applied Biosystems) at the M.D. Anderson Nucleic Acid Core Facility. The genomic DNA sequence of exons 9, 11, 13, 15, and, 17 was analysed as described previously (Chen et al, 2004).
Statistical analysis
The statistical design of the study was based on a modified two-stage Simon's design (Simon, 1989). Twenty-one patients were to be enrolled and treated in the first stage of the study and 20 in the second stage, summing up to 41 patients. If the response rate was 5% (1 out of 21 patients) or less at the end of the first stage, the study would be terminated. If two or more patients responded, accrual would continue to a total of 41 patients. If the response rate at the end of the study was 10% (4 out of 41) or less, the regimen would be rejected for further study as a single agent for metastatic melanoma. If the response rate was higher, the drug would be recommended for further study. These calculations assumed there was 90% or greater probability that the regimen would be recommended for further study if the true efficacy rate was 20% or greater, and less than 5% probability if the true efficacy rate was 5% or less.
Results
Patient characteristics
From January 2002 to October 2003, 22 patients were enrolled and 21 were treated with imatinib. One patient with early progressive central nervous system metastases was not treated. The pretreatment characteristics of the patients are listed in Table 1. All 21 patients were evaluable for response and toxicity. Two patients (10%) had stage III melanoma with multiple unresectable in-transit lesions, and all others (90%) had stage IV melanoma.
Table 1. Patient characteristics.
| Characteristic | No. of patients (%) |
|---|---|
| Total patients enrolled for the study | 22 |
| Total patients treated | 21 |
| Evaluable patients | 21 (100) |
| Sex | |
| Male | 13 (62) |
| Female | 8 (38) |
| Age (years) | |
| Median | 58 |
| Range | 33–83 |
| ECOG performance status | |
| 0 | 6 (29) |
| 1 | 14 (67) |
| 2 | 1 (5) |
| Disease stage | |
| III | 2 (10) |
| IV | 19 (90) |
| (M1a) | 2 (10) |
| (M1b) | 6 (29) |
| (M1c) | 11 (52) |
| Serum lactate dehydrogenase level | |
| Normal | 18 (86) |
| Higher than upper limit of normal | 3 (14) |
| Prior treatment | |
| None | 8 (38) |
| Interferon-α therapy (adjuvant) | 8 (38) |
| Isolated limb perfusion | 2 (10) |
| Biologic | 2 (10) |
| Chemotherapy | 4 (19) |
| Pathologic type of primary melanoma | |
| Superficial spreading melanoma | 5 (24) |
| Nodular melanoma | 2 (10) |
| Acral lentiginous melanoma | 2 (10) |
| Melanoma of the soft part | 1 (5) |
| Unknown primary | 3 (14) |
| Unclassified or unspecified | 8 (38) |
| Site of primary melanoma | |
| Upper extremities | 3 (14) |
| Thumb – terminal phalanx | 1 (5) |
| Lower extremities | 9 (43) |
| Plantar surface | 5 (24) |
| Scalp/face/neck | 2 (10) |
| Torso | 4 (19) |
| Unknown | 3 (14) |
| Site of metastases | |
| Dermis/subcutaneous tissue | 17 (81) |
| Lymph nodes | 10 (48) |
| Lung | 14 (67) |
| Liver | 3 (14) |
| Bone | 3 (14) |
| Brain | 3 (14) |
| Number of organs with metastases | |
| Median | 3 |
| Range | 1–5 |
ECOG=Eastern Cooperative Oncology Group.
All 22 patients enrolled had tumours expressing at least one target PTK. The baseline immunohistochemical data for the 21 treated patients who qualified the patients for enrollment are summarised in Table 2.
Table 2. Receptor expression and clinical response.
| Patient | c-kit | PDGFR-α | PDGFR-β | c-abl | ARG | Response |
|---|---|---|---|---|---|---|
| (A) Pretreatment | ||||||
| 1 | 3/2 | 0/0 | 2/1 | 0/0 | 0/0 | PD |
| 2 | 3/2 | 3/1 | 3/1 | 3/2 | 0/0 | SD |
| 3 | 0/0 | 0/0 | 3/1 | 2/1 | 0/0 | SD |
| 4 | 3/2 | 0/0 | 0/0 | 0/0 | 1/1 | PD |
| 5 | 3/1 | 0/0 | 3/1 | 0/0 | 0/1 | PD |
| 6 | 3/2 | 0/0 | 0/0 | 0/0 | 2/1 | SD |
| 7 | 3/2 | 3/2 | 3/2 | 3/1 | 0/0 | PD |
| 8 | 3/3 | 0/0 | 3/1 | 2/2 | 0/0 | PR |
| 9 | 3/2 | 3/2 | 3/3 | 3/2 | 2/1 | PD |
| 10 | 2/1 | 2/1 | 0/0 | 2/1 | 0/0 | PD |
| 11 | 2/2 | 0/1 | 0/1 | 2/3 | 2/3 | PD |
| 12 | 1/1 | 0/1 | 1/1 | 1/1 | 2/2 | PD |
| 13 | 3/2 | 0/0 | 1/1 | 2/1 | 1/1 | PD |
| 14 | 3/1 | 3/1 | 3/1 | 3/3 | 3/2 | PD |
| 15 | 3/2 | 2/1 | 2/1 | 2/1 | 0/0 | PD |
| 16 | 3/1 | 0/1 | 0/1 | 1/1 | 0/0 | PD |
| 17 | 2/2 | 2/1 | 3/1 | 2/2 | 1/1 | PD |
| 18 | 3/2 | 3/2 | 3/2 | 3/2 | 3/2 | PD |
| 19 | 2/1 | 3/3 | 2/1 | 1/1 | 0/0 | SD |
| 20 | 2/1 | 2/1 | 2/1 | 0/0 | 1/1 | PD |
| 21 | 0/0 | 2/1 | 1/1 | 2/1 | 0/0 | PD |
| Patient | c-kit | PDGFR-α | PDGFR-β | c-abl | ARG | Response |
| (B) During second week of treatment | ||||||
| 1 | 2/1↓ | 0/0– | 0/0↓ | 2/1↑ | 0/0– | PD |
| 2 | SD | |||||
| 3 | 0/0– | 0/0– | 0/0↓ | 2/2↑ | 2/1↑ | SD |
| 4 | 3/2– | 0/0– | 0/0– | 0/0– | 0/0↓ | PD |
| 5 | PD | |||||
| 6 | 3/2– | 0/0– | 0/0– | 2/1↑ | 2/1– | SD |
| 7 | PD | |||||
| 8 | 3/3– | 0/0– | 0/0↓ | 0/0↓ | 0/0– | PR |
| 2/2 * | 0/0 * | 0/0 * | 0/0 * | 0/0 * | ||
| 9 | 0/0↓ | 1/1↓ | 1/1↓ | 1/1↓ | 0/0↓ | PD |
| 10 | 2/2↑ | 1/1↓ | 0/0– | 2/1– | 0/0– | PD |
| 11 | 3/2↑ | 0/2↑ | 2/1↑ | 2/1↓ | 3/1– | PD |
| 12 | 2/2↑ | 0/1– | 3/2↑ | 0/0↓ | 1/1↓ | PD |
| 13 | 0/0↓ | 0/1↑ | 0/0↓ | 0/0↓ | 0/1↓ | PD |
| 14 | PD | |||||
| 15 | PD | |||||
| 16 | PD | |||||
| 17 | PD | |||||
| 18 | 1/1↓ | 0/0↓ | 0/0↓ | 0/1↓ | 0/1↓ | PD |
| 19 | 0/0↓ | 0/0↓ | 0/0↓ | 0/0↓ | 0/0– | SD |
| 20 | PD | |||||
| 21 | 1/1↑ | 1/2– | 0/1↓ | 2/1– | 0/0– | PD |
ARG=abl-related gene; PD=progressive disease; PDGFR=platelet-derived growth factor receptor; PR=partial response; SD=stable disease.
The first number is an indicator of the percentage of cells staining positive for the protein tyrosine kinase (PTK) (0, 0–5%; 1, 6–25%; 2, 26–75%; 3, >75%); the second number denotes the staining intensity (1, weak; 2, moderate; 3, strong).
Blank spaces indicate no tissue available for analysis.
↑ denotes increase in protein expression during treatment.
↓ denotes decrease in protein expression during treatment.
– denotes no change in protein expression during treatment.
* denotes PTK expression at the time of relapse (patient 8).
Treatment
The 21 patients received a total of 33 courses of imatinib (median, one course per patient; range, 1–9). Three patients were unable to complete the first course of therapy because of rapid disease progression; one of them died of a progressing haemorrhagic brain metastasis. Thirteen and four patients completed one and two courses, respectively. The sole patient with responding disease tolerated nine consecutive courses of treatment over a period of 1 year. One patient with a history of rheumatoid arthritis required a dose reduction to 300 mg twice a day because of grade 3 polyarthritis. No other patient required a dose reduction.
Responses
Clinical responses are listed in Table 2. Among the 21 patients treated, 4 had disease stabilisation, which lasted no more than 12 weeks. Only one patient (5%) had a partial response, which lasted for 12.8 months. That patient was a 66-year-old man whose disease had progressed while he had been receiving intratumoral HLA-B7 plasmid gene transfer treatment. He had a near-complete response to imatinib in numerous metastases of the cutaneous and subcutaneous tissues, inguinal and iliac lymph nodes, and lungs. After just 6 weeks of treatment, a PET scan revealed a marked reduction of metabolic activity in all disease sites (Figure 1). Follow-up computed tomography (CT) scans did not confirm ongoing major response in this patient until after 12 weeks of treatment. Serial physical exams with photographs confirmed gradual depigmentation and complete resolution of all palpable skin nodules and follow-up CT scans showed near-complete resolution in nodal and lung metastases after 6 months. The treatment was discontinued when he developed new brain metastases and a recurrence in the lymph nodes. This patient was later treated with temozolomide and paclitaxel achieving a brief partial response, but subsequently died of disease progression 26 months after the beginning of imatinib treatment.
Figure 1.
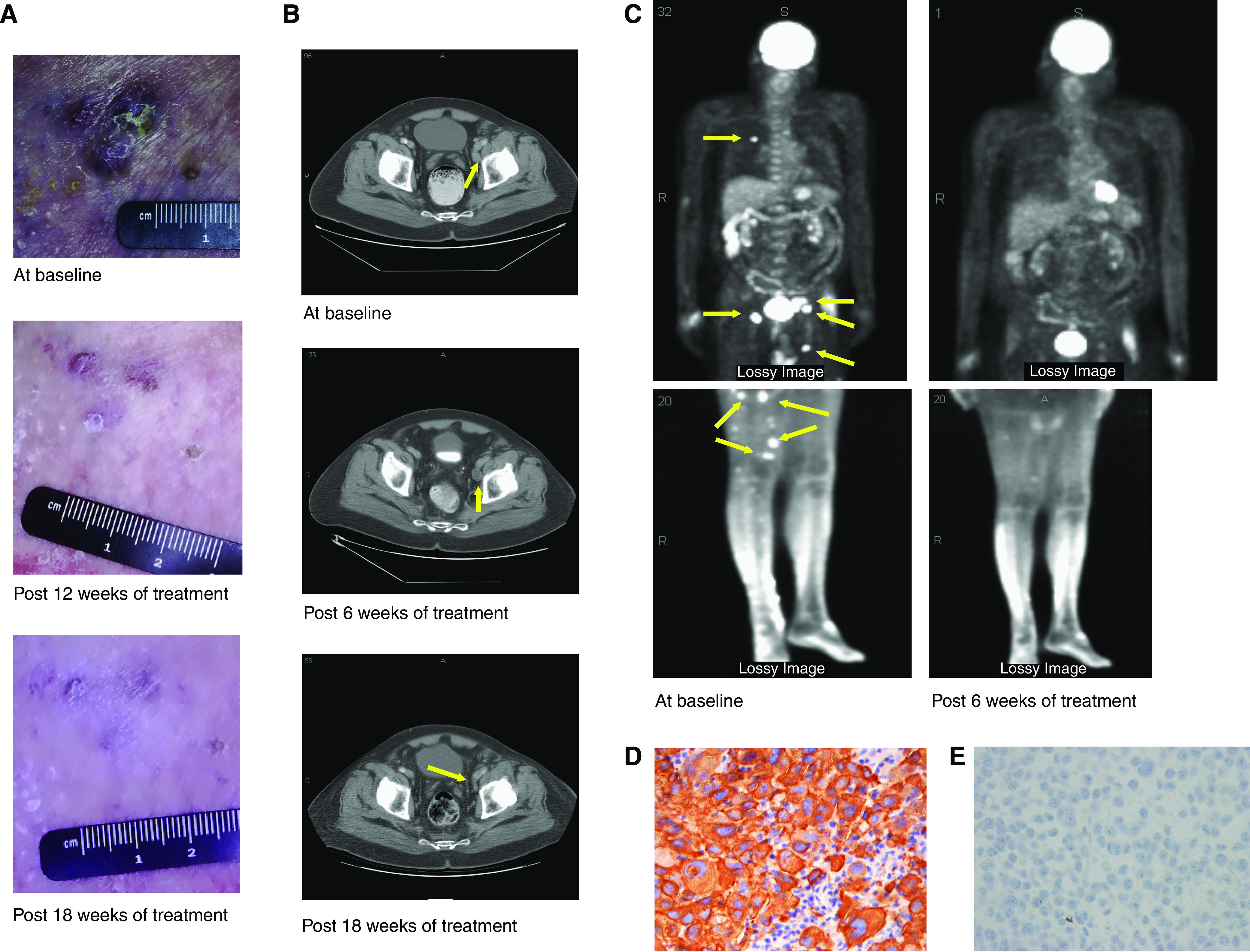
Clinical and radiological studies of a partial response to imatinib. All metastatic lesions shrank. (A) Response of in-transit metastases on right thigh. (B) Computed tomographic scan showing response of left external iliac lymph node (arrow). (C) Positron emission tomographic scans showing decrease in fluorodeoxyglucose uptake in all lesions (arrows). (D) Photomicrograph of the strong c-kit expression in the tumour of the responder. (E) Photomicrograph of a case of negative c-kit expression.
With progression of disease confirmed in all patients, the median time to disease progression was 1.4 months (range, 0.6–12.8), and the median overall survival duration was 7.5 months (range, 0.7–25.8+), with two patients alive at 19 months.
Toxicity
Adverse events are listed in Table 3 by toxicity grade and frequency. No patient required treatment discontinuation for toxicity. Fatigue and oedema were the only grade 3 or 4 adverse events that occurred in more than 10% of the patients, and these were reversible upon discontinuation of treatment.
Table 3. Toxicities observed with imatinib treatment (number of patients with toxicity).
| Adverse event | Grade 1/2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Fatigue | 14 | 3 | 1 |
| Anaemia | 12 | — | 1 |
| Oedema | 13 | 3 | — |
| Nausea | 13 | 2 | — |
| Muscle weakness | 6 | 2 | — |
| Anorexia | 14 | 1 | — |
| Lymphopoenia | 10 | 1 | — |
| Vomiting | 9 | 1 | — |
| Skin erythema/pruritis | 9 | 1 | — |
| Constipation | 7 | 1 | — |
| Dyspepsia | 7 | 1 | — |
| Neutropoenia | 4 | 1 | — |
| Ocular effects (visual changes) | 8 | — | — |
| Arthralgia | 1 | 1 | — |
| Dyspnoea | 7 | — | — |
| Diarrhoea | 7 | — | — |
| Pleural effusion | 7 | ||
| Diaphoresis | 6 | — | — |
| Cough | 6 | — | — |
| Haemorrhage | 6 | — | — |
| Thrombocytopoenia | 3 | — | — |
| Fever | 3 | — | — |
| Serum creatinine elevation | 3 | — | — |
| Peripheral neuropathy | 2 | — | — |
| Petechia | 2 | — | — |
| Ascites | 1 | — | — |
Correlative studies
We examined PTK expression in melanoma cells at baseline and during the second week of treatment (Table 2). Among the 21 patients treated, all with evaluable baseline tumour specimens, 16 also had specimens obtained during the second week of treatment. Thirteen of the second-week specimens were evaluable for PTK expression. With only one major response, no definite association could be made between the baseline immunohistochemical data and the observed clinical activity (Table 2A). Nevertheless, the patient with the major response had strong c-kit receptor staining in over 75% of the tumour cells – the strongest staining pattern observed among all the pretreatment specimens.
Among the evaluable 13 paired baseline and follow-up specimens, there were no consistent staining pattern changes observed after 2 weeks of treatment. Most often, however, there was a decrease in PTK receptor staining intensity (Table 2B). In the patient with the major response, c-kit receptor staining did not change, and there was complete loss of PDGFR-β and abl staining 2 weeks into treatment.
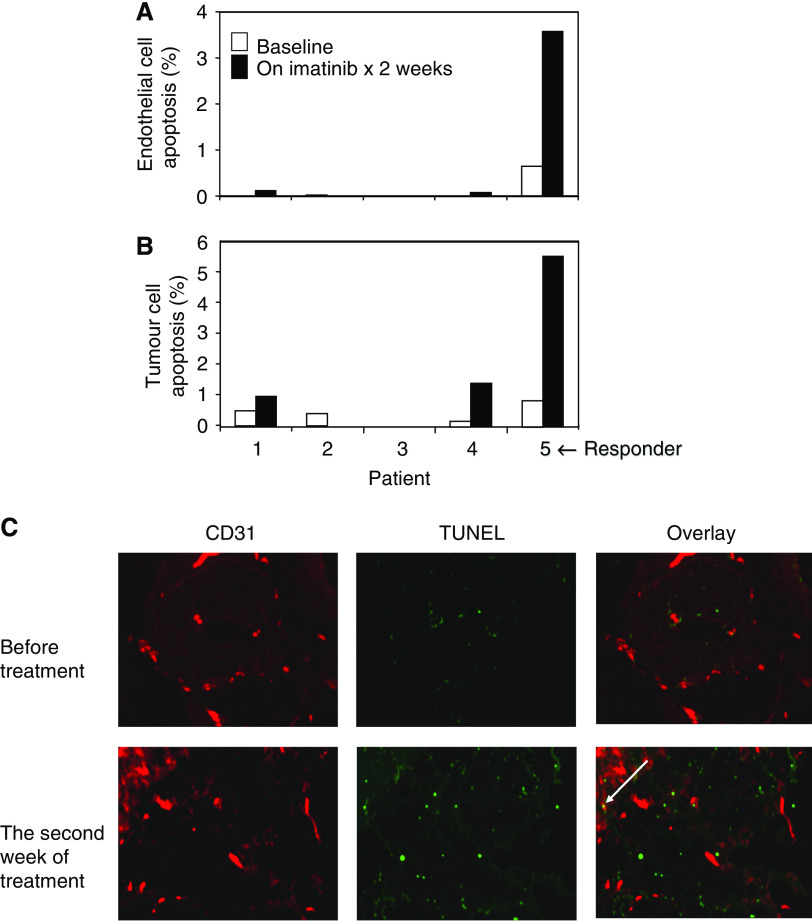
The responding patient's tumours also showed an increase in the percentage of apoptotic endothelial and tumour cells after only 2 weeks of imatinib treatment, based on the CD31-TUNEL dual immunofluorescence studies, suggesting a very early impact of treatment on the tumour specimen (Figure 2).
Figure 2.
Tumour and endothelial cell apoptosis before treatment and during the second week of imatinib treatment. (A) Tumour cell apoptosis in five patients; patient 5 had a partial response. (B) Endothelial cell apoptosis in five patients. (C) Photomicrographs of the TUNEL stains for both melanoma and endothelial cells in the responder. Red in the CD31 column represents the endothelial cells, and green in the TUNEL column represents apoptotic cells. Yellow (indicated by an arrow) in the overlay column represents endothelial cells undergoing apoptosis.
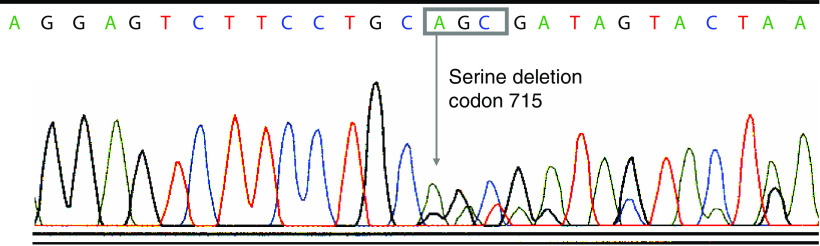
We analysed pretreatment tumour samples from five patients, including the only responder, for c-kit mutation for both genomic DNA and cDNAs. None of our melanoma specimens analysed, including those from the responding patient, contained genomic mutations of c-kit in exon 9 or 11, which is frequently mutated in imatinib-responsive gastrointestinal stromal tumours (Heinrich et al, 2003). Genomic sequences of c-kit in exons 13, 15, and 17 were also analysed, and none of the specimens contained mutations in these sites. Of interest, the cDNA to RNA from the responder's tumour displayed alternative splicing in exon 15 of the c-kit kinase II domain (Figure 3). This alternate splice form had a 3 bp deletion of the codon for serine (codon 715) at the very beginning of exon 15. The alternate splice form encodes the SER715− isoform of c-kit (Crosier et al, 1993; Lasota et al, 2002). However, this finding was not specific for the responding patient; four non-responding patients also had this alternative splice in their tumours.
Figure 3.
Chromatogram of the sequence for the alternative splicing seen in c-kit mRNA from the responding patient. The c-kit cDNA sequence from the responder displayed a deletion of the first three nucleotides of exon 15, which encoded a serine (codon 715).
There was no evidence of an immunological infiltrate in the responding metastases.
Discussion
In this trial, imatinib was given orally at 400 mg twice a day without interruption. Wyman et al (2006) reported that imatinib administered at 400 mg twice a day caused unexpectedly severe toxicity with frequent grade 3 or 4 adverse events, requiring dose reduction and even treatment cessation. In another phase II study of imatinib, in which 18 patients with treatment-refractory advance melanoma were treated, Ugurel et al (2005) showed that there was no clinical response among 16 evaluable patients. However, in our study, 400 mg of imatinib given twice a day was fairly well tolerated. The toxicity of imatinib observed in our study was similar to previously reported clinical studies (van Oosterom et al, 2001; Verweij et al, 2003). Most adverse events, especially skin rash and pleural effusion, were self-limiting without drug discontinuation.
In our study, there was early progression of disease in the majority of patients and only one partial response, precluding the study from entering the second stage of accrual. This lack of efficacy was also observed in the other clinical trial testing the same dose and schedule of imatinib (Wyman et al, 2006). In that trial, the 26 patients enrolled with metastatic melanoma had a higher percentage of visceral metastases (stage M1c) and had received more prior systemic therapy compared with the patients enrolled in our study (58 vs 32% and 73 vs 27%, respectively). More grade 3 or 4 nausea, vomiting, fatigue, fluid retention with oedema, anaemia, hyponatraemia, and hypoglycaemia were observed in the second trial, perhaps as a result of these poorer prognostic characteristics. No clinical responses were reported. Hence, there has been only one partial response reported among the 46 evaluable patients treated in these two trials for an aggregate response rate of 2% and a median time to progression of less than 8 weeks.
Since all tumours screened in our study expressed at least one of the five target PTKs, based on positive immunohistochemical staining in at least 25% of the cells, the 25% threshold was not selective for predicting clinical response. Furthermore, there was only one clinical response among the 21 patients; therefore, positive IHC results, as defined in our study, were not predictive of response to imatinib. Nevertheless, in tumours from the one patient who had a meaningful and prolonged clinical response, over 75% of the cells had marked c-kit staining. None of the other patients' tumours had such intense staining; thus the threshold for positive staining, if used to select patients for treatment, could be set at a higher value in future studies. On the other hand, no change in the strong c-kit staining during the second week of treatment in the responding melanoma could be evidence that total expression of c-kit is not the critical marker for imatinib activity. Similarly, the decrease in staining intensity for PDGFR-β and c-abl in the responding tumour could be discounted as important leads as this pattern of staining was also seen in the non-responding specimens.
Despite the lack of correlative biological changes observed with imatinib in this patient, we believe that the lone response was not a coincidence because the response was observed in multiple sites including multiple in-transit lesions, deep inguinal nodes, and lung, and lasted more than a year on therapy. In addition, it is worth noting that the responder's primary disease was acral lentiginous melanoma. Recently, Curtin et al (2006) demonstrated that nearly 30–40% subtypes of melanoma, such as acral lentiginous, mucosal melanoma, or cutaneous melanoma, on chronic sun-damaged skin, harbour c-kit mutation and/or aberration, such as a gene amplification or an increased gene copy number. Although no genomic mutation in c-kit was found in the tumour of the responder in our trial, it is possible that the tumour contained either an increased c-kit gene copy number or its gene amplification, which might have activated the c-kit signal pathway. We found the alternative splice in exon 15, which encodes the SER715− isoform of c-kit in tumour samples, but this variant, which has been reported in other tumour types by a number of investigators (Crosier et al, 1993; Ashman, 1999; Lasota et al, 2002), did not predict the response to imatinib.
The increase in apoptosis of both tumour and endothelial cells in the responding patient implies that imatinib may effectively inactivate important signal transduction pathways in endothelial cells as well. For instance, PDGFRs are known to be expressed both on melanoma and on endothelial cells (Beitz et al, 1991; Barnhill et al, 1996; Shen et al, 2003). The nature of the imatinib-sensitive signals that mediate survival of endothelial cells is being investigated. We are currently investigating the phosphorylation status of the target PTKs and the effect of imatinib on the inactivation of these targets in melanoma and endothelial cells of tumours from the patients in this study.
As reported in CML and GIST, resistance to imatinib was observed in the responder after 1 year of treatment. Many mechanisms have been suggested for this as well as ways to circumvent evolution of resistance in a previously exploitable pathway, including, but not limited to, substituting other PTK inhibitors or combining imatinib with other agents that affect other signal transduction steps. In addition, imatinib resistance might result from kinase-activating mutations of B-Raf, which is downstream of the target receptor kinases of imatinib. Davies et al (2002) recently showed that approximately 66% of melanomas contain somatic missense mutations, located within the kinase domain of B-Raf, with the ability to transform NIH3T3 cells. These mutations can constitutively activate the mitogen-activated protein kinase pathway, regardless of imatinib's ability to inactivate upstream receptor kinases. It may be necessary to select patients without such B-Raf mutations to increase the rate of clinical response to imatinib in patients with metastatic melanoma.
There is also increasing evidence that combinations of imatinib and chemotherapeutic agents, such as taxane or gemcitabine, have synergistic antitumour activity in vitro and in vivo (Pietras et al, 2002; Hwang et al, 2003; Mathew et al, 2004).
In conclusion, single-agent imatinib had only minimal clinical efficacy in patients with metastatic melanoma. The success of imatinib as a targeted therapy for advanced melanoma will depend on more stringent selection of patients with exploitable tumour targets and its use either sequentially or in combination with other agents. Currently, imatinib is under clinical investigation in patients with advanced melanoma harbouring somatic alterations of c-kit, and its result is eagerly anticipated.
Acknowledgments
This study was supported by National Cancer Institute Grants N01 CM-17003 and CA16672, and Core Grant no. CA16672.
References
- Ashman LK (1999) The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol 31: 1037–1051 [DOI] [PubMed] [Google Scholar]
- Barnhill RL, Xiao M, Graves D, Antoniades HN (1996) Expression of platelet-derived growth factor (PDGF)-A, PDGF-B and the PDGF-α receptor, but not the PDGF-β receptor, in human malignant melanoma in vivo. Br J Dermatol 135: 898–904 [DOI] [PubMed] [Google Scholar]
- Beitz JG, Kim IS, Calabresi P, Frackelton Jr AR (1991) Human microvascular endothelial cells express receptors for platelet-derived growth factor. Proc Natl Acad Sci USA 88: 2021–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ, Lydon NB (1996) Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res 56: 100–104 [PubMed] [Google Scholar]
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Regenass U, Lydon NB (1995) Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc Natl Acad Sci USA 92: 2558–2562 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen LL, Trent JC, Wu EF, Fuller GN, Ramdas L, Zhang W, Raymond AK, Prieto VG, Oyedeji CO, Hunt KK, Pollock RE, Feig BW, Hayes KJ, Choi H, Macapinlac HA, Hittelman W, Velasco MA, Patel S, Burgess MA, Benjamin RS, Frazier ML (2004) A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res 64: 5913–5919 [DOI] [PubMed] [Google Scholar]
- Crosier PS, Ricciardi ST, Hall LR, Vitas MR, Clark SC, Crosier KE (1993) Expression of isoforms of the human receptor tyrosine kinase c-kit in leukemic cell lines and acute myeloid leukemia. Blood 82: 1151–1158 [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, Bastian BC (2006) Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 24: 4340–4346 [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Druker BJ, Lydon NB (2000) Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest 105: 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21: 4342–4349 [DOI] [PubMed] [Google Scholar]
- Hwang RF, Yokoi K, Bucana CD, Tsan R, Killion JJ, Evans DB, Fidler IJ (2003) Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res 9: 6534–6544 [PubMed] [Google Scholar]
- Lasota J, Kopczynski J, Majidi M, Miettinen M, Sarlomo-Rikala M (2002) Apparent KIT Ser(715) deletion in GIST mRNA is not detectable in genomic DNA and represents a previously known splice variant of KIT transcript. Am J Pathol 161: 739–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew P, Fidler IJ, Logothetis CJ (2004) Combination docetaxel and platelet-derived growth factor receptor inhibition with imatinib mesylate in prostate cancer. Semin Oncol 31: 24–29 [DOI] [PubMed] [Google Scholar]
- Okuda K, Weisberg E, Gilliland DG, Griffin JD (2001) ARG tyrosine kinase activity is inhibited by STI571. Blood 97: 2440–2448 [DOI] [PubMed] [Google Scholar]
- Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A (2002) Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res 62: 5476–5484 [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J (2000) Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289: 1938–1942 [DOI] [PubMed] [Google Scholar]
- Shen SS, Zhang PS, Eton O, Prieto VG (2003) Analysis of protein tyrosine kinase expression in melanocytic lesions by tissue array. J Cutan Pathol 30: 539–547 [DOI] [PubMed] [Google Scholar]
- Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10: 1–10 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Ugurel S, Hildenbrand R, Zimpfer A, La Rosee P, Paschka P, Sucker A, Keikavoussi P, Becker JC, Rittgen W, Hochhaus A, Schadendorf D (2005) Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer 92: 1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, Silberman S, Nielsen OS (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358: 1421–1423 [DOI] [PubMed] [Google Scholar]
- Verweij J, van Oosterom A, Blay JY, Judson I, Rodenhuis S, van der Graaf W, Radford J, Le Cesne A, Hogendoorn PC, di Paola ED, Brown M, Nielsen OS (2003) Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 39: 2006–2011 [PubMed] [Google Scholar]
- Wyman K, Atkins MB, Prieto V, Eton O, McDermott DF, Hubbard F, Byrnes C, Sanders K, Sosman JA (2006) Multicenter phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer 106: 2005–2011 [DOI] [PubMed] [Google Scholar]