Abstract
Cancer is the leading cause of disease-related death in teenagers and young adults aged 13–24 years (TYAs) in England. We have analysed national 5-year relative survival among more than 30 000 incident cancer cases in TYAs. For cancer overall, 5-year survival improved from 63% in 1979–84 to 74% during 1996–2001 (P<0.001). However, there were no sustained improvements in survival over time among high-grade brain tumours and bone and soft tissue sarcomas. Survival patterns varied by age group (13–16, 17–20, 21–24 years), sex and diagnosis. Survival from leukaemia and brain tumours was better in the youngest age group but in the oldest from germ-cell tumours (GCTs). For lymphomas, bone and soft tissue sarcomas, melanoma and carcinomas, survival was not significantly associated with age. Females had a better survival than males except for GCTs. Most groups showed no association between survival and socioeconomic deprivation, but for leukaemias, head and neck carcinoma and colorectal carcinoma, survival was significantly poorer with increasing deprivation. These results will aid the development of national specialised service provision for this age group and identify areas of clinical need that present the greatest challenges.
Keywords: cancer survival, trends, Teenagers and Young Adults, national cancer statistics, socioeconomic deprivation
In 2004 in England, over 50% of cancers were diagnosed at age 70 years and above, with only 0.5% in 15–24-year-olds (Office for National Statistics, 2006). However, cancer is the leading disease-related cause of death in this age range (Geraci et al, 2007). There is growing recognition that young cancer patients have special physical, social and educational needs in addition to appropriate disease-specific treatment. Risks of developing treatment-induced second malignancies and organ dysfunction are critical considerations in the young. Loss of fertility and other organ-specific cytotoxic effects and disruption to education, vocational and professional training can have a profound influence on future life (National Collaborating Centre for Cancer, 2005).
Detailed national survival data for this age group have not been reported hitherto but are important for service planning and as a baseline for monitoring progress. We now describe survival trends over time and patterns of survival by age, sex and socioeconomic deprivation for a 23-year series of 13- to 24-year-olds with cancer in England.
Materials and methods
Teenagers and young adults aged 13–24 years (TYAs) diagnosed with malignancy in England, during the period 1979–2001, followed up to 31 December 2003, were included in this study. National cancer registration data on individual eligible cases were supplied by the National Cancer Intelligence Centre, Office for National Statistics, London (ONS), including dates of birth, diagnosis and follow-up, sex, histological type and primary site of cancer, Townsend deprivation index score (TDI) and vital status. Cases with vital status unknown (patient record not traced at the National Health Service Central Register) were excluded as were cases with a survival time of zero (diagnosed and died on the same day). These exclusion criteria were those applied by Coleman et al (1999). Cases lost to follow-up, for example, emigrated, were included up to date last known to be alive.
For cases registered from 1979 to 1994, cancer morphology was coded according to the International Classification of Diseases for Oncology, first edition (ICD-O 1) (World Health Organization, 1976), and cancer site according to the ninth revision of the International Classification of Diseases (ICD 9) (World Health Organization, 1977). For cases registered during the period 1995–2001, diagnoses were similarly coded according to ICD-O, second edition (ICD-O 2) (Percy et al, 1990), and ICD tenth revision (ICD-10) (World Health Organization, 1992). The cases were classified into 10 main diagnostic groups and 2–7 subgroups per main group as described previously (Birch et al, 2002). This classification is recognised internationally as a suitable vehicle for analysing data on TYAs (Barr et al, 2006).
We examined survival for each diagnostic subgroup by age at diagnosis (13–16, 17–20, 21–24 years), TDI, sex and calendar period (1979–1984, 1985–1989, 1990–1995, 1996–2001). Cases were divided into five groups, from the most affluent to most deprived, on the basis of the quintile of the distribution of TDIs for census ward of residence. TDIs are derived from levels of four census variables: car ownership; house ownership; overcrowding and unemployment within wards, giving a measure of material deprivation (Townsend et al, 1998).
Five-year relative survival in each diagnostic group was calculated by dividing observed by expected survival among comparable groups in the general population (Ederer et al, 1961). The 5-year expected survival was derived from the age, sex, deprivation and calendar year-specific national mortality rates for England (Coleman et al, 1999). Relative survival by age, sex, calendar period and TDI was examined using Poisson regression (Dickman et al, 2004, Breslow and Day, 1987). Specified diagnostic subgroups with 250 (0.8%) or more cases as well as smaller groups of interest were examined individually. The significance level was set at 5%. Analyses were carried out using the statistical software package Stata, Version 9 (StataCorp LP, 2005).
Results
Survival time was available for 31 876 (94.8%) of 33 625 potentially eligible patients. During the period from 1993 (when such information became available) to 2001, 142 of 217 (65.4%) with zero survival time were registered by death certificate only. The remaining cases were hospital registrations who were diagnosed and died on the same day.
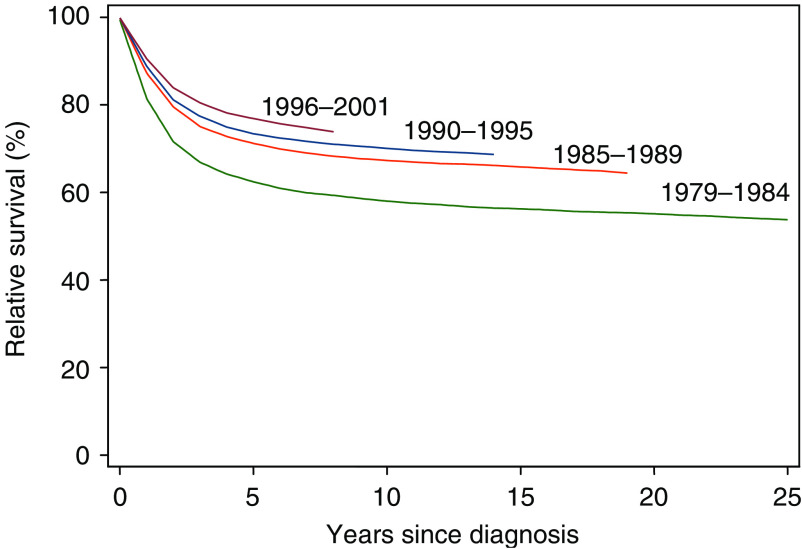
Figure 1 shows that overall 5-year relative survival steadily increased throughout the study period (P<0.001) from 63% in 1979–1984 to 77% in 1996–2001. The most marked increase was between the two earliest periods.
Figure 1.
Relative survival of cancer patients aged 13–24 years, diagnosed between 1979 and 2001 in England, by calendar period.
Table 1 shows that 5-year relative survival was significantly better for females than males except for germ cell tumours (GCTs) and central nervous system (CNS) tumours. The pattern of survival with age varied between diagnostic groups. For GCTs in 13- to 16-years-olds, 17- to 20-year-olds and 21- to 24-year-olds, survival was 80, 87 and 90% respectively. However, for leukaemia and CNS tumours, survival was better in the youngest group (P<0.001). For lymphomas, bone sarcomas, soft tissue sarcomas (STSs), melanoma and carcinomas, survival was not significantly associated with age. For leukaemia and carcinomas, the most deprived groups had the lowest survival (P=0.048 and 0.008, respectively). All diagnostic groups showed improvements in 5-year relative survival during the study period of between 9% (CNS) and 21% (leukaemia), except STS for which no improvement was seen.
Table 1. Five-year relative survival (%) of patients diagnosed at age 13–24 during 1979–2001 in England by main diagnostic group.
|
All
|
Leukaemia
|
Lymphoma
|
CNS
|
Bone sarcomas
|
STS
|
GCTs
|
Melanoma
|
Carcinomas
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | |
| Sex | |||||||||||||||||||||||||||
| Male | 17 339 | 69 | <0.001 | 2129 | 42 | 0.016 | 4607 | 80 | <0.001 | 1777 | 59 | 0.122 | 1170 | 46 | 0.019 | 950 | 52 | 0.012 | 4022 | 89 | 0.730 | 928 | 78 | <0.001 | 1548 | 63 | <0.001 |
| Female | 14 433 | 73 | 1393 | 47 | 3583 | 84 | 1382 | 62 | 769 | 52 | 793 | 58 | 520 | 86 | 1768 | 89 | 3931 | 76 | |||||||||
| Age (years) | |||||||||||||||||||||||||||
| 13–16 | 6682 | 65 | <0.001 | 1303 | 47 | <0.001 | 1644 | 82 | 0.772 | 1092 | 68 | <0.001 | 815 | 48 | 0.611 | 495 | 58 | 0.455 | 381 | 80 | <0.001 | 244 | 89 | 0.158 | 600 | 70 | 0.920 |
| 17–20 | 10 107 | 70 | 1194 | 42 | 2948 | 81 | 922 | 61 | 716 | 46 | 611 | 51 | 1386 | 87 | 786 | 84 | 1363 | 73 | |||||||||
| 21–24 | 14 983 | 74 | 1025 | 40 | 3598 | 82 | 1145 | 53 | 408 | 54 | 637 | 55 | 2775 | 90 | 1666 | 85 | 3516 | 72 | |||||||||
| Deprivation | |||||||||||||||||||||||||||
| Most affluent | 6353 | 71 | 0.001 | 700 | 45 | 0.048 | 1705 | 83 | 0.073 | 653 | 61 | 0.914 | 408 | 50 | 0.904 | 346 | 51 | 0.844 | 916 | 87 | 0.212 | 595 | 86 | 0.861 | 928 | 73 | 0.008 |
| 2 | 6353 | 71 | 725 | 46 | 1635 | 82 | 661 | 60 | 412 | 48 | 320 | 55 | 919 | 89 | 584 | 85 | 1011 | 73 | |||||||||
| 3 | 6355 | 72 | 668 | 42 | 1663 | 82 | 648 | 62 | 366 | 47 | 339 | 60 | 914 | 89 | 571 | 85 | 1101 | 73 | |||||||||
| 4 | 6355 | 71 | 723 | 43 | 1570 | 82 | 599 | 60 | 394 | 48 | 384 | 53 | 884 | 89 | 514 | 83 | 1168 | 73 | |||||||||
| Most deprived | 6356 | 70 | 706 | 41 | 1617 | 80 | 598 | 60 | 359 | 51 | 354 | 53 | 909 | 89 | 432 | 87 | 1271 | 69 | |||||||||
| Year of diagnosis | |||||||||||||||||||||||||||
| 1979–1984 | 7509 | 62 | <0.001 | 935 | 33 | <0.001 | 2031 | 77 | <0.001 | 763 | 54 | <0.001 | 544 | 39 | <0.001 | 424 | 53 | 0.152 | 924 | 80 | <0.001 | 454 | 74 | <0.001 | 1309 | 64 | <0.001 |
| 1985–1989 | 7482 | 71 | 789 | 43 | 2064 | 82 | 718 | 61 | 446 | 55 | 426 | 53 | 1063 | 85 | 606 | 86 | 1224 | 71 | |||||||||
| 1990–1995 | 8488 | 73 | 929 | 47 | 2121 | 83 | 869 | 64 | 468 | 52 | 447 | 55 | 1220 | 92 | 850 | 86 | 1432 | 74 | |||||||||
| 1996–2001 | 8293 | 77 | 869 | 54 | 1974 | 86 | 809 | 63 | 481 | 51 | 446 | 56 | 1335 | 94 | 786 | 90 | 1514 | 78 | |||||||||
CNS=central nervous system tumours; GCTs=germ-cell tumours; STS=soft tissue sarcoma.
Tables 2, 3, 4, 5 and 6 present the results of analyses by the major subtypes within main diagnostic groups.
Table 2. Five-year relative survival (%) of patients with haematological malignanciesa diagnosed at age 13–24 during 1979–2001 in England.
|
ALL
|
AML
|
CML
|
NHL
|
HL
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | |
| Sex | |||||||||||||||
| Male | 1163 | 43 | 0.019 | 671 | 36 | 0.074 | 178 | 50 | 0.690 | 1552 | 65 | 0.206 | 3055 | 87 | 0.026 |
| Female | 633 | 50 | 565 | 42 | 116 | 54 | 789 | 68 | 2794 | 89 | |||||
| Age (years) | |||||||||||||||
| 13–16 | 869 | 50 | <0.001 | 333 | 40 | 0.536 | 54 | 43 | 0.372 | 599 | 70 | 0.033 | 1045 | 89 | 0.550 |
| 17–20 | 581 | 44 | 439 | 39 | 94 | 54 | 816 | 65 | 2132 | 88 | |||||
| 21–24 | 346 | 37 | 464 | 38 | 146 | 54 | 926 | 66 | 2672 | 88 | |||||
| Deprivation | |||||||||||||||
| Most affluent | 361 | 48 | 0.066 | 255 | 39 | 0.253 | 45 | 73 | 0.173 | 457 | 62 | 0.813 | 1248 | 90 | 0.190 |
| 2 | 400 | 48 | 240 | 43 | 53 | 43 | 452 | 69 | 1183 | 86 | |||||
| 3 | 313 | 48 | 260 | 35 | 52 | 47 | 453 | 69 | 1210 | 86 | |||||
| 4 | 371 | 43 | 252 | 41 | 62 | 51 | 457 | 66 | 1113 | 89 | |||||
| Most deprived | 351 | 42 | 229 | 36 | 82 | 50 | 522 | 65 | 1095 | 87 | |||||
| Year of diagnosis | |||||||||||||||
| 1979–1984 | 490 | 37 | <0.001 | 311 | 27 | <0.001 | 64 | 30 | 0.002 | 506 | 52 | <0.001 | 1525 | 85 | <0.001 |
| 1985–1989 | 416 | 45 | 259 | 34 | 71 | 62 | 569 | 70 | 1495 | 86 | |||||
| 1990–1995 | 457 | 48 | 351 | 43 | 77 | 51 | 629 | 68 | 1492 | 89 | |||||
| 1996–2001 | 433 | 55 | 315 | 50 | 82 | 66 | 637 | 72 | 1337 | 93 | |||||
ALL=acute lymphoid leukaemia; AML=acute myeloid leukaemia; CML=chronic myeloid leukaemia; HL=Hodgkin lymphoma; NHL=non-Hodgkin lymphoma.
Excluding 196 cases with other rare and unspecified haematological malignancies.
Table 3. Five-year relative survival (%) of patients with selected CNS tumours diagnosed at age 13–24 during 1979–2001 in England.
|
Astrocytoma
|
Other glioma
|
Ependymoma
|
PNET
|
Other specified and unspecified
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | |
| Sex | |||||||||||||||
| Male | 869 | 60 | 0.439 | 376 | 53 | 0.125 | 141 | 76 | 0.291 | 191 | 51 | 0.295 | 200 | 60 | 0.092 |
| Female | 749 | 59 | 251 | 61 | 110 | 84 | 117 | 57 | 155 | 69 | |||||
| Age (years) | |||||||||||||||
| 13–16 | 574 | 71 | <0.001 | 183 | 62 | 0.249 | 94 | 77 | 0.196 | 129 | 60 | 0.058 | 112 | 68 | 0.383 |
| 17–20 | 480 | 61 | 179 | 56 | 77 | 81 | 89 | 49 | 97 | 66 | |||||
| 21–24 | 564 | 47 | 265 | 53 | 80 | 82 | 90 | 49 | 146 | 59 | |||||
| Deprivation | |||||||||||||||
| Most affluent | 335 | 59 | 0.830 | 129 | 59 | 0.921 | 52 | 83 | 0.246 | 62 | 53 | 0.750 | 75 | 64 | 0.623 |
| 2 | 328 | 60 | 123 | 57 | 54 | 85 | 74 | 55 | 82 | 54 | |||||
| 3 | 324 | 61 | 134 | 57 | 52 | 76 | 65 | 59 | 73 | 68 | |||||
| 4 | 312 | 61 | 131 | 49 | 44 | 78 | 48 | 50 | 64 | 67 | |||||
| Most deprived | 319 | 57 | 110 | 62 | 49 | 75 | 59 | 49 | 61 | 67 | |||||
| Year of diagnosis | |||||||||||||||
| 1979–1984 | 359 | 56 | 0.199 | 184 | 49 | 0.002 | 64 | 71 | 0.014 | 78 | 50 | 0.118 | 78 | 49 | 0.006 |
| 1985–1989 | 347 | 62 | 153 | 55 | 62 | 74 | 66 | 46 | 90 | 69 | |||||
| 1990–1995 | 443 | 60 | 159 | 64 | 61 | 89 | 77 | 59 | 129 | 65 | |||||
| 1996–2001 | 469 | 59 | 131 | 61 | 64 | 85 | 87 | 61 | 58 | 74 | |||||
PNET=medulloblastoma and primitive neuroectodermal tumours.
Table 4. Five-year relative survival (%) of patients with bone and soft tissue sarcomas diagnosed at age 13–24 during 1979–2001 in England.
|
Osteosarcoma
|
Ewing sarcoma
|
RMS
|
Other specified STS
|
Unspecified STS
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | |
| Sex | |||||||||||||||
| Male | 638 | 44 | 0.008 | 356 | 37 | 0.990 | 241 | 31 | 0.329 | 403 | 57 | 0.446 | 188 | 51 | 0.069 |
| Female | 407 | 53 | 215 | 39 | 161 | 38 | 397 | 61 | 133 | 58 | |||||
| Age (years) | |||||||||||||||
| 13–16 | 480 | 47 | 0.810 | 247 | 44 | 0.021 | 172 | 42 | <0.001 | 191 | 67 | 0.065 | 80 | 59 | 0.159 |
| 17–20 | 394 | 48 | 207 | 30 | 159 | 30 | 276 | 56 | 112 | 55 | |||||
| 21–24 | 171 | 48 | 117 | 38 | 71 | 25 | 333 | 56 | 129 | 51 | |||||
| Deprivation | |||||||||||||||
| Most affluent | 216 | 48 | 0.996 | 118 | 42 | 0.974 | 92 | 33 | 0.733 | 157 | 58 | 0.258 | 62 | 50 | 0.063 |
| 2 | 216 | 46 | 127 | 35 | 71 | 41 | 149 | 63 | 58 | 45 | |||||
| 3 | 198 | 46 | 117 | 37 | 76 | 34 | 148 | 61 | 68 | 62 | |||||
| 4 | 215 | 48 | 114 | 34 | 82 | 28 | 177 | 57 | 77 | 56 | |||||
| Most deprived | 200 | 48 | 95 | 41 | 81 | 36 | 169 | 55 | 56 | 57 | |||||
| Year of diagnosis | |||||||||||||||
| 1979–1984 | 311 | 38 | <0.001 | 152 | 26 | 0.005 | 92 | 33 | 0.430 | 153 | 58 | 0.294 | 71 | 44 | 0.007 |
| 1985–1989 | 235 | 55 | 124 | 41 | 122 | 39 | 168 | 56 | 77 | 49 | |||||
| 1990–1995 | 246 | 50 | 138 | 43 | 99 | 33 | 217 | 57 | 98 | 61 | |||||
| 1996–2001 | 253 | 49 | 157 | 42 | 89 | 29 | 262 | 62 | 75 | 59 | |||||
RMS=rhabodomyosarcoma; STS=soft tissue sarcoma.
Table 5. Five-year relative survival (%) of patients with GCTs diagnosed at age 13–24 during 1979–2001 in England.
|
Testis
|
Ovary
|
CNS
|
Others
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | P | N | % | P | N | % | P | N | % | P | |
| Sex | ||||||||||||
| Male | 3788 | 90 | 132 | 73 | 0.260 | 102 | 51 | <0.001 | ||||
| Female | 417 | 87 | 26 | 81 | 77 | 85 | ||||||
| Age (years) | ||||||||||||
| 13–16 | 155 | 83 | <0.001 | 142 | 83 | 0.120 | 63 | 76 | 0.329 | 21 | 57 | 0.348 |
| 17–20 | 1120 | 89 | 145 | 87 | 54 | 80 | 67 | 64 | ||||
| 21–24 | 2513 | 92 | 130 | 90 | 41 | 66 | 91 | 68 | ||||
| Deprivation | ||||||||||||
| Most affluent | 755 | 89 | 0.267 | 97 | 82 | 0.716 | 35 | 77 | 0.413 | 29 | 45 | 0.246 |
| 2 | 775 | 90 | 72 | 90 | 37 | 81 | 35 | 71 | ||||
| 3 | 763 | 92 | 81 | 86 | 33 | 69 | 37 | 65 | ||||
| 4 | 742 | 91 | 84 | 89 | 22 | 78 | 36 | 69 | ||||
| Most deprived | 753 | 91 | 83 | 85 | 31 | 68 | 42 | 72 | ||||
| Year of diagnosis | ||||||||||||
| 1979–1984 | 734 | 83 | <0.001 | 105 | 72 | <0.001 | 29 | 66 | 0.178 | 56 | 63 | 0.083 |
| 1985–1989 | 908 | 87 | 86 | 85 | 28 | 64 | 41 | 59 | ||||
| 1990–1995 | 1010 | 93 | 109 | 94 | 57 | 83 | 44 | 71 | ||||
| 1996–2001 | 1136 | 96 | 117 | 94 | 44 | 77 | 38 | 71 | ||||
Table 6. Five-year relative survival (%) of patients with selected carcinoma diagnosed at age 13–24 during 1979–2001 in England.
|
Head and necka
|
Lung
|
Female breast
|
Ovary
|
Cervix
|
Colorectal
|
Other GU
|
Other GI
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | N | % | P | N | 5 years | P | N | 5 years | P | |
| Sex | ||||||||||||||||||||||||
| Male | 305 | 70 | 0.001 | 80 | 46 | 0.052 | 268 | 52 | <0.001 | 312 | 83 | 0.002 | 177 | 25 | 0.956 | |||||||||
| Female | 271 | 82 | 66 | 63 | 458 | 61 | 572 | 79 | 886 | 79 | 281 | 69 | 206 | 72 | 166 | 20 | ||||||||
| Age (years) | ||||||||||||||||||||||||
| 13–16 | 143 | 74 | 0.375 | 17 | 64 | 0.433 | 10 | 70 | 0.041 | 43 | 78 | 0.425 | 5 | 60 | 0.293 | 86 | 60 | 0.090 | 43 | 67 | 0.003 | 48 | 28 | 0.055 |
| 17–20 | 186 | 72 | 32 | 56 | 57 | 75 | 164 | 84 | 75 | 87 | 164 | 60 | 138 | 73 | 104 | 27 | ||||||||
| 21–24 | 247 | 80 | 97 | 51 | 391 | 58 | 365 | 76 | 806 | 78 | 299 | 62 | 337 | 82 | 191 | 19 | ||||||||
| Deprivation | ||||||||||||||||||||||||
| Most affluent | 102 | 82 | 0.034 | 24 | 57 | 0.109 | 77 | 57 | 0.985 | 111 | 72 | 0.258 | 103 | 82 | 0.703 | 108 | 66 | 0.001 | 99 | 81 | 0.299 | 53 | 24 | 0.842 |
| 2 | 111 | 75 | 23 | 65 | 104 | 62 | 101 | 85 | 142 | 76 | 100 | 71 | 93 | 77 | 61 | 26 | ||||||||
| 3 | 111 | 78 | 27 | 63 | 78 | 65 | 112 | 78 | 188 | 78 | 113 | 59 | 110 | 82 | 68 | 20 | ||||||||
| 4 | 122 | 76 | 35 | 59 | 104 | 56 | 117 | 76 | 206 | 81 | 98 | 65 | 104 | 79 | 82 | 21 | ||||||||
| Most deprived | 130 | 69 | 37 | 32 | 95 | 62 | 131 | 83 | 247 | 78 | 130 | 48 | 112 | 74 | 79 | 24 | ||||||||
| Year of diagnosis | ||||||||||||||||||||||||
| 1979–1984 | 146 | 67 | 0.003 | 39 | 46 | 0.046 | 103 | 53 | 0.055 | 119 | 75 | 0.013 | 199 | 69 | 0.012 | 127 | 54 | 0.003 | 132 | 73 | 0.091 | 93 | 19 | <0.001 |
| 1985–1989 | 120 | 74 | 28 | 50 | 113 | 63 | 119 | 76 | 219 | 81 | 111 | 58 | 124 | 83 | 83 | 22 | ||||||||
| 1990–1995 | 137 | 78 | 40 | 50 | 127 | 62 | 138 | 73 | 243 | 84 | 124 | 58 | 160 | 78 | 83 | 19 | ||||||||
| 1996–2001 | 173 | 83 | 39 | 67 | 115 | 64 | 196 | 87 | 225 | 80 | 187 | 70 | 102 | 83 | 84 | 31 | ||||||||
Excluding thyroid.
Table 2 shows 5-year survival of patients with haematological malignancies. For acute lymphoid leukaemia (ALL), females had a better survival than males (50 vs 43%, P=0.019), survival decreased with increasing age (P<0.001) and increased by 18% during the study period from 37 to 55% (P<0.001). For acute myeloid leukaemia (AML), there was an even greater improvement over time, from 27 to 50% but from a lower starting point than ALL. There were no significant differences in survival from AML by age and sex. For chronic myeloid leukaemia, there was a marked improvement in survival between the time periods 1979–1984 and 1985–1989 but no consistent further improvement. Although there was a significant trend with TDI for leukaemia overall (Table 1) there was no significant trend for any subtype of leukaemia.
For non-Hodgkin lymphoma (NHL), 13- to 16-year-olds had a better survival than 17- to 24-year-olds (P=0.033). There was a marked improvement in survival between 1979–84 and 1985–89 but little subsequent improvement. For Hodgkin lymphoma (HL), female patients had a small survival advantage over males (P=0.026), with significant improvements over time (P<0.001), reaching 93% in the latest period. There was no significant trend in survival with TDI for either NHL or HL.
Table 3 shows 5-year relative survival of patients with selected CNS tumours. Only astrocytomas showed decreased survival with increasing age (P<0.001), and this group showed no improvement over the study period. This pattern was driven by high-grade astrocytoma (HGA), which is more common in older age groups (data not shown). For HGA, 5-year survival did not improve during the study period (P=0.85) and a very low survival rate of 14% was seen in the latest period. However, for low-grade astrocytoma, 5-year survival improved from 76% in 1979–1984 to 90% in 1990–1995 but with no further improvement (P=0.005). The ‘other glioma’ group, mainly oligodendroglioma and ependymoma, showed marked improvements from 1979 to 1995 but no subsequent improvement. For medulloblastoma and supratentorial primitive neuroectodermal tumours, there were no significant differences in survival by sex, age and time period of diagnosis. No CNS tumour group showed a trend with TDI.
Table 4 shows 5-year relative survivals of patients with bone tumours and STS. For osteosarcoma, females had a better prognosis than males (P=0.008) and survival improved significantly between 1979–84 and 1985–89 but with no improvements more recently. Thirteen- to sixteen-year-olds with Ewing sarcoma did better than 17- to 24-year-olds (P=0.021), with a significant improvement in survival over time (P=0.005), again particularly marked between 1979–1984 and 1985–1989. Rhabdomyosarcoma (RMS) survival decreased with increasing age (P<0.001), with no improvement over time and 5-year survival of only 29% in the latest period. For other specified types of STS combined, there was no significant improvement in survival over time. For unspecified STSs, survival improved significantly between 1979 and 1995 but not subsequently. There were no significant trends in survival with TDI for any specified types of bone and STS.
Table 5 shows 5-year relative survivals among patients with GCTs by primary site. For testicular GCTs, survival increased with advancing age at diagnosis (P<0.001). There was a consistent improvement in 5-year survival over time (P<0.001), reaching 96% in the latest period. Ovarian GCTs showed similar patterns of survival. For CNS GCTs, there were no significant differences in survival by sex, age and period of diagnosis. For GCTs of other sites, females had a substantially superior 5-year survival (P<0.001). None of the subgroups showed a trend with TDI.
Table 6 shows 5-year relative survivals of patients with carcinomas of selected sites. For head and neck carcinomas (excluding thyroid), females had a better survival than males (P=0.001). There was a trend of decreasing survival with increasing deprivation, but no trend with age. Survival steadily improved over time (P=0.003). Thyroid carcinoma showed a 97% or higher survival throughout the study period, with no significant variations in age, sex and deprivation (data not shown). For carcinoma of lung, there was a significant improvement over time (P=0.046) and females had a higher survival (P=0.052). For carcinoma of breast, survival decreased with increasing age but the number of cases below age 21 years was very small. There was a borderline significant trend for improved survival over time (P=0.055). For ovarian carcinoma, there was a marked improvement in survival between 1990–1995 and 1996–2001. This may in part reflect changes in coding between ICD-O1 and ICD-O2 so that additional, lower-grade ovarian tumours were included in the 1996–2001 data. No trend with age was seen. Carcinoma of cervix showed a marked improvement between 1979–84 and 1985–89 (P=0.012), but survival has remained at the same level since. For colorectal carcinomas, females had a substantially better survival than males (P<0.001). Survival increased significantly over time, particularly in the most recent period. The most deprived group had the lowest survival (P=0.001). Numbers of carcinomas of other sites were too small for separate analysis.
Discussion
This study presents the first national data for England on cancer survival among TYAs. It is now acknowledged that the special needs of cancer patients aged 0–18 years would be best served by principal treatment centres providing age-appropriate facilities and managed by dedicated multidisciplinary teams (National Collaborating Centre for Cancer, 2005). For 19- to 24-year-olds, unhindered access to such expert teams is recommended. It has been suggested that in this age group in the United States, low recruitment to clinical trials contributes to the comparatively poor improvements in cancer survival (Bleyer et al, 2007). A recent study has compared clinical trial inclusion rates of children with those of TYAs in Great Britain who have cancers relevant to both age ranges and for which phase III trials are in progress (leukaemia, lymphoma, CNS tumours, sarcoma and testicular GCT). Results for cases diagnosed in 2005–2007 show that 56% of total incident cases aged 5–14 years are entered into trials compared with only 20% of 15- to 24-year-olds. Trial inclusion for CNS tumours was particularly low (Whelan and Fern, 2008). The baseline data on survival trends presented here are of importance in monitoring future progress in cancer survival in TYAs and assessing the impact of new specialist TYA cancer units, including recruitment to clinical trials.
In this study, we have analysed survival by predefined morphological groups of cancers, appropriate to the TYA age range, using national data covering more than 30 000 incident cases over a 23-year period. Overall, there were marked improvements in survival during the study period especially for all subgroups of leukaemia and NHL. However, for certain other groups, the results are less encouraging. Both osteosarcoma and Ewing sarcoma showed a step change in survival between the two early periods but then no further increase after 1989. There was no improvement for RMS and other STS. Although survival among children diagnosed with osteosarcoma up to 1997 in Britain was only slightly better than that in TYAs reported here, children with Ewing sarcoma and RMS showed more marked improvements and better survivals than their TYA counterparts (Stiller et al, 2006; Pastore et al, 2006). During 1993–1997, in children 5-year survival was 67% for Ewing sarcoma and 65% for RMS, but in TYAs during 1996–2001, the respective survivals were 42 and 29%. For RMS, this may partly be due to a higher proportion of TYA patients with more aggressive histologies, but this cannot entirely explain the poor outcomes in TYAs with bone and soft tissue sarcomas in general.
High-grade CNS tumours in TYAs showed little or no improvements in survival during the study period, and this was also the case for British children up to 1997 with comparable tumours (Peris-Bonet et al, 2006). Clearly, the clinical management of high-grade CNS tumours in young people presents a major challenge. In contrast, these results show consistently high survival rates for GCTs, with equivalent improvement in 5-year survival for both testicular and ovarian tumours. Across all ages, nearly all testicular tumours are of germ-cell origin, so that the high survival rates previously reported for testicular cancer can be interpreted as survival from GCTs of the testis. However, most ovarian cancers are carcinomas, and survival rates for ovarian cancer (Coleman et al, 1999) do not reflect survival from ovarian GCTs. This is particularly important in TYAs, as GCTs are the predominant ovarian malignancy in this age group (Birch et al, 2003).
About 80% of cancers overall are carcinomas, but these constitute only 16% of TYA cancers (Alston et al, 2007). Carcinomas of most sites in the present series show favourable survival rates compared with cancers at those sites across all ages (Coleman et al, 1999). This suggests that the TYA cases may have histologically lower-grade tumours than older cases and/or differ biologically. However, survival from breast carcinoma in TYAs is similar to breast cancer in general (Coleman et al, 1999). Females have higher survival rates for most types of cancer, and this holds true for TYAs; possible explanations include earlier presentation in females and less aggressive tumour biology.
Cancer survival rates among older patients in England and Wales are strongly influenced by socioeconomic status (Coleman et al, 1999), but only small non-significant differences were seen in children. In this study, most cancers showed no association between TDI and survival, but for leukaemias and carcinomas overall, there were significant trends towards poorer survival with increasing deprivation, particularly for colorectal and head and neck carcinomas. These latter cancers, which show marked associations between survival and deprivation in older patients (Coleman et al, 1999), are aetiologically linked to lifestyle factors also associated with deprivation, such as tobacco smoking and poor diet (IARC, 1986; Key et al, 2004). These factors may influence survival due to general poor health. Other considerations include speed of seeking medical healthcare, referral patterns and clinical management in socioeconomically deprived areas.
In conclusion, although there were marked increases in survival over time for all cancers combined, for some diagnostic groups, little or no improvements were seen. These results provide baseline data against which to compare outcomes in patients treated in the developing specialist TYA cancer units and in those entered into clinical trials. The data serve to identify the patient groups that present the greatest clinical challenges.
Acknowledgments
This research was funded by Cancer Research UK and CLIC Sargent. JM Birch is a Cancer Research UK Professorial Fellow, University of Manchester. TOB Eden is Teenage Cancer Trust, Professor of Teenage and Young Adult Cancer, University of Manchester. We are grateful to Professor Michel Coleman for providing the national population mortality data and to Dr Lorna Fern and Dr Jeremy Whelan for sight of their article before publication. Data on incident cancer cases used in this study were provided by the nine regional cancer registries in England.
References
- Alston RD, Rowan S, Eden TOB, Moran A, Birch JM (2007) Cancer incidence patterns by region and socioeconomic deprivation in teenagers and young adults in England. Brit J Cancer 96: 1760–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RD, Holowaty EJ, Birch JM (2006) Classification schemes for tumors diagnosed in adolescents and young adults. Cancer 106: 1425–1430 [DOI] [PubMed] [Google Scholar]
- Birch JM, Alston RD, Kelsey AM, Quinn MJ, Babb P, McNally RJ (2002) Classification and incidence of cancers in adolescents and young adults in England 1979–1997. Br J Cancer 87(11): 1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch JM, Alston RD, Quinn M, Kelsey AM (2003) Incidence of malignant disease by morphological type in young persons aged 12–24 years in England, 1979–1997. Eur J Cancer 39: 2622–2631 [DOI] [PubMed] [Google Scholar]
- Bleyer A, Budd T, Montello M (2007) Older adolescents and young adults with cancer and clinical trials: lack of participation and progress in North America. In: Cancer in Adolescents and Young Adults. Chapter 5, pp 71–81
- Breslow NE, Day NE (1987) Statistical methods in cancer research Vol. II. The design and analysis of cohort studies. IARC Sci Publ 82: 1–406 [PubMed] [Google Scholar]
- Coleman MP, Babb P, Damiecki P, Grosclaude P, Honjo S, Jones J, Knerer G, Pitard A, Quinn M, Sloggett A, De Stavola B (1999) Cancer Survival Trends in England and Wales, 1971–1995: Deprivation and NHS Region. London: The Stationary Office [Google Scholar]
- Dickman PW, Sloggett A, Hills M, Hakulinen T (2004) Regression models for relative survival. Stat Med 23(1): 51–64 [DOI] [PubMed] [Google Scholar]
- Ederer F, Axtell LM, Cutler SJ (1961) The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr 6: 101–121 [PubMed] [Google Scholar]
- Geraci M, Birch JM, Alston RD, Moran A, Eden TOB (2007) Cancer mortality in 13–29 year olds in England and Wales, 1981–2005. Brit J Cancer 97(11): 1588–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (1986) Tobacco smoking. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. 38: 127–135, International Agency for Research on Cancer, Lyon, France [PubMed] [Google Scholar]
- Key TJ, Schatzkin A, Willett EC, Allen NE, Spencer EA, Travis RC (2004) Diet, nutrition and the prevention of cancer. Public Health Nutr 7: 187–200 [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Cancer, Guidance on cancer services, improving outcomes in children and young people with cancer (2005) National Institute for Health and Clinical Excellence. London. ISBN: 1-84629-067-8 [Google Scholar]
- Office for National Statistics (2006) Cancer statistics, registrations. Registrations of cancer diagnosed in 2004, England. Series MB1 no 35. London: Office for National Statistics [Google Scholar]
- Percy C, Van Holten V, Muir CS (1990) International classification of diseases for oncology: ICD-O 2nd ed.. Geneva: World Health Organization [Google Scholar]
- Pastore G, Peris-Bonet R, Carli M, Martínez-García C, Sánchez de Toledo J, Steliarova-Foucher E (2006) Childhood soft tissue sarcomas incidence and survival in European children (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 42: 2136–2149 [DOI] [PubMed] [Google Scholar]
- Peris-Bonet R, Martínez-Garcia C, Lacour B, Petrovich S, Giner-Ripoll B, Navajas A, Steliarova-Foucher E (2006) Childhood central nervous system tumours – incidence and survival in Europe (1978–1997): report from Automated Childhood Cancer Information System project. Eur J Cancer 42: 2064–2080 [DOI] [PubMed] [Google Scholar]
- StataCorp (2005) Stata Statistical Software: Release 9. TX: StataCorp LP [Google Scholar]
- Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E (2006) Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer 42: 2124–2135 [DOI] [PubMed] [Google Scholar]
- Townsend P, Phillimore P, Beattie A (1998) Health and Deprivation. Inequality and the North. London: Croom-Helm [Google Scholar]
- Whelan J, Fern LA (2008) Poor rates of accrual of teenagers and young adults into clinical trials in the UK. Keynote comment. Lancet Oncol 9(4): 306–307 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1976) ICD-O: International Classification of Diseases for Oncology. Geneva: World Health Organization [Google Scholar]
- World Health Organization (1977) International Statistical Classification of Diseases, Injuries and Causes of Death. 9th revision Geneva: World Health Organization [Google Scholar]
- World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems. 10th revision Geneva: World Health Organization [Google Scholar]