Abstract
Natural antisense transcripts are at least partially complementary to their sense transcripts. Cis-Sense/Antisense pairs (cis-SAs) have been extensively characterized and known to play diverse regulatory roles, whereas trans-Sense/Antisense pairs (trans-SAs) in animals are poorly studied. We identified long trans-SAs in human and nine other animals, using ESTs to increase coverage significantly over previous studies. The percentage of transcriptional units (TUs) involved in trans-SAs among all TUs was as high as 4.13%. Particularly 2896 human TUs (or 2.89% of all human TUs) were involved in 3327 trans-SAs. Sequence complementarities over multiple segments with predicted RNA hybridization indicated that some trans-SAs might have sophisticated RNA–RNA pairing patterns. One-fourth of human trans-SAs involved noncoding TUs, suggesting that many noncoding RNAs may function by a trans-acting antisense mechanism. TUs in trans-SAs were statistically significantly enriched in nucleic acid binding, ion/protein binding and transport and signal transduction functions and pathways; a significant number of human trans-SAs showed concordant or reciprocal expression pattern; a significant number of human trans-SAs were conserved in mouse. This evidence suggests important regulatory functions of trans-SAs. In 30 cases, trans-SAs were related to cis-SAs through paralogues, suggesting a possible mechanism for the origin of trans-SAs. All trans-SAs are available at http://trans.cbi.pku.edu.cn/.
INTRODUCTION
Natural antisense transcripts are at least partially complementary to their endogenous sense RNAs. Transcripts in a cis-sense/antisense (cis-SA) pair are transcribed from the opposite strands at the same genomic locus and thus display perfect RNA–RNA sequence complementarity, whereas transcripts from a trans-sense/antisense (trans-SA) pair are transcribed from different genomic loci and may have imperfect sequence complementarity (1). Cis-antisense transcripts have been extensively studied both computationally (2–5) and experimentally, and found to play a variety of regulatory roles including involvement in imprinting (6), alternative splicing (7) and transcriptional interference (8). Studies of trans-antisense RNAs (also termed trans-encoded RNAs or trans-acting RNAs) have mainly focused on small RNAs such as small interfering RNAs (siRNAs) and microRNAs (miRNAs) which function in a trans base-pairing mechanism with their targets and play important regulatory roles such as in mRNA degradation and translational repression (9).
There is evidence suggesting that long trans-antisense RNAs may also perform versatile regulatory functions (10). In bacteria, there are several examples of functional long trans- antisense transcripts (11–13). In eukaryotes, to date in only three cases has the activity of long trans-antisense been experimentally characterized: Lymnaea anti-NOS prevents the translation of the nNOS protein from its encoding mRNA, resulting in substantial suppression of nNOS enzyme activity (14,15); variant δ of mouse Msh4 forms double-stranded RNA (dsRNA) with Hspa5, possibly inducing Hspa5 RNA degradation and resulting in cell death (16); MBP's antisense RNA reduces the expression of the MBP gene, either by inhibition of MBP transport from the nucleus or by selective degradation of the MBP–antisense-MBP RNA duplex in myelin-deficient mutant mice (17).
Three groups have used computational methods to identify long trans-SAs from mRNAs and full-length cDNAs in human and Arabidopsis: Lehner et al. identified 92 putative trans-SA transcript pairs in human mRNA sequences (18); Li et al. identified 161 trans-SA transcript pairs in human RefSeq mRNAs (10) and Wang et al. identified 1320 trans-SA transcript pairs in Arabidopsis from full-length cDNA sequences (19). Studies on long trans-SAs are far from complete, especially given that these analyses have not utilized the more extensively available EST sequences. Therefore, the prevalence of trans-SAs together with other features has not been elucidated. Moreover, there have been no studies in animal species other than human.
A number of long noncoding RNAs were found to function as cis-antisense transcripts to regulate their overlapping sense genes (20,21). It was recently reported that around 98% of all transcripts in human were likely to be noncoding RNAs (22). The functions of most long noncoding RNAs remain unknown. It is important to explore whether they may function through trans-acting RNA–RNA interaction.
The goal of our present work was to: (i) estimate the prevalence of trans-SAs in different animals; (ii) classify the pairing and overlapping patterns of trans-SAs that may correspond to different regulatory mechanisms; (iii) investigate how many long noncoding RNAs might function by means of trans- RNA–RNA interaction in RNA-mediated gene regulation; (iv) determine in what biological events TUs in trans-SAs were most often involved; (v) study whether some, if not all, of trans-SAs may have correlated expression profiles; (vi) find trans-SAs that were conserved between different species and (vii) investigate how trans-SA originated by analyzing the relationship between trans-SAs and cis-SAs.
MATERIALS AND METHODS
Identification of trans-SAs
We identified trans-SAs in human and nine other animal species: Mus musculus (mouse), Rattus norvegicus (rat), Bos taurus (cattle), Canis lupus familiaris (dog), Gallus gallus (chicken), Danio rerio (zebrafish), Drosophila melanogaster (fly), Caenorhabditis elegans (worm) and Ciona intestinalis (sea squirt). These species were chosen because they allowed a range of cross-species comparisons with human and had relatively complete genomic sequences and abundant ESTs.
Lessons from previous studies of cis-SAs indicated that more SAs could be identified using ESTs in addition to mRNAs, rather than mRNAs alone (4,5). Using ESTs, however, is computationally more challenging because there are an-order-of-magnitude more ESTs than mRNAs and ESTs tend to have lower quality than mRNAs. Identifying trans-SAs is also more challenging than identifying cis-SAs because trans-SAs are transcribed from separate genomic loci. Previous work often clustered ESTs/mRNAs based on genomic overlap. This, however, could accidentally cluster neighboring genes into one. An additional confounding factor is that many genes in higher organisms are alternatively spliced. All these aspects indicated the need for a new and accurate identification pipeline of trans-SAs.
Our trans-SA identification pipeline was summarized in Supplementary Figure S1 and described below. We first downloaded from NATsDB (4) all EST/mRNA sequences which were (i) mapped unambiguously to the genome and (ii) had corrected orientations. For quality control, NATsDB applied stringent criteria to filter ESTs/mRNAs by aligning ESTs/mRNAs to genome, filtering low-quality mapping, selecting best unique mapping and inferring the correct orientation of ESTs/mRNAs (4,5). By comparing with human full-length cDNAs in the H-Invitational database (23), we found that our procedure inferred the correct orientation for 186 219 (or 99.5%) out of the 187 156 transcripts in the database, achieving an accuracy similar to other reports (2,24).
In the next step, our trans-SA identification pipeline refined exon/intron boundaries. BLAT cannot always ensure precise genome mapping for low-quality ESTs, resulting in some ambiguous exon boundaries (25). Our pipeline refined exon boundaries by integrating multiple evidence including standard splicing sites (GT-AG), sequence type (mRNA or EST), boundary frequency and chromosomal mapping quality: (i) If one boundary encoded a standard splicing site whereas another did not, the former was selected as the correct boundary. (ii) If the first criterion did not apply, the boundary inferred from mRNAs was used in preference to that from ESTs. Our pipeline also allowed users to manually rank their input sequences and boundaries according to their own estimate of quality. (iii) If the second criterion also failed, for instance, if all input sequences were ESTs and the user did not provide any ranking, the correct boundary was selected to be the one which occurred most frequently among the input sequences. (iv) If two boundaries occurred with the same frequency, the correct boundary was defined as the one inferred from the sequence with higher mapping identity.
A gene may encode multiple transcripts due to alternative splicing and a transcript may correspond to multiple ESTs due to redundant sequencing. Thus, following Riken's definition, we grouped ESTs/mRNAs into transcripts and clustered alternatively spliced transcripts into one transcriptional unit (TU), if the ESTs/mRNAs shared exonic overlap of at least one nucleotide and had the same chromosomal orientation. Grouping ESTs/mRNAs into TUs was advantageous over previous pipelines that grouped them into genomic clusters (2,3,5) or ‘transcriptional forests’ (26) because a cluster or a transcriptional forest may accidentally include multiple TUs because of the existence of hundreds of triplet and quadruplet cases of cis-SAs that we had previously discovered (5).
We used the Splicing Variant Analysis Platform (SVAP) (http://svap.cbi.pku.edu.cn) to assemble ESTs/mRNAs into alternatively spliced transcripts using splice graphs. Similar to existing variant finders such as ASPIC (27) and ESTGene (28), SVAP represented a splice graph as a directed acyclic graph (DAG), using nodes to represent exons and edges to represent relationship between exons. SVAP walked through all possible paths of the splicing DAG and created a variant for each path. To filter out possible false positives, SVAP discarded those variants with at least one exon that was not covered by any sequence evidence (24).
The result of SVAP was a huge collection of isoforms assembled into TUs, from which we then identified trans- and cis-SAs. We ran BLASTN using the collection of isoforms as queries against their reverse complement sequences (by setting BLASTN parameter −S to 2). Previous work has used two different criteria to define trans-SAs: (i) an e-value cutoff of 10−9 (18) and (ii) an e-value cutoff of 10−9 with an identity threshold of 98% (10). We chose the former criteria because the sequence identity between the partners of two validated trans-SAs in Lymnaea, antiNOS-1/Lym-nNOS and antiNOS-2/Lym-nNOS, was both 80%, lower than the identity threshold in the latter criteria (14). miRNAs are also known to form imperfect base-pairing with their trans targets. If a sequence and the reverse complement sequence of another sequence had a pairwise BLASTN e-value lower than 10−9 and were mapped to nonoverlapping genomic coordinates, they were considered a trans-SA pair. Otherwise, if they had overlapping genomic coordinates, they were considered a cis-SA pair. We then condensed trans-SA pairs into trans-SA TU pairs, which were reported and analyzed in this manuscript.
Finally, we applied additional filters to further ensure the high quality of the trans-SAs identified. First, we removed trans-SAs that had any pairing regions mapped to known repeats (defined by UCSC Genome Browser) (29). Second, we mapped the trans-SA TUs to known pseudogenes downloaded from www.pseudogene.org and discarded all TUs having overlapping chromosomal coordinates with a pseudogene. Third, to avoid false TUs resulting from incorrectly inferred transcript orientation, we followed the strategy published by Engstrom et al. (24) and discarded a TU if the number of transcripts mapped to it were less than a threshold t. t was set to be the smallest integer greater than 2 for which P(Bin(N, P) t) ≤ 0.01, where N was the total number of ESTs/mRNAs mapped to the TU, P was the estimated rate of misorientation of ESTs/mRNAs (0.5% in our case) and Bin stood for binomial distribution (24). This stringent filter offered an additional benefit of eliminating possible noisy transcription or genomic sequence contamination because these were less likely to have large number of transcripts.
Determination of coding potential
Our pipeline for determining coding potential of a TU was summarized in Supplementary Figure S2. We first identified protein-coding TUs based on NCBI gene annotations (30). For unannotated TUs, we used Coding Potential Calculator (CPC) to predict their coding potential (31). CPC extracts six features from the transcript's sequence and inputs these features into a support vector machine (SVM) classifier. Using several reference databases as testing datasets, the accuracy of CPC was shown to be over 91%, outperforming other existing prediction algorithms (31). If CPC predicted that a TU had both noncoding RNAs and protein-coding RNAs, as some noncoding RNAs are known to be transcribed from protein-coding genes (32), we considered this TU as protein-coding. Only those TUs in which all RNAs were predicted to lack coding potential were considered as noncoding.
Calculation of hybridizing potential of trans-SAs by DINAMelt
DINAMelt, a RNA hybridization prediction program (33,34), used an in silico RNA hybridization model to predict the hybridizing regions between two RNAs and was able to handle matched pairs, mismatches and symmetric and asymmetric interior loops. The thermodynamic parameters of oligonucleotides by DINAMelt had good agreement with experimental data (34) and it had been successfully used in many area such as the design of gene probes and microarray oligonucleotides and the prediction of miRNA targets (35). The parameters of DINAMelt were set as default: the concentrations of two molecules were equal and two molecules were hybridized at 37°C.
Finding functional categories and pathways enriched in trans-SAs
To study the functional categories involved, we mapped all TUs to Entrez Gene (30) and retrieved their functional categorization based on Gene Ontology (GO) (36) annotations available in Entrez Gene. We then used GO::TermFinder (37) to find statistically enriched GO terms. Assuming a hypergeometric distribution, GO::TermFinder compared the number of trans-SA TUs that fell into each functional category against the number of TUs in the whole genome that fell into the same functional category and calculated a P-value for each enriched GO term. GO::TermFinder also implemented a 1000-simulation-based correction for multiple hypotheses testing for every P-value. The functional categories with corrected P-value ≤ 0.01 were considered statistically enriched in trans-SAs.
To study the pathways involved, we used the KOBAS software (38) to assign TUs to metabolic pathways based on sequence similarity to sequences in known pathways in the KEGG database (39). KOBAS then compared the number of trans-SA TUs in each pathway against the number of all TUs in the genome in the same pathway, assuming a hypergeometric distribution (38). To reduce Type-1 errors, KOBAS performed an FDR correction. Pathways with q-value ≤ 0.01 were considered to be statistically enriched.
Analysis of expression profiles of trans-SAs
Significant positive or negative expression correlation could give hints on antisense RNAs’ concordant or reciprocal regulation (40). We used the enlarged human SAGE library consisting of 309 samples on GPL4 platform, downloaded from NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL4). We reconstructed 22 ‘tissue-type’ SAGE libraries (adrenal cortex, blood, bone, brain, breast, colon, eye, kidney, liver, lung, muscle, ovary, placenta, prostate, skin, stem cell, stomach, thyroid, pancreas, peritoneum, sciatic nerve and sperm). After being normalized to tags per million (tpm), only tags that could be mapped unambiguously to TUs by SAGEMap (http://www.ncbi.nlm.nih.gov/SAGE/) were used. The expression level of a TU in a tissue library was set to the sum of tpm of all SAGE tags containing the TU in that tissue library (41). Only TUs with at least 3 tpm across all the tissues were kept in order to eliminate potential sequencing errors in low-abundance SAGE tags (42). We adopted the correlation coefficient r following the same strategy proposed by RIKEN group (40). We made one important improvement: we tested whether a correlation coefficient r was statistically significantly different from what one would expect by chance in which the population correlation coefficient ρ was equal to zero. We calculated the P-value using t-test. To reduce Type-1 errors, we then performed an FDR correction (43) with a stringent cutoff of q-value ≤ 0.01.
Conservation of trans-SAs between human and mouse
We sought to investigate whether any trans-SAs were conserved between human and mouse. We first mapped TUs across species by NCBI HomoloGene (44) and BLASTN search (5): (i) If both partners of a human trans-SA could be mapped to partners of a mouse trans-SA by HomoloGene, this trans-SA was considered conserved between human and mouse. (ii) If one partner of a human trans-SA could be mapped to a partner of a mouse trans-SA by HomoloGene but the other partner could not, we calculated the sequence similarity between the unmapped partners using pair-wise BLASTN. If BLASTN e-value < 10−10, identity > 80% and alignment length >100 nt (5), we considered this trans-SA pair to be conserved between human and mouse. (iii) For the remaining human trans-SAs with neither partner mapped by HomoloGene, we performed all-against-all BLASTN search against mouse trans-SAs. A trans-SA was considered conserved between human and mouse if both partners passed the above BLASTN similarity cutoff.
We next considered a second evidence of conservation, complementary to the first one, by human-mouse BLASTZ genome alignment that identified homologous regions of the human and mouse genomes by alignment of large neutrally evolving regions, allowing nucleotide substitutions (45). We extracted the borders for all human TUs involved in trans-SAs and identified their homologous genomic regions in mouse, using the human-mouse BLASTZ CHAIN pair-wise alignment from the UCSC Genome Browser Database (assembly hg18, mm8) (29). We chose the CHAIN alignment because it permitted a region of the human genome to be aligned to more than one region in the mouse genome (46). If the genomic regions of a human trans-SA and a mouse trans-SA were homologous, the trans-SA was considered conserved between human and mouse.
RESULTS
A new pipeline identified trans-SAs in 10 species
Using the new pipeline, we identified trans-SAs in human and nine other animal species. Table 1 showed the number and percentage of trans-SAs identified. The percentage of TUs involved in trans-SAs among all TUs was as high as 4.13%. In particular, in human, we found that 2896 TUs (or 2.89% of all human TUs) were involved in 3327 trans-SAs (sometimes one TU may be involved in two or more trans-SAs). Our results showed that the number of human trans-SAs had previously been severely underestimated. Lehner et al. (18) and Li et al. (10) identified 92 and 161 human trans-SAs on the transcript level, respectively, whereas we identified thousands on the TU level. As another example, in mouse, we found that 1519 TUs (or 3.13%) were involved in trans-SAs. The chromosomal distribution of TUs involved in human trans-SAs could be viewed at http://trans.cbi.pku.edu.cn/html/trans_overview.php?spe=hs. A segment on human chromosome 10 was illustrated in Figure 1.
Table 1.
Input and output statistics of trans-SAs in 10 species
| Species | GoldenPath genome version | Number of orientation-reliable sequences mapped to exact genomic location from NATsDB | Total number of TUs | Number of trans-SA TU pairs | Number of TUs involved in trans-SAsb | Proportion of TUs involved in trans-SAs among all TUs (%) |
|---|---|---|---|---|---|---|
| Human | hg18 | 4 494 665 | 99 919 | 3327 | 2896 | 2.89 |
| Mouse | mm8 | 2 100 305 | 57 416 | 1519 | 1799 | 3.13 |
| Fly | dm2 | 310 319 | 15 375 | 76 | 122 | 0.79 |
| Worm | ce2 | 291 395 | 19 815 | 41 | 69 | 0.35 |
| Sea squirt | ci2 | 414 454 | 14 273 | 1216 | 589 | 4.13 |
| Rata | rn4 | 463 787 | 40 428 | 110 | 188 | 0.47 |
| Cattlea | bosTau2 | 536 939 | 27 102 | 251 | 302 | 1.11 |
| Doga | canFam2 | 203 772 | 16 956 | 31 | 54 | 0.32 |
| Chickena | galGal2 | 299 931 | 21 426 | 37 | 66 | 0.31 |
| Zebrafisha | danRer4 | 522 259 | 23 809 | 185 | 282 | 1.18 |
aDue to the relatively small amount of available ESTs compared to their genome size for these species, the absolute numbers reported for these five species may be low and the percentages may not be accurate. However, the candidate trans-SAs identified should still be reliable.
bPrevious papers reported the number and percentages of genes forming cis-SAs in terms of clusters or transcriptional forests. Here, we reported the number and percentage in terms of TUs.
Figure 1.
Trans-SAs within a segment on human chromosome 10. Arcs linking two partners of a trans-SA pair were color-coded: red arc linked protein-coding–protein-coding pairs; blue arc linked noncoding–protein-coding pairs and green linked noncoding–noncoding pairs. In particular, a noncoding TU, AA187228, could pair with a coding TU (RP11-564C4.1) as well as a noncoding TU (DN831175).
We stored trans-SAs from all 10 species in a MySQL 5.0 (http://www.mysql.com/) relational database and developed a web interface of the database using PHP (http://www.php.net/) and GD (http://www.boutell.com/gd/) graphical libraries. The database, named Trans-SAMap, is freely available at http://trans.cbi.pku.edu.cn/. Users could interactively browse the database or search the database by TUs, Entrez Gene synonyms (44), mRNA/EST accession numbers, chromosomal locations and sequences (by BLAST similarities). Each trans-SA was displayed graphically, showing the overlapping patterns and other annotations.
One-fourth of human trans-SAs involved noncoding RNAs
We next examined whether and how many human trans-SAs involved noncoding TUs. We classified trans-SAs into three types: protein-coding–protein-coding pairs (p–p pairs), noncoding–protein-coding pairs (n–p pairs) and noncoding–noncoding pairs (n–n pairs). In human, 332 noncoding TUs were involved in 830 trans-SAs (24.9% of all human trans-SAs), including 753 n–p pairs and 77 n–n pairs. On average, each noncoding TU was involved in 2.5 trans-SAs, indicating that some noncoding RNAs might not only have trans-antisense activities but might also potentially regulate multiple targets.
In 247 human n–p pairs, the protein-coding TU had coding regions (CDS) annotation. Among them, in 172 (69.6%) pairs, the partner noncoding TU overlapped with CDS of the protein-coding TU, 46 (18.6%) with 3′ UTR and 29 (11.8%) with 5′ UTR. Different locations of the overlapping in trans-SAs may be related to different regulatory mechanisms (1,10). Overlapping with CDS of a sense transcript may destroy the sense transcript through an RNA interference (RNAi) mechanism (18) or interfere with the sense transcript's interaction with their trans-acting proteins; an overlap in the 3′UTR might affect the sense-mRNA's stability in cytoplasm (47) or its transport out of the nucleus (1) and an overlap in the 5′UTR might regulate mRNA translation initiation in a manner similar to, for example, what occurs with RNA III (48).
Trans-SAs may form sophisticated RNA–RNA pairing patterns
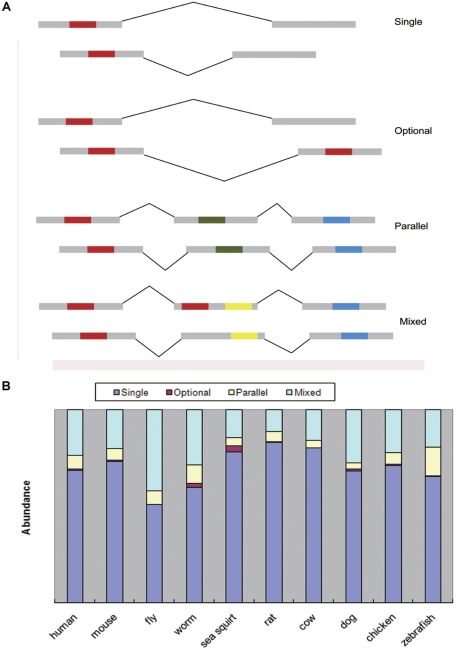
We observed that trans-SAs had diverse sequence complementary patterns, which may relate to their being involved in different regulatory mechanisms. Particularly, for many trans-SAs, there was more than one region that was complementary between two transcripts. Based on different patterns of BLASTN High-Scoring Pairs (HSPs) in a trans-SA, we constructed a new classification schema consisting of four classes of trans-SAs, shown in Figure 2A and described below:
‘Single’: both transcripts contain only one HSP.
‘Optional’: one transcript contains a region that can form HSPs with more than one distinct region in the paired transcript
‘Parallel’: both transcripts contain more than one HSP, forming pairing regions in parallel.
‘Mixed’: a pair of trans-SA transcripts with other, often more complicated, pairing patterns that cannot be classified into any of the above three classes. This class included transcripts that had simultaneously two or more of the above pairing patterns of HSPs (e.g. Figure 2A ‘Mixed’ which is a mixture of ‘Optional’ and ‘Parallel’) as well as transcripts in which the HSPs overlap in their sequence coordinates.
Figure 2.
Trans-SAs may have complex pairing patterns. (A) Trans-SAs are classified into four classes based on their HSP patterns. Grey blocks indicate exons. Folding lines between blocks indicate splicing junctions. Red, blue, yellow and green blocks indicate complementary regions. (B) The abundance of four classes of trans-SAs in 10 species.
We observed that although the ‘Single’ class was predominant, a significant number of trans-SAs fell into the ‘Optional’, ‘Parallel’ and ‘Mixed’ classes (Figure 2B).
The number of pairs with a perfect sequence match and the median length of the overlapping region were listed in Supplementary Table S1. Most trans-SA overlapping regions were longer than 50 bps, which was clearly different from those of small trans-acting RNAs such as miRNAs. The majority of the pairings were imperfect, which was reasonable because, unlike cis-SAs, two partners of a trans-SA were transcribed from different chromosomal loci and small trans-acting RNAs such as miRNAs were known to have imperfect matches with their targets.
We further investigated whether two transcripts in a trans-SA could in fact bind to each other based on the melting profiles calculated by DINAMelt (33,34). The HSPs in 89.42% of trans-SAs in the ‘Single’ class were covered by the hybridizing regions predicted by DINAMelt, indicating that, in addition to being complementary in sequence, they were likely to hybridize in solution. Investigating the other classes, we applied two criteria: a nonstringent one that specified that as long as any HSP was covered by the hybridizing regions predicted by DINAMelt, the trans-SA pair was considered to form dsRNA in solution and a stringent one that required that all HSP regions in both transcripts must be covered by the DINAMelt-predicted pairing regions. If a trans-SA pair satisfied the stringent criteria, then it was more likely that a complex pairing pattern indeed occurred in solution. Applying the nonstringent criteria, we found that 93.81% of the pairs in the ‘Optional’ class, 98.10% in ‘Parallel’ and 96.71% in ‘Mixed’ had at least one HSP covered by the hybridization regions predicted by DINAMelt, indicating that they may form dsRNA. Applying the more stringent criteria, we found that 44.62% of the pairs in the ‘Optional’ class, 19.43% in ‘Parallel’ and 74.45% in ‘Mixed’ had all HSPs in both transcripts covered by the hybridizing regions predicted by DINAMelt, indicating that complex pairing patterns might indeed occur.
TUs in trans-SAs were significantly enriched in nucleic acid binding, ion/protein binding and transporter/signal transducer functions and pathways
As shown in Supplementary Table S2 and S3, TUs in trans-SAs were enriched in functional categories and pathways involved in nucleic acid binding, similar to the functional bias of cis-SAs (5). In addition, TUs in trans-SAs were also more common in ion/protein binding and transporter activities as represented by the GO categories ‘ion binding’, ‘ion transport’, ‘protein binding’, ‘protein transport’, ‘membrane-bound organelle’ and ‘voltage-gated sodium channel complex’ and by the KEGG pathways ‘Protein export’, ‘Adhesion junction’ and ‘Tight junction’. These were categories in which cis-SAs were not commonly found. Enriched functions that were consistent between human and mouse were shown in Table 2.
Table 2.
Enriched Gene Ontology (GO) categories in trans-SAs that were consistent between human and mouse (corrected P-value ≤ 0.01, up to GO level 4)
| Biological Process | Molecular function | Cellular component |
|---|---|---|
| Regulation of cellular process | Metal ion binding | Intracellular |
| Transcription | Cation binding | Intracellular organelle |
| Transport | DNA binding | Intracellular part |
| Organelle organization and biogenesis | Purine nucleotide binding | Intracellular organelle part |
| DNA packaging | RNA binding | Cytoplasm |
| Cellular localization | Hydrolase activity, acting on acid anhydride | Membrane |
| Intracellular transport | Cytoskeletal protein binding | Cytoplasmic part |
| Negative regulation of biological process | Transferase activity, transferring phosphorus-containing group | Membrane part |
| Positive regulation of biological process | Ligase activity, forming carbon–nitrogen bond | Cytoskeletal part |
| Negative regulation of cellular process | Unfolded protein binding | Plasma membrane |
| Protein transport | Ribonucleoprotein complex | |
| Ion transport | Nuclear part | |
| Positive regulation of cellular process | Organelle lumen | |
| Regulation of cell cycle | Nuclear lumen | |
| Vesicle-mediated transport | Chromosomal part | |
| Organ development | Membrane fraction | |
| Response to DNA damage stimulus | Nucleosome | |
| Cytoplasm organization and biogenesis | Cell fraction | |
| Organelle membrane | ||
| Mitochondrial membrane | ||
| Voltage-gated sodium channel complex | ||
| Endomembrane system | ||
| Organelle inner membrane | ||
| Transcription factor complex |
The detailed P-values were listed in Supplementary Table S2.
Our study revealed, for the first time, the statistical functional bias of trans-SAs in animals. Results were in agreement with those from the small number of previous experimental studies of individual trans-SAs in prokaryotes and plants. For instance, in Escherichia coli, RyhB, a 90 nt noncoding RNA, is found to down-regulate a group of iron-storage and iron-using proteins by trans-acting when iron is limited (49); MicF gene encodes a 93 nt noncoding antisense RNA and regulates target ompF expression via a trans-pairing mechanism that inhibits translation and induces mRNA degradation, thereby affecting stress response cellular processes (50). In plants, transcripts involved in trans-SAs were found to be over-represented in function categories such as signal transducer activity and transporter activity (19).
Some trans-SAs showed significant concordant (positively correlated) or reciprocal (negatively correlated) expression patterns
Significant positive or negative expression correlation between trans-SAs could suggest antisense RNAs’ concordant or reciprocal regulation. Among 392 trans-SAs that co-occurred in at least 3 tissues and were used in subsequent expression analysis, 164 (or 42%) showed significant expression correlation. Three pairs, including two p–p pairs (ZNF227 versus CR593740, HIST1H2BG versus HIST1H2AK) and one n–p pair (CN480497 versus CYP4A11), showed a significant negative expression correlation (q-value ≤ 0.01), whereas remarkably, 161 pairs, including 146 p–p pairs and 15 n–p pairs, showed a significant positive expressional correlation (q-value ≤ 0.01).
Some trans-SAs were conserved between human and mouse
Our multi-species dataset enabled us to identify how many trans-SAs were conserved between human and mouse. Among trans-SAs where both partners had one-to-one mapping by HomoloGene (496 in human and 321 in mouse by orthologous mapping), five p–p trans-SAs in human (1.0%) were conserved in mouse (Table 3, panel A). To test whether the percentage of conserved trans-SAs differed from what one would expect by chance, we extracted all human and mouse genes in HomoloGene and constructed 9600 pseudo human SAs and 10 254 pseudo mouse SAs by pairing one gene to another randomly without repetition. No pseudo human SA pairs were found to be conserved in mouse. Fisher test confirmed that the fraction of conserved trans-SAs significantly differed from what would be expected by chance (P-value ∼2.94e-7). Among trans-SAs where one partner was mapped to HomoloGene and the other was mapped by BLASTN similarity (1801 pairs in human and 832 in mouse), an additional four p–p and two n–p trans-SAs in human were conserved in mouse (Table 3, panel B). Among the remaining trans-SAs both partners of which were not covered in HomoloGene but mapped by BLASTN similarity (1030 pairs in human and 366 in mouse), another twelve human trans-SAs were conserved in mouse (7 conserved p–p pairs and 5 n–p pairs, shown in Table 3, panel C).
Table 3.
List of human trans-SAs that were conserved between human and mouse
| Trans-SA | Gene symbol | Gene ID | TU ID |
|---|---|---|---|
| Panel A | |||
| 1 | FER | 2241 | hs_27889_p.14 |
| THOC2 | 57187 | hs_37092_m.1 | |
| 2 | TUBB3 | 10381 | hs_13578_p.3 |
| LOC401565 | 401565 | hs_36040_m.0 | |
| 3 | KCNA6 | 3742 | hs_7160_p.0 |
| KCNA10 | 3744 | hs_1560_p.3 | |
| 4 | LOC401565 | 401565 | hs_36040_m.0 |
| TUBB4 | 10382 | hs_16192_m.0 | |
| 5 | PPAN | 55337 | hs_16298_p.0 |
| ANGPTL2 | 23452 | hs_35783_m.3 | |
| Panel B | |||
| 1 (n–p)a | GPD1L | 23171 | hs_23040_p.1 |
| CA450491b | hs_7693_m.2* | ||
| 2 (n–p)a | MAFK | 7975 | hs_30950_p.0 |
| DB018139b | hs_15104_p.0* | ||
| 3 | TUBB2C | 10383 | hs_36040_p.0 |
| MTAP | 4507 | hs_34800_p.2 | |
| 4 | H2AFJ | 55766 | hs_7373_p.0 |
| MGC12935 | 84780 | hs_29114_p.0 | |
| 5 | TUBA1C | 84790 | hs_7676_p.2 |
| MGC16703 | 113691 | hs_22137_m.1 | |
| 6 | TUBA1C | 84790 | hs_7676_p.2 |
| LOC730222 | 730222 | hs_2584_m.0 | |
| Panel C | |||
| 1 | BX326232b | hs_11717_m.0 | |
| C14orf37 | 145407 | hs_10399_m.0 | |
| 2 (n–p)a | LOC729226 | 729226 | hs_13114_p.0* |
| LOC390616 | 390616 | hs_4793_m.0 | |
| 3 | BU662546b | hs_13878_p.1 | |
| LOC390616 | 390616 | hs_4793_m.0 | |
| 4 (n–p)b | BG398362b | hs_18569_p.0 | |
| LOC400794 | 400794 | hs_2189_m.2* | |
| 5 | CR602957b | hs_19485_m.8 | |
| C14orf37 | 145407 | hs_10399_m.0 | |
| 6 (n–p)a | LOC400794 | 400794 | hs_2189_m.2* |
| CDC27 | 996 | hs_14521_m.10 | |
| 7 (n–p)a | BQ935655b | hs_8564_p.2 | |
| LOC400794 | 400794 | hs_2189_m.2* | |
| 8 | ADRA1B | 147 | hs_28453_p.0 |
| BE535870b | hs_36406_m.0 | ||
| 9 | BE535870b | hs_36406_m.0 | |
| DB239600b | hs_20661_m.4 | ||
| 10 (n–p)a | BM477121b | hs_36425_m.0 | |
| LOC400794 | 400794 | hs_2189_m.2* | |
| 11 | LOC338739 | 338739 | hs_5542_p.3 |
| BG612114b | hs_30109_p.0 | ||
| 12 | BC036362b | hs_8886_m.6 | |
| BE535870b | hs_36406_m.0 |
Panel A, List of human trans-SAs that were conserved in mouse identified by cross-referencing HomoloGene. Panel B, List of human trans-SAs that were conserved in mouse identified by HomoloGene cross-reference of one TU and pair-wise BLAST similarity of the other TU. Panel C, Human trans-SAs that were conserved in mouse identified by pair-wise BLASTN similarity of both TUs.
a(n–p) indicates a trans-SA pair in which a noncoding TU pairs with a protein-coding TU. The TUs without coding potential were marked with asterisk. When not specified, the trans-SA pair is a protein-coding–protein-coding (p–p) pair. We did not observe any n–n pairs conserved between human and mouse.
bThese TUs have no NCBI GeneID or Gene name. Thus we used an isoform to represent the TU.
Two thousand five hundred and fifty six human trans-SAs could be mapped to mouse using BLASTZ alignment as genome-based evidence of conservation. Among them, 1017 (39.7%) formed trans-SAs in mouse and were considered conserved between human and mouse (Supplementary Table S4), including 826 p–p pairs, 188 n–p pairs and 3 n–n pairs. We shuffled the human and mouse trans-SAs and constructed equal number of human and mouse pseudo trans-SAs. Only 125 out of 2443 pseudo human trans-SAs were found to be conserved in mouse following the same protocol. Fisher test confirmed that the fraction of the conserved trans-SAs we detected was significantly higher than what would be expected by chance in the pseudo dataset (P-value < 2.2e-16). These results demonstrated that the conserved trans-SAs we observed were unlikely a chance event and suggested that they might have important functions.
A proposed mechanism of origination of some trans-SAs via cis-SAs and gene duplication
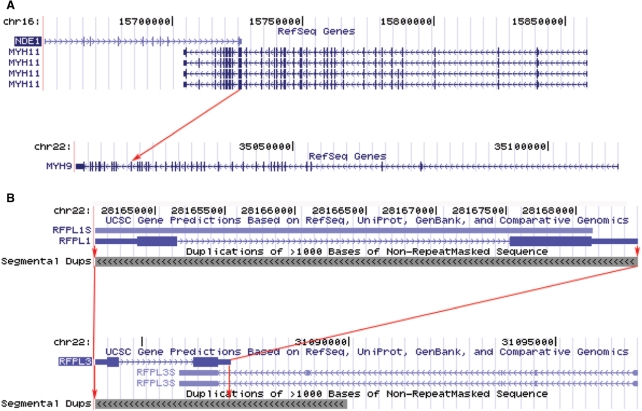
Comparing trans-SAs and cis-SAs in human, we found that 616 TUs involved in trans-SAs also had cis-SA partners. Based on information in GeneCards (51), we found that, for 30 such TUs, their cis-SA partner and trans-SA partner were members of the same gene family (Supplementary Table S5). Given the high abundance of cis-SAs and the relative simplicity with which cis-SAs can originate (52,53), it was more likely that in these dual cases, cis-SAs originated prior to trans-SAs. The following example illustrated one possible scenario of trans-SA origination. Human MYH11 (myosin, heavy polypeptide 11) and MYH9 (myosin, heavy polypeptide 9) are paralogs from the same family (based on GeneCards (51)) encoded at separate chromosomal loci. A third gene, NDE1 (nudE nuclear distribution gene E homolog 1), formed a cis-SA pair with MYH11 through a polyA-sharing mechanism. This cis-SA pair appeared to have originated recently because in both mouse and chicken, NDE1 and MYH11 were simply neighboring genes on the opposite strands without any overlap. Due to the high sequence similarity between MYH11 and MYH9, NDE1 forms a trans-SA pair with MYH9 (shown in Figure 3A).
Figure 3.
Some trans-SAs were related to cis-SAs through paralogues. (A) NDE1 and MYH11 formed a cis-SA pair sharing the last exon of NDE1 (99 bp overlap with perfect complementarity), from UCSC genome browser. The arrow indicates MYH9's paralogous exon to the cis-pairing region of MYH11. NDE1 shares this paralogous exon and thus trans-pairs with MYH9. (B) RFPL1 and RFPL1S formed a cis-SA (293 bp overlap with perfect complementarity). RFPL3 and RFPL3S formed another cis-SA (588 bp overlap with perfect complementarity). RFPL3S and RFPL1 formed a trans-SA (591 bp overlap with an e-value of 0.0 and an identity of 95%). RFPL3 and RFPL1S formed another trans-SA (519 bp overlap with an e-value of 0.0 and an identity of 96%). Red arrows indicate the cis-pairing region of RFPL1 and RFPL1S was duplicated to the locus of RFPL3 and RFPL3S.
A second example involves genes duplicated by segmental duplications. The pairing regions in 500 human trans-SAs (15% of all human trans-SAs) could be mapped to known segmental duplication regions based on UCSC genome browser database. For example, the RFPL (Ret finger protein-like) gene family is known to have undergone a series of duplications from an ancestor containing a RING-B30 domain (54). RFPL1 and RFPL3 are two members of this family, and RFPL1 is believed to be the older of the two evolutionarily (54). RFPL1S formed a cis-SA pair with RFPL1, and on a separate genomic locus, RFPL3S formed a cis-SA pair with RFPL3. Because RFPL1 and RFPL3 shared 95% sequence identity across their full length (54), RFPL1 and RFPL3S constituted a trans-SA pair, while RFPL3 and RFPL1S constituted another trans-SA pair (shown in Figure 3B).
DISCUSSION
Our pipeline had the highest coverage compared to previous works (10,18,19) because of the use of ESTs in addition to mRNAs. At the same time, our pipeline was also rigorous, applying stringent quality control filters. We identified an order-of-magnitude more trans-SAs in human (at least 20 times higher than previous studies (10,18)), as well as trans-SAs in nine other animal species for the first time, allowing new features of trans-SAs to be revealed. For instance, we observed that trans-SAs could potentially form more complex RNA–RNA pairing patterns than previously thought (10,18).
We followed the common practice of filtering out repeats and pseudogenes in order to identify trans-SAs that were most likely to be functional (10,18). However, although repeats were previously thought to be genomic parasites (55) and their transcripts were major targets of RNAi pathways (56), recent evidence has suggested repeats’ roles in the regulation of transcription or post-transcriptional events (57–59). Some repeats were found to be under strong selective constraint (55). Some were found to function as antisense (60,61). Similarly, pseudogenes were often considered evolutionary ‘dead-ends’ and filtered out. However, recently a large number of novel endogenous siRNAs were reported to be derived from a protein-coding gene and a cis-antisense transcript of its pseudogene, indicating the functional role of pseudogene (or at least its cis-antisense) in regulating the parental mRNA's level (62,63). Interestingly, we found that, if we had not filtered out repeats and pseudogenes in our pipeline, the final set of trans-SAs would be 20 times larger. The potential functions of trans-SAs involving repeats and pseudogenes are currently under further investigation.
Our results indicated that long noncoding RNAs, aside from functioning as cis-antisense transcripts such as Air (21) and Xist (20), might also function via a trans-acting mechanism in animals and could regulate more than one target. It was previously known that DsrA, an 87 nt noncoding RNA in Escherichia coli, regulates at least five different genes via trans-acting RNA–RNA interaction by different pairing regions (64). Therefore, a trans-acting mechanism for noncoding RNAs appeared to be capable to regulate more targets than a cis-antisense mechanism in which a noncoding RNA could regulate only one target at the same genomic locus on the opposite strand. This is reminiscent of the difference between short trans-SAs, i.e. miRNAs, which can regulate multiple targets at different genomic loci, and siRNAs, which most often regulate one target at the same genomic locus.
The expression profiles of trans-SAs pointed toward a complexity of regulatory interactions. Well-known short trans-acting RNAs and their targets have been shown to have different patterns of expression correlation. miRNAs could act through at least two mechanisms: imperfect base-pairing within 3′UTR that blocks translation, which does not affect target mRNA's abundance, or perfect base-pairing with miRNA-mediate cleavage and inactivation of target mRNA, which results in a decrease of target mRNA's abundance (65). Data have shown that the long trans-acting RNA, DsrA, has opposite expression effects on two different targets both mediated by RNA–RNA interaction: it decreases H-NS mRNA level but increases RpoS mRNA level (64). Among 392 trans-SAs having expression data in human tissues, we identified 164 trans-SAs that had significant expression correlations that might be involved in inhibition or stabilization effect of antisense transcripts. As for the remaining trans-SA transcripts, they might be implicated in other aspects of RNA function such as translation, RNA editing, trafficking and subcellular localization.
We used the large human SAGE library consisting of 309 samples on GPL4 platform for our expression studies. Admittedly this SAGE data set was still far from complete because it provided enough data for only 12% of all human trans-SAs. Thus, it would be important to continue to use high-throughout technologies to further investigate trans-SAs’ expression profiles. Nevertheless, we were able to find a remarkable 161 trans-SA pairs with positive expression correlation. Positive expression correlation of trans-SAs could be caused by three possible mechanisms. The first was a dsRNA-mediated stabilization function (66,67). Scheele et al. observed that a mammalian noncoding antisense molecule (ncNAT) could increase the abundance of its cis- target mRNA (svPINK1) in a ‘tail-tail’ overlapping pattern (67). In the overlapping region we detected neither AU-rich elements (AREs) nor other rapid decay-associated motifs using UTRscan (68) and theorized a possible mechanism: the ‘tail-tail’ overlap might affect mRNA stability by reducing mRNA decay where transcripts undergo 3′→5′ exonucleolytic decay subsequent to poly(A) shortening (47,69). This mechanism could potentially account for some of the positively-correlated trans-SAs. Among trans-SAs where at least one partner had CDS annotation, 15 p–p pairs overlapped at 3′UTR and two n–p pairs overlapped at 3′UTR. A second possible mechanism was that trans-antisense might interrupt target intramolecular base-pairing (13) and thereby stabilize target mRNA. For example, in Escherichia coli, DsrA pairing stabilizes RpoS mRNA and stimulates RpoS translation, freeing the translation initiation region from intramolecular base-pairing and allowing increased translation and mRNA stability (70). The third mechanism was that trans-antisense might function as a transcription activator (48). In Staphylococcus aureus, aside from being an activator of translation, RNAIII acts on the initiation of transcription of virulence genes by means of intermediary protein factors (71). Similarly, some trans-antisense RNAs in animals might also initiate transcription of their target genes.
We performed several analyses on the evolutionary conservation of trans-SAs between human and mouse and provided evidence for trans-SAs’ conservation. We observed that, as a group, trans-SAs were significantly more conserved between human and mouse than what would be expected by chance. However, the lack of detected conservation of many trans-SAs did not necessarily mean that they were not functional. First, the ENCODE project recently reported that as many as 50% of the experimentally identified functional elements do not show evidence of evolutionary constraint across mammals, especially for many types of noncoding functional elements (72). Second, TUs in some trans-SAs might be under rapid sequence evolution. As widely known, a subset of human protein-coding genes are fast evolving, driven by positive Darwinian selection, such as genes involved in immune response and reproduction (73). Some functional noncoding RNAs were also found to be subject to positive selection when associated with phenotypic radiation (74). Third, some pairs might be species-specific and arose recently after the divergence of human and mouse.
The mechanism of trans-SA origination was not studied before. Gene duplication including segmental duplication may contribute to trans-SA origination. Segmental duplication is known to play fundamental roles in both gene evolution and genomic disease (75). The pairing regions in 500 human trans-SAs (15% of all human trans-SAs) could be mapped to known segmental duplication regions (accounting for 2% of all human segmental duplications). The majority (85%) of human trans-SAs were not related to segmental duplications
In summary, our comprehensive analysis offers not only candidates for further experiments but also important clues to the function and evolution of trans-SAs in multiple animal species.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We are grateful to Drs Manyuan Long, Jane Wu, Louis Tao, Zicai Liang and Ge Gao for insightful suggestions. We thank Dr Laurie Goodman for proofreading the manuscript. This work was supported by China Ministry of Science and Technology 863 Hi-Tech Research and Development Programs (No. 2006AA02Z334, 2006AA02Z314, 2006AA02A312, 2007AA02Z165) and 973 Basic Research Programs (No. 2006CB910404, 2007CB946904), and China Ministry of Education 111 project (No. B06001). This study was also supported in part by the National Institutes of Health (NIH)-Intramural Research Program, National Institute on Drug Abuse. Funding to pay the Open Access publication charges for this article was provided by China Ministry of Education 111 Project (No. B06001).
Conflict of interest statement. None declared.
REFERENCES
- 1.Vanhee-Brossollet C, Vaquero C. Do natural antisense transcripts make sense in eukaryotes? Gene. 1998;211:1–9. doi: 10.1016/s0378-1119(98)00093-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Sun M, Kent WJ, Huang X, Xie H, Wang W, Zhou G, Shi RZ, Rowley JD. Over 20% of human transcripts might form sense-antisense pairs. Nucleic Acids Res. 2004;32:4812–4820. doi: 10.1093/nar/gkh818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yelin R, Dahary D, Sorek R, Levanon EY, Goldstein O, Shoshan A, Diber A, Biton S, Tamir Y, Khosravi R, et al. Widespread occurrence of antisense transcription in the human genome. Nat. Biotechnol. 2003;21:379–386. doi: 10.1038/nbt808. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Li J, Kong L, Gao G, Liu QR, Wei L. NATsDB: Natural Antisense Transcripts DataBase. Nucleic Acids Res. 2007;35:D156–D161. doi: 10.1093/nar/gkl782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Liu XS, Liu QR, Wei L. Genome-wide in silico identification and analysis of cis natural antisense transcripts (cis-NATs) in ten species. Nucleic Acids Res. 2006;34:3465–3475. doi: 10.1093/nar/gkl473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wutz A, Smrzka OW, Schweifer N, Schellander K, Wagner EF, Barlow DP. Imprinted expression of the Igf2r gene depends on an intronic CpG island. Nature. 1997;389:745–749. doi: 10.1038/39631. [DOI] [PubMed] [Google Scholar]
- 7.Munroe SH, Lazar MA. Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA. J. Biol. Chem. 1991;266:22083–22086. [PubMed] [Google Scholar]
- 8.Prescott EM, Proudfoot NJ. Transcriptional collision between convergent genes in budding yeast. Proc. Natl Acad. Sci. USA. 2002;99:8796–8801. doi: 10.1073/pnas.132270899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim VN, Nam JW. Genomics of microRNA. Trends Genet. 2006;22:165–173. doi: 10.1016/j.tig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Li YY, Qin L, Guo ZM, Liu L, Xu H, Hao P, Su J, Shi Y, He WZ, Li YX. In silico discovery of human natural antisense transcripts. BMC Bioinformatics. 2006;7:18. doi: 10.1186/1471-2105-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch M, Elliott T. Role of ppGpp in rpoS stationary-phase regulation in Escherichia coli. J. Bacteriol. 2002;184:5077–5087. doi: 10.1128/JB.184.18.5077-5087.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller T, Franch T, Udesen C, Gerdes K, Valentin-Hansen P. Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 2002;16:1696–1706. doi: 10.1101/gad.231702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good L. Translation repression by antisense sequences. Cell Mol. Life Sci. 2003;60:854–861. doi: 10.1007/s00018-003-3045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korneev S, O'Shea M. Evolution of nitric oxide synthase regulatory genes by DNA inversion. Mol. Biol. Evol. 2002;19:1228–1233. doi: 10.1093/oxfordjournals.molbev.a004183. [DOI] [PubMed] [Google Scholar]
- 15.Korneev SA, Park JH, O'Shea M. Neuronal expression of neural nitric oxide synthase (nNOS) protein is suppressed by an antisense RNA transcribed from an NOS pseudogene. J. Neurosci. 1999;19:7711–7720. doi: 10.1523/JNEUROSCI.19-18-07711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano M, Noda T. Genomic organization of the mouse Msh4 gene producing bicistronic, chimeric and antisense mRNA. Gene. 2004;342:165–177. doi: 10.1016/j.gene.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Okano H, Aruga J, Nakagawa T, Shiota C, Mikoshiba K. Myelin basic protein gene and the function of antisense RNA in its repression in myelin-deficient mutant mouse. J. Neurochem. 1991;56:560–567. doi: 10.1111/j.1471-4159.1991.tb08186.x. [DOI] [PubMed] [Google Scholar]
- 18.Lehner B, Williams G, Campbell RD, Sanderson CM. Antisense transcripts in the human genome. Trends Genet. 2002;18:63–65. doi: 10.1016/s0168-9525(02)02598-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Chua NH, Wang XJ. Prediction of trans-antisense transcripts in Arabidopsis thaliana. Genome Biol. 2006;7:R92. doi: 10.1186/gb-2006-7-10-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauler FM, Koerner MV, Barlow DP. Silencing by imprinted noncoding RNAs: is transcription the answer? Trends Genet. 2007;23:284–292. doi: 10.1016/j.tig.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–813. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 22.Mattick JS. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2:986–991. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamasaki C, Murakami K, Fujii Y, Sato Y, Harada E, Takeda J, Taniya T, Sakate R, Kikugawa S, Shimada M, et al. The H-Invitational Database (H-InvDB), a comprehensive annotation resource for human genes and transcripts. Nucleic Acids Res. 2008;36:D793–799. doi: 10.1093/nar/gkm999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engstrom PG, Suzuki H, Ninomiya N, Akalin A, Sessa L, Lavorgna G, Brozzi A, Luzi L, Tan SL, Yang L, et al. Complex Loci in human and mouse genomes. PLoS Genet. 2006;2:e47. doi: 10.1371/journal.pgen.0020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Zink D, Korn B, Vingron M, Haas SA. Strengths and weaknesses of EST-based prediction of tissue-specific alternative splicing. BMC Genomics. 2004;5:72. doi: 10.1186/1471-2164-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 27.Bonizzoni P, Rizzi R, Pesole G. ASPIC: a novel method to predict the exon-intron structure of a gene that is optimally compatible to a set of transcript sequences. BMC Bioinformatics. 2005;6:244. doi: 10.1186/1471-2105-6-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyras E, Caccamo M, Curwen V, Clamp M. ESTGenes: alternative splicing from ESTs in Ensembl. Genome Res. 2004;14:976–987. doi: 10.1101/gr.1862204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karolchik D, Baertsch R, Diekhans M, Furey TS, Hinrichs A, Lu YT, Roskin KM, Schwartz M, Sugnet CW, Thomas DJ, et al. The UCSC Genome Browser Database. Nucleic Acids Res. 2003;31:51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2007;35:D21–25. doi: 10.1093/nar/gkl986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, Wei L, Gao G. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35:W345–W349. doi: 10.1093/nar/gkm391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerstein MB, Bruce C, Rozowsky JS, Zheng D, Du J, Korbel JO, Emanuelsson O, Zhang ZD, Weissman S, Snyder M. What is a gene, post-ENCODE? History and updated definition. Genome Res. 2007;17:669–681. doi: 10.1101/gr.6339607. [DOI] [PubMed] [Google Scholar]
- 33.Dimitrov RA, Zuker M. Prediction of hybridization and melting for double-stranded nucleic acids. Biophys. J. 2004;87:215–226. doi: 10.1529/biophysj.103.020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markham NR, Zuker M. DINAMelt web server for nucleic acid melting prediction. Nucleic Acids Res. 2005;33:W577–581. doi: 10.1093/nar/gki591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgler C, Macdonald PM. Prediction and verification of microRNA targets by MovingTargets, a highly adaptable prediction method. BMC Genomics. 2005;6:88. doi: 10.1186/1471-2164-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, Sherlock G. GO::TermFinder–open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21:3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 39.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katayama S, Tomaru Y, Kasukawa T, Waki K, Nakanishi M, Nakamura M, Nishida H, Yap CC, Suzuki M, Kawai J, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Sun M, Hurst LD, Carmichael GG, Rowley JD. Genome-wide analysis of coordinate expression and evolution of human cis-encoded sense-antisense transcripts. Trends Genet. 2005;21:326–329. doi: 10.1016/j.tig.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Lash AE, Tolstoshev CM, Wagner L, Schuler GD, Strausberg RL, Riggins GJ, Altschul SF. SAGEmap: a public gene expression resource. Genome Res. 2000;10:1051–1060. doi: 10.1101/gr.10.7.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalmasso C, Broet P, Moreau T. A simple procedure for estimating the false discovery rate. Bioinformatics. 2005;21:660–668. doi: 10.1093/bioinformatics/bti063. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2006;34:D173–D180. doi: 10.1093/nar/gkj158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, Hardison RC, Haussler D, Miller W. Human-mouse alignments with BLASTZ. Genome Res. 2003;13:103–107. doi: 10.1101/gr.809403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kent WJ, Baertsch R, Hinrichs A, Miller W, Haussler D. Evolution's cauldron: duplication, deletion, and rearrangement in the mouse and human genomes. Proc. Natl. Acad. Sci. USA. 2003;100:11484–11489. doi: 10.1073/pnas.1932072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavorgna G, Dahary D, Lehner B, Sorek R, Sanderson CM, Casari G. In search of antisense. Trends Biochem. Sci. 2004;29:88–94. doi: 10.1016/j.tibs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 1995;14:4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl Acad. Sci. USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delihas N, Forst S. MicF: an antisense RNA gene involved in response of Escherichia coli to global stress factors. J. Mol. Biol. 2001;313:1–12. doi: 10.1006/jmbi.2001.5029. [DOI] [PubMed] [Google Scholar]
- 51.Rebhan M, Chalifa-Caspi V, Prilusky J, Lancet D. GeneCards: a novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics. 1998;14:656–664. doi: 10.1093/bioinformatics/14.8.656. [DOI] [PubMed] [Google Scholar]
- 52.Keese PK, Gibbs A. Origins of genes: “big bang” or continuous creation? Proc. Natl Acad. Sci. USA. 1992;89:9489–9493. doi: 10.1073/pnas.89.20.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shintani S, O'HUigin C, Toyosawa S, Michalova V, Klein J. Origin of gene overlap: the case of TCP1 and ACAT2. Genetics. 1999;152:743–754. doi: 10.1093/genetics/152.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seroussi E, Kedra D, Pan HQ, Peyrard M, Schwartz C, Scambler P, Donnai D, Roe BA, Dumanski JP. Duplications on human chromosome 22 reveal a novel Ret Finger Protein-like gene family with sense and endogenous antisense transcripts. Genome Res. 1999;9:803–814. doi: 10.1101/gr.9.9.803. [DOI] [PubMed] [Google Scholar]
- 55.Nishihara H, Smit AF, Okada N. Functional noncoding sequences derived from SINEs in the mammalian genome. Genome Res. 2006;16:864–874. doi: 10.1101/gr.5255506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernstein E, Allis CD. RNA meets chromatin. Genes Dev. 2005;19:1635–1655. doi: 10.1101/gad.1324305. [DOI] [PubMed] [Google Scholar]
- 57.Britten RJ. Mobile elements inserted in the distant past have taken on important functions. Gene. 1997;205:177–182. doi: 10.1016/s0378-1119(97)00399-5. [DOI] [PubMed] [Google Scholar]
- 58.Brosius J. Retroposons–seeds of evolution. Science. 1991;251:753. doi: 10.1126/science.1990437. [DOI] [PubMed] [Google Scholar]
- 59.Peaston AE, Evsikov AV, Graber JH, de Vries WN, Holbrook AE, Solter D, Knowles BB. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell. 2004;7:597–606. doi: 10.1016/j.devcel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Tchurikov NA, Kretova OV. Suffix-specific RNAi leads to silencing of F element in Drosophila melanogaster. PLoS ONE. 2007;2:e476. doi: 10.1371/journal.pone.0000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Lagemaat LN, Medstrand P, Mager DL. Multiple effects govern endogenous retrovirus survival patterns in human gene introns. Genome Biol. 2006;7:R86. doi: 10.1186/gb-2006-7-9-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 63.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lease RA, Belfort M. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc. Natl Acad. Sci. USA. 2000;97:9919–9924. doi: 10.1073/pnas.170281497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moorwood K, Charles AK, Salpekar A, Wallace JI, Brown KW, Malik K. Antisense WT1 transcription parallels sense mRNA and protein expression in fetal kidney and can elevate protein levels in vitro. J. Pathol. 1998;185:352–359. doi: 10.1002/(SICI)1096-9896(199808)185:4<352::AID-PATH119>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 67.Scheele C, Petrovic N, Faghihi MA, Lassmann T, Fredriksson K, Rooyackers O, Wahlestedt C, Good L, Timmons JA. The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC Genomics. 2007;8:74. doi: 10.1186/1471-2164-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pesole G, Liuni S. Internet resources for the functional analysis of 5' and 3' untranslated regions of eukaryotic mRNAs. Trends Genet. 1999;15:378. doi: 10.1016/s0168-9525(99)01795-3. [DOI] [PubMed] [Google Scholar]
- 69.Day DA, Tuite MF. Post-transcriptional gene regulatory mechanisms in eukaryotes: an overview. J. Endocrinol. 1998;157:361–371. doi: 10.1677/joe.0.1570361. [DOI] [PubMed] [Google Scholar]
- 70.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl Acad. Sci. USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furlong RF, Yang Z. Comparative genomics coming of age. Heredity. 2003;91:533–534. doi: 10.1038/sj.hdy.6800372. [DOI] [PubMed] [Google Scholar]
- 74.Pang KC, Frith MC, Mattick JS. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet. 2006;22:1–5. doi: 10.1016/j.tig.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Bailey JA, Yavor AM, Massa HF, Trask BJ, Eichler EE. Segmental duplications: organization and impact within the current human genome project assembly. Genome Res. 2001;11:1005–1017. doi: 10.1101/gr.187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.