Abstract
The DNA cleavage reaction of human topoisomerase IIα is critical to all of the physiological and pharmacological functions of the protein. While it has long been known that the type II enzyme requires a divalent metal ion in order to cleave DNA, the role of the cation in this process is not known. To resolve this fundamental issue, the present study utilized a series of divalent metal ions with varying thiophilicities in conjunction with DNA cleavage substrates that replaced the 3′-bridging oxygen of the scissile bond with a sulfur atom (i.e. 3′-bridging phosphorothiolates). Rates and levels of DNA scission were greatly enhanced when thiophilic metal ions were included in reactions that utilized sulfur-containing substrates. Based on these results and those of reactions that employed divalent cation mixtures, we propose that topoisomerase IIα mediates DNA cleavage via a two-metal-ion mechanism. In this model, one of the metal ions makes a critical interaction with the 3′-bridging atom of the scissile phosphate. This interaction greatly accelerates rates of enzyme-mediated DNA cleavage, and most likely is needed to stabilize the leaving 3′-oxygen.
INTRODUCTION
Topoisomerase IIα is essential for the proliferation of human cells (1–5). The enzyme removes knots, tangles and torsional stress from the genetic material, plays critical roles in replicative processes, and is required for the segregation of sister chromatids during mitosis and meiosis (1–5).
Topoisomerase IIα acts by passing a double helix through a transient double-stranded break that it generates in a separate segment of DNA (2,3,5–7). The ability of the enzyme to cleave and ligate DNA is central to all of its catalytic functions (2,3,5–7). Topoisomerase IIα has two identical subunits, each of which contains an active site tyrosine at position 805 (2,3,5–8). Each tyrosine is responsible for cutting one strand of the double helix, and forms a covalent bond with the 5′-terminal phosphate that results from scission (8–10). A 3′-terminal –OH moiety also is generated by the action of the enzyme. The two scissile bonds are located across the major groove, four bases apart from one another, such that the DNA cleavage event results in four-base, 5′-overhanging cohesive ends (8,9).
Beyond the role of the DNA cleavage event in the normal physiological functions of topoisomerase IIα, this reaction is the primary cellular target for several widely prescribed anticancer agents (2,5,11–14). These drugs, typified by compounds such as etoposide and doxorubicin, are front-line therapy for a variety of hematopoietic, germ-line and solid malignancies (2,5,11–14). Although topoisomerase II-targeted anticancer agents are derived from a number of structural classes, they all function by increasing cellular levels of the covalent enzyme–cleaved DNA complex (i.e. cleavage complex) that is generated during the scission event (2,5,11–14).
Despite the importance of topoisomerase II-mediated DNA cleavage to the regulation of DNA topology and the treatment of human cancers, many aspects of the reaction are poorly understood. To this point, it has long been known that the type II enzyme requires a divalent metal ion in order to cleave DNA (8,9,15–17), but the role of the cation is unclear.
Commonly, enzymes that cleave nucleic acids, including exo- and endonucleases, utilize divalent cations in their active sites to stabilize both attacking (i.e. amino acids and/or water) and leaving groups (i.e. DNA backbone oxygen atoms) (18–37). However, the number of metal ions per active site and the specific roles of these cations in stabilizing leaving groups (bridging versus nonbridging oxygen) varies. For example, based on structural studies, it has been proposed that several DNA polymerases utilize two divalent cations for both their 3′→5′ proofreading exonuclease and 5′→3′ polymerization reactions (18,21). Kinetic studies with these enzymes suggest that interactions between one of the active site divalent cations and the bridging oxygen of the DNA backbone play an important role in supporting catalysis (21,24). Although some ribozymes utilize two metal ions to catalyze self-splicing, others require only one (20,27,38–43). Furthermore, interactions with both the bridging and nonbridging oxygens have been described (27,39,42). Finally, structural studies imply that the restriction endonuclease, BglII, uses only a single divalent cation and that it coordinates with the nonbridging oxygen of the scissile phosphate (29,44).
Based on mutagenesis and enzymological studies with DNA gyrase, Noble and Maxwell suggested that the prokaryotic type II topoisomerase utilizes a two-metal-ion mechanism similar to that of DNA polymerases (32). However the specific roles of the divalent cations in DNA gyrase were not characterized.
Recently, a crystal structure of the catalytic core of yeast topoisomerase II complexed with DNA was solved (45). While the structure showed only a single Mg2+ atom per active site, the enzyme–DNA complex was noncovalent in nature. Furthermore, it was noted that the active site tyrosine was too far away from the scissile bond to be in a cleavage competent configuration (45). Consequently, the number of divalent metal ions in the active site of eukaryotic type II topoisomerases and their role(s) in the DNA cleavage reaction remain open questions.
To resolve these fundamental issues, the present study utilized a series of divalent metal ions with varying thiophilicities in conjunction with DNA cleavage substrates that replaced the 3′-bridging oxygen of the scissile bond with a sulfur atom (i.e. 3′-bridging phosphorothiolates). Results indicate that human topoisomerase IIα mediates DNA cleavage via a two-metal-ion mechanism and that one of the required divalent cations makes critical interactions with the bridging atom of the scissile bond during this process.
MATERIALS AND METHODS
Enzymes and materials
Human topoisomerase IIα was expressed in Saccharomyces cerevisiae and purified as described previously (46–48).
Two 50-base oligonucleotide duplexes were designed using previously identified topoisomerase II cleavage sites from pBR322. These sites correspond to sequences designated as sites 1 and 2 by Fortune et al. (49). Wild-type oligonucleotide sequences were generated using an Applied Biosystems (Foster City, CA, USA) DNA synthesizer. The 50-mer site 1 sequences for the top and bottom strands were 5′-AGCGGTATCAGCTCACTCAAAGGC↓GGTAATACGGTTATCCACAGAATCAG-3′ and 5′-CTGATTCTGTGGATAACCGTAT↓TACCGCCTTTGAGTGAGCTGATACCGCT-3′, respectively (arrow denotes point of cleavage). The 50-mer site 2 top and bottom sequences were 5′-TTGGTATCTGCGCTCTGCTGAAGCC↓AGTTACCTTCGGAAAAAGAGTTGGT-3′ and 5′-ACCAACTCTTTTTCCGAAGGT↓AACTGGCTTCAGCAGAGCGCAGATACCAA-3′, respectively.
DNA containing a single 3′-bridging phosphorothiolate linkage was synthesized as described previously (50). The location of the phosphorothiolate was either at the normal scissile bond (sites 1 and 2) or one position 3′ from the normal scissile bond (site 1b). Substrates containing a racemic phosphorothioate in place of the nonbridging scissile bond oxygens of the bottom strand of site 2 were synthesized by Operon. In some cases, oligonucleotides contained a site-specific nick at the scissile bond of the top strand.
[γ-32P]ATP (∼5000 Ci/mmol) was obtained from MP Biomedicals (Irvine, CA, USA). Single-stranded oligonucleotides were labeled on their 5′ termini using T4 polynucleotide kinase (New England Biolabs, Ipswich, MA, USA). Following labeling and gel purification, complementary oligonucleotides were annealed by incubation at 70°C for 10 min and cooling to 25°C.
Topoisomerase II-mediated DNA cleavage
DNA cleavage assays were carried out by a modification of the procedure of Fortune et al. (49). Oligonucleotide substrates were always 5′-end-labeled. Unless otherwise stated, DNA cleavage reactions contained 200 nM human topoisomerase IIα and 100 nM double-stranded oligonucleotide in a total of 20 μl of cleavage buffer: 10 mM Tris–HCl (pH 7.9), 135 mM KCl, 5 mM divalent cation, 0.1 mM EDTA and 2.5% glycerol. In some cases, the concentration of divalent cation (MgCl2, MnCl2, CaCl2 or CoCl2) was varied and/or combinations of the cations were used. Studies incorporating experiments that monitored DNA cleavage over a range that included divalent cation concentrations below 1 mM utilized cleavage buffer that lacked EDTA. Reactions were initiated by the addition of enzyme and were incubated for 0–30 min at 37°C. DNA cleavage products were trapped by the addition of 2 μl of 10% SDS followed by 2 μl of 250 mM NaEDTA (pH 8.0). Proteinase K (2 μl of 0.8 mg/ml) was added to digest the enzyme, and cleavage products were resolved by electrophoresis in a 14% denaturing polyacrylamide gel. To inhibit oxidation of cleaved oligonucleotides containing 3′-terminal –SH moieties and the formation of multimers in the gel, 100 mM DTT was added to the sample loading buffer (50). DNA cleavage products were visualized and quantified using a Bio-Rad (Hercules, CA, USA) Molecular Imager.
In order to determine rate constants for DNA cleavage, pre-equilibrium reactions were monitored for 5 ms to 3 s using a KinTek (Austin, TX) model RQF-3 chemical quench flow apparatus. Cleavage was initiated by rapidly mixing equal volumes of two independent solutions. The first contained a noncovalent complex formed between human topoisomerase IIα and 32P-labeled oligonucleotide in cleavage buffer that lacked divalent cation. The second solution contained cleavage buffer in which the divalent cation concentration was 2 times higher than normal (10 mM). The two solutions were mixed at 37°C, and DNA cleavage was quenched with 1% SDS (v/v final concentration). Products were processed and analyzed as described earlier. The observed rate constant of DNA cleavage was determined by a fit of the data to the double exponential equation y = b1(1−e−k1X) + b2(1−e−k2X), where y is the percent of DNA cleavage, b is the amplitude, k is the observed rate constant and X is the time.
RESULTS AND DISCUSSION
DNA cleavage mediated by human topoisomerase IIα is promoted by an interaction between a divalent metal ion and the bridging atom of the scissile phosphate
Although the DNA cleavage reaction of human type II topoisomerases is central to their physiological functions and their roles as targets for anticancer drugs (8,9,15–17,32), the mechanism of the reaction has not been fully described. The only cofactor that is required for DNA scission is a divalent metal ion, which appears to be Mg2+ in vivo (9,15–17,32). However, the contribution of the divalent cation to enzyme function is not understood. Therefore, the role of the metal ion in the DNA cleavage reaction mediated by human topoisomerase IIα was characterized.
Several nucleases that generate a 3′-OH and 5′-phosphate during cleavage require an interaction between a metal ion and the 3′-bridging atom of the scissile phosphate (22,28). Consequently, as a first step in exploring the requirement for a divalent cation in the DNA cleavage reaction of human topoisomerase IIα, the interaction between the divalent cation and the 3′-bridging atom was assessed. Experiments took advantage of novel phosphorothiolate-containing oligonucleotide substrates that substituted a 3′-bridging sulfur for the oxygen atom at the scissile phosphate (i.e. S–P scissile bond) (50). Topoisomerase IIα cleaves these oligonucleotides with all of the characteristics of wild-type substrates, with the following exception: since the resulting 3′-terminal –SH moiety is a poor nucleophile at phosphorous, 3′-bridging phosphorothiolates do not support ligation (50). As a result, these substrates isolate the forward DNA scission event from ligation, and therefore allow high levels of cleavage complexes to accumulate (50). This is in contrast to wild-type oligonucleotides with 3′-bridging oxygen atoms (i.e. O–P scissile bond), which establish a rapid DNA cleavage–ligation equilibrium and maintain low levels of cleavage complexes (50). Due to the characteristics of DNA scission with these substrates, both rates and levels of cleavage can be determined with S–P oligonucleotides. However, only levels of cleavage can be monitored with O–P oligonucleotides in ‘bench top’ experiments.
Interactions between the divalent cation and the 3′-bridging atom of the scissile phosphate were determined by comparing the ability of topoisomerase IIα to cleave DNA substrates containing either a 3′-bridging oxygen or sulfur atom in the presence of metal ions of varying ‘hardness’ (i.e. thiophilicity). The ions that were used for this study were Ca2+, Mg2+, Co2+ and Mn2+. Within this series, Co2+ and Mn2+ are the ‘softest’, or most thiophilic metals, and Mg2+ and Ca2+ are harder, or less thiophilic (51–53). Softer metal ions often prefer sulfur over oxygen as an inner-sphere ligand, while hard metals usually coordinate more readily with oxygen (21,24,27,38–42,51–53).
The DNA cleavage reaction of human topoisomerase IIα generates a 3′-oxygen leaving group in a divalent metal ion-dependent manner. If there is a direct interaction between the metal ion and the leaving group that facilitates catalysis, relative rates (or levels) of scission with substrates containing a 3′-sulfur in place of the oxygen should increase in the presence of soft (thiophilic) metals (21,24,27,38–42). Conversely, less cleavage should be generated in reactions that contain hard metals (21,24,27,38–42).
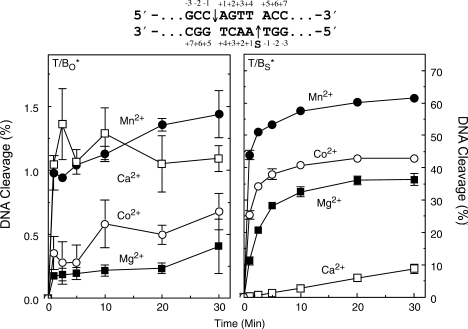
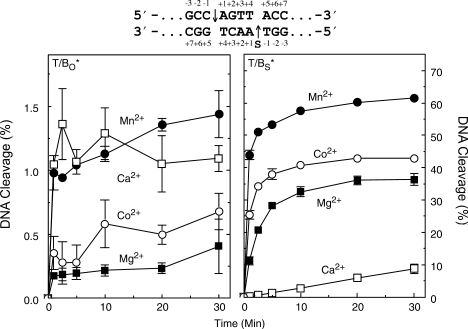
The first set of experiments established the capacity of divalent metal ions to support topoisomerase IIα-mediated cleavage of a wild-type O–P substrate (site 2 oligonucleotide). Consistent with previous work with plasmid substrates (17), Mn2+, Ca2+ and Co2+ all generated higher levels of DNA cleavage than Mg2+ (Figure 1, left panel). Levels of cleavage complexes formed in the presence of Mn2+ or Ca2+ were ∼4 times higher than those observed with Mg2+, and those with Co2+ were ∼2 times higher.
Figure 1.
Cleavage of oligonucleotide substrates mediated by human topoisomerase IIα in the presence of different divalent metal ions. Results for wild-type (O–P, T/BO*, left) and phosphorothiolate (S–P, T/BS*, right) substrates are shown. The asterisk denotes the 5′-end-labeled strand. The wild-type duplex containing an O–P scissile bond (subscript O) is denoted by T/BO* for the labeled bottom strand. The phosphorothiolate substrate containing an S–P scissile bond (subscript S) is denoted by T/BS* for the labeled phosphorothiolate bottom strand. DNA cleavage was carried out in the presence of 5 mM Mg2+ (closed square), Mn2+ (closed circle), Ca2+ (open square) or Co2+ (open circle). The cleavage sequence (site 2) is depicted above the graphs with the scissile bonds denoted on the top and bottom strands by arrows. ‘S’ on the bottom strand denotes the location of the phosphorothiolate modification. Error bars represent the standard deviation (SD) of at least three independent experiments.
The divalent cation preference for reactions that monitored cleavage of the equivalent S–P oligonucleotide is shown in the right panel of Figure 1. Rates and levels of cleavage observed in the presence of the thiophilic metal ions, Mn2+ and Co2+, were still higher than those generated with Mg2+. However, results with Ca2+, a hard metal, were markedly different. The initial velocity of DNA cleavage in the presence of Ca2+ was 1–2 orders of magnitude slower than that seen with Mg2+. Similar results were found using a different matched pair of O–P and S–P substrates (site 1 oligonucleotide; data not shown).
The precipitous drop in Ca2+-supported cleavage of the S–P, as compared to the O–P substrate, together with the maintenance of high DNA cleavage rates and levels with Mn2+ and Co2+, strongly suggest that there is an important interaction between the metal ion and the 3′-bridging atom of the scissile bond in the active site of human topoisomerase IIα.
In order to investigate this interaction in greater detail, rates of DNA cleavage with both the O–P and S–P substrates were compared. Since wild-type substrates attain a cleavage–ligation equilibrium too quickly to ascertain rates in bench top experiments, a rapid chemical quench system was employed. Unfortunately, over the course of these assays (0–3 s), levels of cleavage of wild-type O–P oligonucleotides were too low to generate reliable rate constants. Therefore, a nick was introduced at the scissile bond on the opposite strand from the one being monitored. These nicked oligonucleotides do not act as ‘suicide substrates’, and rapidly reach a cleavage–ligation equilibrium when opposite an O–P scissile bond (50). However, they maintain ∼10-fold higher levels of cleavage complexes at equilibrium (50).
As a prelude to rapid quench experiments, bench top assays were carried out with these nicked substrates. Results were similar to those obtained with the corresponding un-nicked oligonucleotides (Figure 2). Accordingly, Mn2+, Ca2+ and Co2+ all supported higher levels of DNA cleavage than Mg2+ with the O–P substrate (Figure 2, left panel). Furthermore, rates and levels of cleavage observed in the presence of Mn2+ and Co2+ were higher than those generated with Mg2+ using the S–P substrate, and the initial velocity of DNA cleavage supported by Ca2+ was >10 times slower than that seen with Mg2+ (Figure 2, right panel). Once again, these findings support the conclusion that the divalent metal ion interacts with the bridging atom of the scissile bond and promotes the DNA cleavage reaction of topoisomerase IIα.
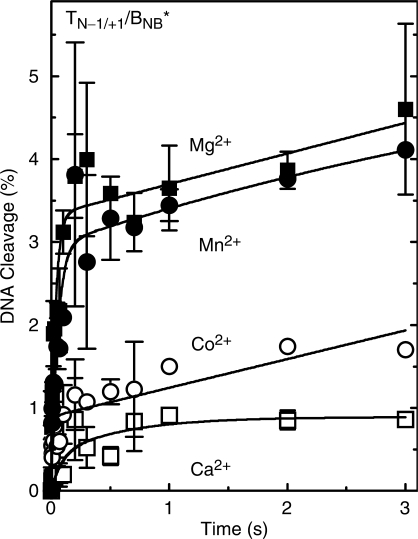
Figure 2.
Cleavage of nicked oligonucleotide substrates by topoisomerase IIα in the presence of different divalent metal ions. The nick is located at the scissile bond on the unlabeled strand (–1/+1 position). Results for the O–P (TN−1/+1/BO*, left) and S–P (TN−1/+1/BS*, right) nicked substrates are shown. DNA cleavage was carried out in the presence of 5 mM Mg2+ (closed square), Mn2+ (closed circle), Ca2+ (open square) and Co2+ (open circle). The cleavage sequence (site 2) is depicted above the graphs with the scissile bonds denoted on the top and bottom strands by arrows. ‘N’ on the top strand denotes the location of the single-stranded nick, and ‘S’ on the bottom strand denotes the location of the phosphorothiolate modification. Error bars represent the SD of at least three independent experiments.
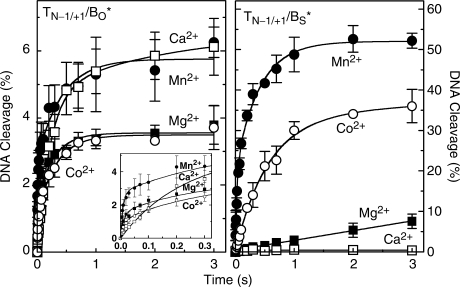
Rapid quench kinetic data for the cleavage of the O–P and S–P substrates are shown in Figure 3. The earliest time point that could be monitored was 5 ms, and reactions were followed up to 3 s. The data were best fit using a double exponential rate equation. The mechanistic basis for this fit is unclear at the present time.
Figure 3.
Pre-equilibrium cleavage of nicked oligonucleotide substrates by topoisomerase IIα in the presence of different divalent metal ions. Reaction times varied from 5 ms to 3 s. Results for the O–P (TN−1/+1/BO*, left) and S–P (TN−1/+1/BS*, right) nicked substrates are shown. The nick was located at the scissile bond on the unlabeled strand (–1/+1 position, see Figure 2). DNA cleavage was carried out in the presence of 5 mM Mg2+ (closed square), Mn2+ (closed circle), Ca2+ (open square) and Co2+ (open circle). A double exponential curve is plotted for each reaction. The inset in the left panel shows reaction times up to 300 ms. Error bars represent the SD of at least three independent experiments.
Although DNA cleavage levels in the presence of Co2+ and Ca2+ ultimately were as high or higher than observed with Mg2+ and the nicked O–P substrate, reaction velocities at the earliest time points were somewhat slower (left panel). Consequently, rate constants calculated for these divalent metal ions were ∼0.77 and 0.050 relative to that derived for Mg2+ (Table 1). In contrast, the calculated rate constant for Mn2+ was ∼1.3 times greater than that for Mg2+.
Table 1.
Observed double exponential rate constants for metal ion-promoted DNA cleavage mediated by human topoisomerase IIα
| Cation | Oligonucleotidea |
||
|---|---|---|---|
| O–P | S–P | RP/SP | |
| kobs, M−1 s−1 (kobs/kobs Mg2+)b | |||
| Mg2+ | 92 ± 20 (1.0) | 5.1 × 10−7 ± 0.15 (1.0) | 29 ± 7.0 (1.0) |
| Mn2+ | 120 ± 26 (1.3) | 35 ± 6.2 (6.8 × 107) | 19 ± 5.6 (0.66) |
| Co2+ | 71 ± 20 (0.77) | 1.8 ± 0.99 (3.5 × 106) | 70 ± 26 (2.4) |
| Ca2+ | 4.6 ± 1.0 (0.050) | ND | 7.3 ± 3.8 (0.25) |
ND, not determined (i.e. could not be calculated).
aWild-type, O–P; 3′-bridging phosphorothiolate, S–P and racemic nonbridging phosphorothioate, RP/SP.
bData represent values for kobs ± SD based on three independent experiments. Included in parenthesis is the relative observed rate constant compared to the value for Mg2+ (kobs/kobs Mg2+).
Results with the nicked S–P oligonucleotide (Figure 3, right panel) paralleled those seen in bench top experiments. Relative to the rate constant derived for Mg2+, those for the thiophilic divalent cations, Mn2+ and Co2+, were ∼6.8 × 107 and 3.5 × 106 times greater, respectively (Table 1). Conversely, rates for Ca2+-supported reactions were so low that a rate constant could not be calculated. As above, these data indicate that the metal ion interacts with the bridging atom of the scissile bond and that this interaction is important for the DNA cleavage event mediated by topoisomerase IIα.
Divalent metal ion interactions with the nonbridging atom of the scissile phosphate
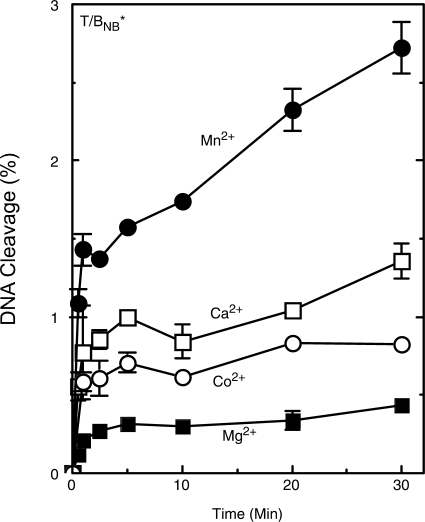
Interactions between a metal ion and the nonbridging oxygen atom of the scissile phosphate have been observed or postulated for a number of enzymes that cleave or join nucleic acids (18,21,29,32,44). In order to determine whether such an interaction takes place in the active site of human topoisomerase IIα, a racemic phosphorothioate substrate (site 2 oligonucleotide) that substituted a sulfur atom for either the RP or SP nonbridging oxygen atom of the scissile phosphate was employed. The first experiment was carried out on the bench top and monitored DNA cleavage with an un-nicked oligonucleotide (Figure 4). Results were similar to those observed in Figure 1 for the wild-type substrate that contained no sulfur atoms. It is notable that nonbridging phosphorothiolates also have little effect on levels of DNA cleavage mediated by E. coli gyrase (54).
Figure 4.
Cleavage of an oligonucleotide substrate containing a nonbridging (ND) phosphorothioate by topoisomerase IIα in the presence of different divalent metal ions. Reactions included a racemic mixture of phosphorothioate substrates in which the nonbridging oxygen atoms at the scissile phosphate on the labeled strand were replaced by sulfur atoms. DNA cleavage was carried out in the presence of 5 mM Mg2+ (closed square), Mn2+ (closed circle), Ca2+ (open square) and Co2+ (open circle). Error bars represent the SD of at least three independent experiments.
The second experiment utilized the rapid quench kinetic system in conjunction with a nicked phosphorothioate substrate to generate rate constants for DNA cleavage supported by the four divalent cations (Figure 5). Once again, results were similar to those calculated with the nicked O–P oligonucleotide (Table 1). Relative to Mg2+, calculated rate constants for Mn2+, Co2+ and Ca2+ were ∼0.66, 2.4 and 0.25, respectively.
Figure 5.
Pre-equilibrium cleavage of a nicked oligonucleotide substrate containing a nonbridging (ND) phosphorothioate by topoisomerase IIα in the presence of different divalent metal ions. Reaction times varied from 5 ms to 3 s. The substrate is the same as used in Figure 4 except that there is a nick at the scissile bond of the unlabeled strand (–1/+1 position). DNA cleavage was carried out in the presence of 5 mM Mg2+ (closed square), Mn2+ (closed circle), Ca2+ (open square) and Co2+ (open circle). A double exponential curve is plotted for each reaction. Error bars represent the SD of at least three independent experiments.
The above data with the racemic phosphorothioate substrate cannot rule out an interaction between a divalent cation and one of the nonbridging oxygen atoms of the scissile phosphate. However, if such an interaction exists in the active site of topoisomerase IIα, its effect on the DNA cleavage reaction of the enzyme appears to be equivocal.
Human topoisomerase IIα uses two divalent metal ions for DNA cleavage
A number of nucleic acid enzymes, including polymerases, nucleases, ribozymes and DNA gyrase have been postulated to use two divalent metal ions in their active sites (19,20,22,23,25–27,29–31,33–37). In the case of most protein-based enzymes, evidence comes primarily from crystallographic studies and the kinetic analysis of mutant enzymes in which amino acids that interact with active site metal ions are altered (18,19,21,25,26,29,30,32). This latter approach was used with DNA gyrase (32). In the case of ribozymes and the 3′→5′ exonuclease activity of E. coli DNA polymerase I, strong evidence for the use of two metal ions comes from kinetic analysis of RNA sequences that replaced oxygen atoms of the scissile phosphate with sulfur atoms (20,27,38–43). We employed this latter strategy to determine whether human topoisomerase IIα utilizes one or two divalent metal ions during its DNA cleavage reaction.
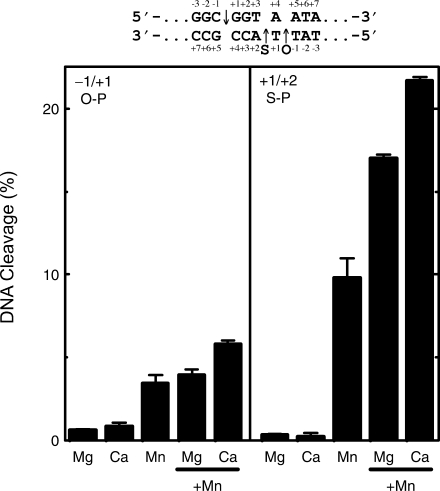
As a first approach, a cleavage substrate (shown in Figure 6) was used that contained a 3′-bridging phosphorothiolate located one base 3′ (+1/+2 position) to the normal scissile bond (–1/+1 position). Site 1b, as opposed to site 2, was employed for this study because it contained a thymine residue at the +1 position. Due to the chemistry involved in generating the 3′-bridging phosphorothioamidite, only modified thymines were available (50).
Figure 6.
Cleavage of a phosphorothiolate linkage that is located one base 3′ to the intrinsic scissile bond. DNA cleavage levels were quantified at both the original O–P scissile bond (–1/+1, O–P, left) and the S–P bond (+1/+2, S–P, right). The sequence (site 1b) is shown above the graphs, and the location of the wild-type scissile bond (‘O’) and the phosphorothiolate bond (‘S’) are denoted. Reactions included Mg2+, Ca2+ or Mn2+ alone, as well as mixtures of Mg2+ and Mn2+, or Ca2+ and Mn2+. All reactions had a total divalent cation concentration of 5 mM (2.5 mM of each divalent cation for reactions with two different divalent cations). Results shown are for a 30 min time point. Error bars represent the SD of at least three independent experiments.
DNA cleavage mediated by topoisomerase IIα was monitored simultaneously at the normal (–1/+1) O–P scissile bond as well as the off-site (+1/+2) S–P bond. Since these bonds are adjacent to one another, the enzyme is forced to choose between the two sites.
Mg2+, Ca2+ and Mn2+ were used for these experiments. Time courses for cleavage were followed up to 30 min. Data for the 30-min time point are displayed in Figure 6. Results for cleavage at the normal O–P scissile bond are shown in the left panel. Consistent with the data from Figure 1, 5 mM Mn2+ supported higher levels of DNA scission than either Mg2+ or Ca2+. A mixture (2.5 + 2.5 mM) of Mg2+ and Mn2+ produced levels of DNA cleavage that were similar to that of 5 mM Mn2+ alone. However, the corresponding mixture of Ca2+ and Mn2+ supported cleavage that was ∼1.7 times greater than that seen with Mn2+ alone. This result suggests that topoisomerase IIα may utilize two divalent metal ions in its cleavage reaction.
More dramatic results were seen at the off-site bond (Figure 6, right panel). Of the three divalent cations employed, only 5 mM Mn2+ was able to support efficient cleavage at the +1/+2 S–P site. This probably is due to the fact that Mn2+ is considerably softer than either Mg2+ or Ca2+ (21,24,27,38–42), and is therefore able to alter the cleavage specificity of topoisomerase IIα in the presence of a 3′-bridging sulfur. Mixtures (2.5 + 2.5 mM) of Mg2+ + Mn2+ or Ca2+ + Mn2+ resulted in levels of cleavage that were ∼1.7- or 2.2-fold higher than observed with 5 mM Mn2+ alone. As above, this finding argues for the use of two divalent metal ions in the active site of topoisomerase IIα.
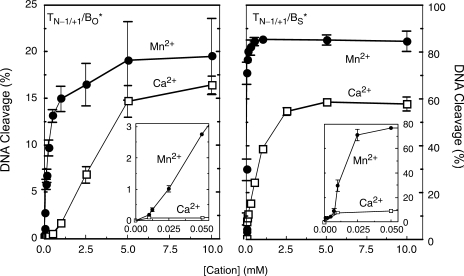
In light of the above results, two additional approaches were employed to further explore whether two metal ions are involved in catalysis. In the first, DNA cleavage was monitored over a range of divalent cation concentrations. These experiments utilized the site 2 substrate that contained a nick at the scissile bond (–1/+1 position) on the opposite strand. As discussed earlier, this substrate generates higher levels of DNA cleavage than observed with the fully wild-type oligonucleotide (50). Mn2+ (a soft cation) and Ca2+ (a hard cation) were used for these studies. As seen in Figure 7, the concentration of Mn2+ that was required to promote DNA scission mediated by topoisomerase IIα was considerably lower than that seen with Ca2+.
Figure 7.
Metal ion concentration dependence for DNA cleavage of wild-type and phosphorothiolate substrates by topoisomerase IIα. Results for the nicked (–1/+1 position) O–P (TN−1/+1/BO*, left) and S–P substrate (TN−1/+1/BS*, right) are shown. Left panel, Mn2+ (closed circles) or Ca2+ (open squares) were titrated from 10 μM to 10 mM. Reactions were incubated for 30 min prior to stopping the reaction. The inset shows DNA cleavage at concentrations up to 50 μM. Right panel, Mn2+ (closed circles) or Ca2+ (open squares) were titrated from 1 μM (for Mn2+) or 10 μM (for Ca2+) to 10 mM. Reactions were incubated for 30 min prior to stopping the reaction. The inset shows concentrations up to 50 μM. Error bars represent the SEM of two independent experiments or the SD of at least three independent experiments.
When cleavage of the O–P oligonucleotide was monitored (Figure 7, left panel), both divalent cations displayed a biphasic concentration dependence. In addition, the initial phase that was seen with Ca2+ was more pronounced than observed with Mn2+. When cleavage of the S–P oligonucleotide was monitored (Figure 7, right panel), the opposite was seen. While the initial phase observed with Mn2+ became more prominent, that with Ca2+ essentially disappeared. The biphasic metal ion concentration dependence implies that there are two divalent cation sites in the DNA cleavage–ligation site of topoisomerase IIα and that both need to be filled in order to support scission. The finding that the initial (i.e. high affinity) phase with the S–P substrate became more prominent with the softer metal ion (Mn2+) and was lost with the harder metal ion (Ca2+), suggests that the first site that is filled by the divalent cation is the one that interacts with the bridging atom of the scissile bond.
A second approach was employed to confirm that two metal ions are required to support DNA cleavage mediated by human topoisomerase IIα. These studies took advantage of the enhanced affinity of Mn2+ and the decreased affinity of Ca2+ for substrates with 3′-bridging sulfur atoms. Experiments compared levels and rates of DNA scission monitored in the presence of Ca2+, Mn2+ or a combination of the two divalent cations. Near saturating concentrations of Ca2+ (5 and 2.5 mM with the O–P and S–P oligonucleotides, respectively) were paired with concentrations of Mn2+ that bound the scissile bond site without appreciably filling the second site (25 and 5 µM with the O–P and S–P oligonucleotides, respectively). These limiting concentrations of Mn2+ supported levels of enzyme-mediated DNA cleavage that were ∼10-fold lower than generated at the concentrations of Ca2+ that were used (Figure 7).
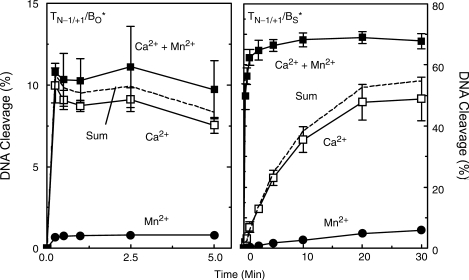
Combining metal ions in reactions that utilized a wild-type substrate had little effect (Figure 8, left panel). Levels of DNA cleavage were only slightly higher than predicted by adding the amount of cleavage generated in the presence of the two individual divalent cations. This result was not surprising, since cleavage of the O–P bond of the site 1b oligonucleotide (Figure 6) was stimulated only moderately by the presence of the two metal ions. Similarly, levels of cleavage mediated by wild-type DNA gyrase were not enhanced in the presence of two different metal ions as compared to the individual cations (32). Stimulation of DNA cleavage by two divalent cations was observed only when residues that interacted with metal ions in the active site of the prokaryotic type II enzyme were altered (32).
Figure 8.
Cleavage of oligonucleotide substrates by topoisomerase IIα in the simultaneous presence of two different divalent metal ions. Results for the nicked (–1/+1 position) O–P substrate (TN−1/+1/BO*, left) and S–P substrate (TN−1/+1/BS*, right) are shown. Left panel, time courses (up to 5 min) were carried out in the presence of 5 mM Ca2+ alone (open squares), 25 μM Mn2+ alone (closed circles) or a mixture of 5 mM Ca2+ and 25 μM Mn2+ (closed squares). The calculated sum of the data from reactions containing Ca2+ or Mn2+ alone also is plotted (dashed line, ‘Sum’). Right panel, time courses (up to 30 min) were carried out in the presence of 2.5 mM Ca2+ alone (open squares), 5 μM Mn2+ alone (closed circles) or a mixture of 2.5 mM Ca2+ and 5 μM Mn2+ (closed squares). Once again, the calculated sum of the data from reactions containing Ca2+ or Mn2+ alone also is plotted (dashed line, ‘Sum’). Error bars represent the SD of three independent experiments or in some cases the SEM of two independent experiments.
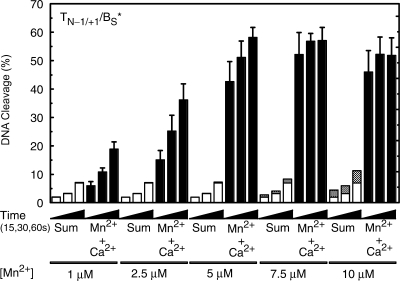
In contrast to results with O–P DNA, a dramatic difference was seen in reactions that utilized the S–P substrate (Figure 8, right panel). The initial velocity of DNA scission in reactions that contained both 2.5 mM Ca2+ and 5 µM Mn2+ (∼3.5% s−1) was nearly 40 times faster than the calculated rate derived from the sum of cleavage observed in the presence of the individual metal ions (∼0.09% s−1). Furthermore, a large enhancement in the rate and level of DNA scission (compared to calculated sums) was observed when 2.5 mM Ca2+ was combined with a range of Mn2+ concentrations (1–10 µM) that partially filled or saturated the high affinity scissile bond site (Figure 9). It is notable that once the scissile bond site was saturated with Mn2+ (7.5 µM), the inclusion of higher levels of Mn2+ did not stimulate DNA cleavage further.
Figure 9.
Cleavage of nicked S–P (TN−1/+1/BS*) oligonucleotide substrates by topoisomerase IIα in the presence of different divalent metal ions. DNA cleavage reactions were carried out for 15, 30 or 60 s in the presence of 2.5 mM Ca2+ alone (open bars), 1–10 μM Mn2+ alone (stippled bars), or a mixture of 2.5 mM Ca2+ and 1–10 μM Mn2+ (Ca2+ + Mn2+, closed bars). The calculated sum of the enzyme-mediated DNA cleavage from reactions containing either Ca2+ or Mn2+ alone is also shown (Sum, stacked open and stippled bars, respectively). All data represent the average of at least three independent experiments. Error bars for reactions carried out in the presence of Ca2+ + Mn2+ are shown. Error bars for reactions carried out in the presence of Ca2+ or Mn2+ alone are not shown for simplicity. However, the SD for these latter reactions never exceeded 1.7%.
Taken together, these results provide strong evidence that human topoisomerase IIα utilizes two metal ions in its cleavage–ligation active site and that both must be present in order for the enzyme to cleave the DNA backbone.
A two–metal-ion model for DNA cleavage mediated by human topoisomerase IIα
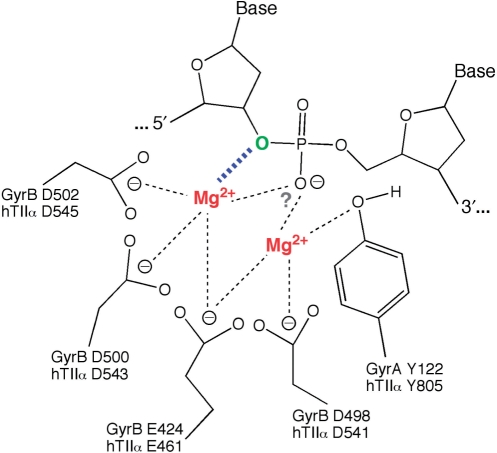
A model for the role of the divalent metal ion in the DNA cleavage reaction of human topoisomerase IIα is proposed in Figure 10. Amino acids that are postulated to interact with the metal ions in the active site of topoisomerase IIα are D541, D543, D545 and E461. These assignments are based on previous enzymological studies of altered E. coli gyrase proteins in which homologous residues were mutated (32), as well as structural studies of prokaryotic topoisomerase III, primase (dnaG) and other DNA enzymes that contain the Toprim domain (55–57). Moreover, they are consistent with a recent crystal structure of the yeast topoisomerase II catalytic core in a complex with nicked DNA (45). In this structure, residues homologous to D541, D543 and E461 of the human enzyme were in close proximity to the observed Mg2+ atom. However, as noted earlier, the yeast topoisomerase II-DNA complex was noncovalent in nature and the active site tyrosine was too far away from the scissile bond to promote cleavage. Mutagenesis studies with topoisomerase IIα currently are underway to address the validity of these assignments in the human enzyme.
Figure 10.
A two-metal-ion model for DNA cleavage by human topoisomerase IIα. Details are described in the text. Amino acids that are postulated to interact with the metal ions in the active site of topoisomerase IIα are D541, D543, D545 and E461. Corresponding amino acids in the A subunit of E. coli DNA gyrase are shown for comparison. The model postulates that one of the metal ions (left, shown in red) makes a critical interaction with the 3′-bridging atom of the scissile phosphate (bond shown in blue), which most likely is needed to stabilize the leaving 3′-oxygen (shown in green). While a second metal ion (right, shown in red) is required for DNA scission, our data provide no direct insight into its specific role during cleavage. However, based on a model proposed for E. coli DNA gyrase (32), this divalent cation may stabilize the DNA transition state and/or help deprotonate the active site tyrosine (Y805). Finally, if an interaction between one of the metal ions (or both) and the nonbridging atoms of the scissile phosphate exists, it does not appear to have a major effect on rates of DNA scission. The figure was adapted from Ref. (32).
Our model for topoisomerase IIα has two salient features. The first is a critical interaction between one of the divalent metal ions and the 3′-bridging atom of the scissile phosphate. This interaction greatly accelerates rates of enzyme-mediated DNA cleavage and most likely is needed to stabilize the leaving 3′-oxygen. While kinetic studies cannot rule out an interaction between a metal ion and the nonbridging atoms of the scissile phosphate, such an interaction (if it exists) does not appear to have a major effect on rates of DNA scission.
The other important feature is a requirement for a second divalent metal ion in the DNA cleavage–ligation active site of the human type II enzyme. Although our data provide strong evidence for the presence of this second metal ion, they offer no direct insight into the specific role that it plays during DNA scission. However, based on a model proposed for E. coli DNA gyrase (32), we postulate that this divalent cation may stabilize the DNA transition state and/or help deprotonate the active site tyrosine (Y805).
In conclusion, while type II topoisomerases require a divalent metal ion in order to cleave DNA, the role of the cation in the scission process was not understood. Based on the studies described above, we propose that human topoisomerase IIα cleaves DNA using a two-metal-ion mechanism in which one of the divalent cations makes a critical interaction with the 3′-bridging atom of the scissile phosphate and facilitates the cleavage event.
ACKNOWLEDGEMENTS
We would like to express our appreciation to Dr F. Peter Guengerich for use of the rapid quench apparatus. We also would like to thank Dr Robert Eoff for instrument training and helpful discussions regarding rapid quench kinetics. We are grateful to Dr Omari J. Bandele, Amanda C. Gentry and Steven L. Pitts for critical reading of the manuscript. This article was provided by National Institutes of Health grant GM33944 J.E.D. was a trainee under National Institutes of Health grant T32 CA09592.
Funding to pay the Open Access publication charges for this article was provided by National Institutes of Health grant GM33944.
Conflict of interest statement. None declared.
REFERENCES
- 1.Nitiss JL. Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochim. Biophys. Acta. 1998;1400:63–81. doi: 10.1016/s0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 2.Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog. Nucleic Acid Res. Mol. Biol. 2000;64:221–253. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- 3.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 4.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 5.McClendon AK, Osheroff N. DNA topoisomerase II, genotoxicity, and cancer. Mutat. Res. 2007;623:83–97. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JC. Moving one DNA double helix through another by a type II DNA topoisomerase: the story of a simple molecular machine. Quart. Rev. Biophys. 1998;31:107–144. doi: 10.1017/s0033583598003424. [DOI] [PubMed] [Google Scholar]
- 7.Berger JM, Gamblin SJ, Harrison SC, Wang JC. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 8.Liu LF, Rowe TC, Yang L, Tewey KM, Chen GL. Cleavage of DNA by mammalian DNA topoisomerase II. J. Biol. Chem. 1983;258:15365–15370. [PubMed] [Google Scholar]
- 9.Sander M, Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J. Biol. Chem. 1983;258:8421–8428. [PubMed] [Google Scholar]
- 10.Zechiedrich EL, Christiansen K, Andersen AH, Westergaard O, Osheroff N. Double-stranded DNA cleavage/religation reaction of eukaryotic topoisomerase II: evidence for a nicked DNA intermediate. Biochemistry. 1989;28:6229–6236. doi: 10.1021/bi00441a014. [DOI] [PubMed] [Google Scholar]
- 11.Li TK, Liu LF. Tumor cell death induced by topoisomerase-targeting drugs. Annu. Rev. Pharmacol. Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 12.Pommier Y, Marchand C. Interfacial inhibitors of protein-nucleic acid interactions. Curr. Med. Chem. Anticancer Agents. 2005;5:421–429. doi: 10.2174/1568011054222337. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- 14.Martincic D, Hande KR. Topoisomerase II inhibitors. Cancer Chemother. Biol. Response Modif. 2005;22:101–121. doi: 10.1016/s0921-4410(04)22005-1. [DOI] [PubMed] [Google Scholar]
- 15.Osheroff N. Role of the divalent cation in topoisomerase II mediated reactions. Biochemistry. 1987;26:6402–6406. doi: 10.1021/bi00394a015. [DOI] [PubMed] [Google Scholar]
- 16.Osheroff N, Zechiedrich EL. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: trapping the covalent enzyme-DNA complex in an active form. Biochemistry. 1987;26:4303–4309. doi: 10.1021/bi00388a018. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin EL, Byl JA, Osheroff N. Cobalt enhances DNA cleavage mediated by human topoisomerase II alpha in vitro and in cultured cells. Biochemistry. 2004;43:728–735. doi: 10.1021/bi035472f. [DOI] [PubMed] [Google Scholar]
- 18.Beese LS, Friedman JM, Steitz TA. Crystal structures of the Klenow fragment of DNA polymerase I complexed with deoxynucleoside triphosphate and pyrophosphate. Biochemistry. 1993;32:14095–14101. doi: 10.1021/bi00214a004. [DOI] [PubMed] [Google Scholar]
- 19.Sarnovsky RJ, May EW, Craig NL. The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. EMBO J. 1996;15:6348–6361. [PMC free article] [PubMed] [Google Scholar]
- 20.Peracchi A, Beigelman L, Scott EC, Uhlenbeck OC, Herschlag D. Involvement of a specific metal ion in the transition of the hammerhead ribozyme to its catalytic conformation. J. Biol. Chem. 1997;272:26822–26826. doi: 10.1074/jbc.272.43.26822. [DOI] [PubMed] [Google Scholar]
- 21.Curley JF, Joyce CM, Piccirilli JA. Functional evidence that the 3′-5′ exonuclease domain of Escherichia coli DNA polymerase I employs a divalent metal ion in leaving group stabilization. J. Am. Chem. Soc. 1997;119:12691–12692. [Google Scholar]
- 22.Viadiu H, Aggarwal AK. The role of metals in catalysis by the restriction endonuclease BamHI. Nat. Struct. Biol. 1998;5:910–916. doi: 10.1038/2352. [DOI] [PubMed] [Google Scholar]
- 23.Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 24.Brautigam CA, Steitz TA. Structural principles for the inhibition of the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I by phosphorothioates. J. Mol. Biol. 1998;277:363–377. doi: 10.1006/jmbi.1997.1586. [DOI] [PubMed] [Google Scholar]
- 25.Brautigam CA, Sun S, Piccirilli JA, Steitz TA. Structures of normal single-stranded DNA and deoxyribo-3′-S-phosphorothiolates bound to the 3′-5′ exonucleolytic active site of DNA polymerase I from Escherichia coli. Biochemistry. 1999;38:696–704. doi: 10.1021/bi981537g. [DOI] [PubMed] [Google Scholar]
- 26.Allingham JS, Pribil PA, Haniford DB. All three residues of the Tn 10 transposase DDE catalytic triad function in divalent metal ion binding. J. Mol. Biol. 1999;289:1195–1206. doi: 10.1006/jmbi.1999.2837. [DOI] [PubMed] [Google Scholar]
- 27.Sontheimer EJ, Gordon PM, Piccirilli JA. Metal ion catalysis during group II intron self-splicing: parallels with the spliceosome. Genes Dev. 1999;13:1729–1741. doi: 10.1101/gad.13.13.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galburt EA, Chevalier B, Tang W, Jurica MS, Flick KE, Monnat RJ, Jr, Stoddard BL. A novel endonuclease mechanism directly visualized for I-PpoI. Nat. Struct. Biol. 1999;6:1096–1099. doi: 10.1038/70027. [DOI] [PubMed] [Google Scholar]
- 29.Lukacs CM, Kucera R, Schildkraut I, Aggarwal AK. Understanding the immutability of restriction enzymes: crystal structure of BglII and its DNA substrate at 1.5 A resolution. Nat. Struct. Biol. 2000;7:134–140. doi: 10.1038/72405. [DOI] [PubMed] [Google Scholar]
- 30.Horton JR, Cheng X. PvuII endonuclease contains two calcium ions in active sites. J. Mol. Biol. 2000;300:1049–1056. doi: 10.1006/jmbi.2000.3938. [DOI] [PubMed] [Google Scholar]
- 31.Yean SL, Wuenschell G, Termini J, Lin RJ. Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature. 2000;408:881–884. doi: 10.1038/35048617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble CG, Maxwell A. The role of GyrB in the DNA cleavage-religation reaction of DNA gyrase: a proposed two metal-ion mechanism. J. Mol. Biol. 2002;318:361–371. doi: 10.1016/S0022-2836(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Arora K, Beard WA, Wilson SH, Schlick T. Critical role of magnesium ions in DNA polymerase beta's closing and active site assembly. J. Am. Chem. Soc. 2004;126:8441–8453. doi: 10.1021/ja049412o. [DOI] [PubMed] [Google Scholar]
- 34.Perry JJ, Yannone SM, Holden LG, Hitomi C, Asaithamby A, Han S, Cooper PK, Chen DJ, Tainer JA. WRN exonuclease structure and molecular mechanism imply an editing role in DNA end processing. Nat. Struct. Mol. Biol. 2006;13:414–422. doi: 10.1038/nsmb1088. [DOI] [PubMed] [Google Scholar]
- 35.Stahley MR, Strobel SA. Structural evidence for a two-metal-ion mechanism of group I intron splicing. Science. 2005;309:1587–1590. doi: 10.1126/science.1114994. [DOI] [PubMed] [Google Scholar]
- 36.Steitz TA. Visualizing polynucleotide polymerase machines at work. EMBO J. 2006;25:3458–3468. doi: 10.1038/sj.emboj.7601211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radhakrishnan R, Arora K, Wang Y, Beard WA, Wilson SH, Schlick T. Regulation of DNA repair fidelity by molecular checkpoints: “gates” in DNA polymerase beta's substrate selection. Biochemistry. 2006;45:15142–15156. doi: 10.1021/bi061353z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sontheimer EJ, Sun S, Piccirilli JA. Metal ion catalysis during splicing of premessenger RNA. Nature. 1997;388:801–805. doi: 10.1038/42068. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Strobel SA. Thiophilic metal ion rescue of phosphorothioate interference within the Tetrahymena ribozyme P4-P6 domain. RNA. 1999;5:1399–1407. doi: 10.1017/s135583829999115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshinari K, Taira K. A further investigation and reappraisal of the thio effect in the cleavage reaction catalyzed by a hammerhead ribozyme. Nucleic Acids Res. 2000;28:1730–1742. doi: 10.1093/nar/28.8.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo LY, Piccirilli JA. Leaving group stabilization by metal ion coordination and hydrogen bond donation is an evolutionarily conserved feature of group I introns. Biochim. Biophys. Acta. 2001;1522:158–166. doi: 10.1016/s0167-4781(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 42.Szewczak AA, Kosek AB, Piccirilli JA, Strobel SA. Identification of an active site ligand for a group I ribozyme catalytic metal ion. Biochemistry. 2002;41:2516–2525. doi: 10.1021/bi011973u. [DOI] [PubMed] [Google Scholar]
- 43.Das SR, Piccirilli JA. General acid catalysis by the hepatitis delta virus ribozyme. Nat. Chem. Biol. 2005;1:45–52. doi: 10.1038/nchembio703. [DOI] [PubMed] [Google Scholar]
- 44.Galburt EA, Stoddard BL. Restriction endonucleases: one of these things is not like the others. Nat. Struct. Biol. 2000;7:89–91. doi: 10.1038/72450. [DOI] [PubMed] [Google Scholar]
- 45.Dong KC, Berger JM. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature. 2007;450:1201–1205. doi: 10.1038/nature06396. [DOI] [PubMed] [Google Scholar]
- 46.Worland ST, Wang JC. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1989;264:4412–4416. [PubMed] [Google Scholar]
- 47.Elsea SH, Hsiung Y, Nitiss JL, Osheroff N. A yeast type II topoisomerase selected for resistance to quinolones. Mutation of histidine 1012 to tyrosine confers resistance to nonintercalative drugs but hypersensitivity to ellipticine. J. Biol. Chem. 1995;270:1913–1920. doi: 10.1074/jbc.270.4.1913. [DOI] [PubMed] [Google Scholar]
- 48.Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase II. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 49.Fortune JM, Dickey JS, Lavrukhin OV, Van Etten JL, Lloyd RS, Osheroff N. Site-specific DNA cleavage by Chlorella virus topoisomerase II. Biochemistry. 2002;41:11761–11769. doi: 10.1021/bi025802g. [DOI] [PubMed] [Google Scholar]
- 50.Deweese JE, Burgin AB, Osheroff N. Using 3′-bridging phosphorothiolates to isolate the forward DNA cleavage reaction of human topoisomerase IIα. Biochemistry. 2008;47:4129–4140. doi: 10.1021/bi702194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson RG. Acids and Bases. Science. 1966;151:172–177. doi: 10.1126/science.151.3707.172. [DOI] [PubMed] [Google Scholar]
- 52.Pecoraro VL, Hermes JD, Cleland WW. Stability constants of Mg2+ and Cd2+ complexes of adenine nucleotides and thionucleotides and rate constants for formation and dissociation of MgATP and MgADP. Biochemistry. 1984;23:5262–5271. doi: 10.1021/bi00317a026. [DOI] [PubMed] [Google Scholar]
- 53.Sigel RKO, Song B, Sigel H. Stabilities and structures of metal ion complexes of adenosine 5′-O-thiomonophosphate (AMPS2-) in comparison with those of its parent nucleotide (AMP2-) in aqueous solution. J. Am. Chem. Soc. 1997;119:744–755. [Google Scholar]
- 54.Dobbs ST, Cullis PM, Maxwell A. The cleavage of DNA at phosphorothioate internucleotidic linkages by DNA gyrase. Nucleic Acids Res. 1992;20:3567–3573. doi: 10.1093/nar/20.14.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aravind L, Leipe DD, Koonin EV. Toprim–a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 1998;26:4205–4213. doi: 10.1093/nar/26.18.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Changela A, DiGate RJ, Mondragon A. Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature. 2001;411:1077–1081. doi: 10.1038/35082615. [DOI] [PubMed] [Google Scholar]
- 57.Changela A, DiGate RJ, Mondragon A. Structural studies of E. coli topoisomerase III-DNA complexes reveal a novel type IA topoisomerase-DNA conformational intermediate. J. Mol. Biol. 2007;368:105–118. doi: 10.1016/j.jmb.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]