Abstract
Macromolecular conjugates of tamoxifen could perhaps be used to circumvent some of the limitations of the extensively used breast cancer drug. To test the feasibility of these conjugates, a 4-hydroxytamoxifen analog was conjugated to a diaminoalkyl linker and then conjugated to activated esters of a poly(methacrylic acid) polymer synthesized by atom transfer radical polymerization. A polymer conjugated to the 4-hydroxytamoxifen analog with a six carbon linker showed high affinity for both estrogen receptor alpha and estrogen receptor beta and potent antagonism of the estrogen receptor in cell-based transcriptional reporter assays. These results suggest that the conjugation of 4-hydroxytamoxifen to a polymer results in a macromolecular conjugate that can display ligand in a manner that can be recognized by estrogen receptor and still act as a potent antiestrogen in cells.
Introduction
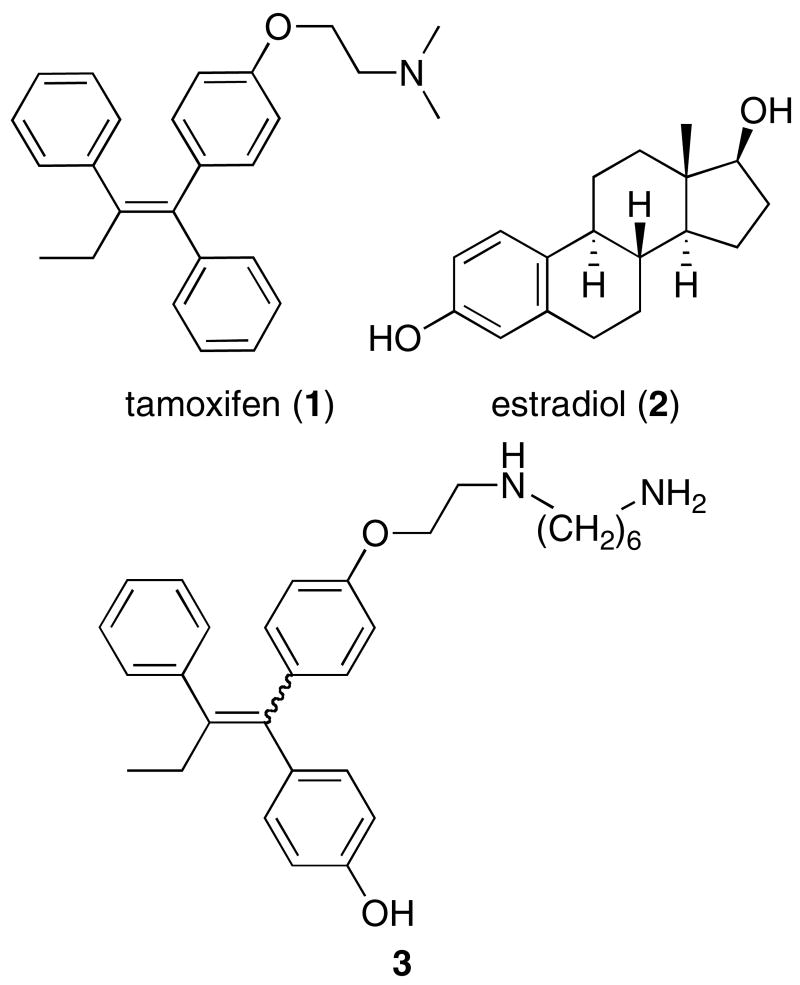
Tamoxifen (Figure 1, compound 1), one of the most effective anticancer drugs ever, acts as an antiestrogen in breast tissue, blocking the activity of endogenous estrogens, most notably estradiol (2), at the estrogen receptor (ER) 1. There are a number of issues that limit tamoxifen's effectiveness related to the effects of tamoxifen in other tissues. Tamoxifen acts as an estrogen in the uterus, which increases the risk of uterine proliferative disorders in women taking the drug. In addition, tamoxifen's antiestrogen activity in the central nervous system can lead to hot flashes, one of the common complaints from women taking tamoxifen 2. Finally, breast cancer cells usually develop resistance to tamoxifen despite the fact that the majority of antiestrogen-resistant tumors remain ER-positive 3. There have been a number of attempts to increase tamoxifen's effectiveness using macromolecular delivery agents such as liposomes and nanocapsules that package tamoxifen analogs inside of them 4, 5, but nothing has been attempted with covalently conjugated macromolecules. Tamoxifen could be conjugated to a macromolecule that maintains the drug's antiestrogen activity in breast tissue, but prevents it from crossing the blood-brain barrier 6, thus preventing tamoxifen from causing hot flashes. Not only could a covalent tamoxifen macromolecular conjugate potentially have different tissue targeting abilities, but it could also be used to potentially target different subcellular locations inside a cell. Increasing evidence suggests that estrogens and antiestrogens can elicit cellular responses such as the stimulation of kinase signaling cascades through mechanisms that arise from different locations in the cell such as the plasma membrane 7-10. The antiestrogenic effects of tamoxifen in the breast and the estrogenic effects of tamoxifen in the uterus appear to stem from different signal transduction pathways 11. A tamoxifen conjugate with the right selectivity could still maintain desired activity in the breast while avoiding deleterious side effects in the uterus. We report the development of a new set of chemical tools that incorporate tamoxifen analogs into poly(methacrylic acid) polymers to serve as high affinity, macromolecular probes of estrogen receptor function and discover that these large molecular weight conjugates still maintain nuclear receptor modulatory activity.
Figure 1.
Structure of tamoxifen (1), estradiol (2) and the 4-hydroxytamoxifen analog used for conjugation (3) The analog exists as a 1:1 mixture of E and Z isomers that readily interconvert
Experimental Procedures
General Procedures
All reagents were purchased from Sigma-Aldrich. Routine proton nuclear magnetic resonance spectra (1H NMR) were obtained on a Bruker DRX500 (500 MHz) instrument. 1H NMR chemical shifts are reported as δ values in parts per million (ppm) downfield from internal tetramethylsilane. NMR instruments were provided by the Shared Resource center of the Purdue Cancer Center. The plasmids used in the reporter gene assays, pSG5-ERα, pSG5-ERβ and estrogen response element (ERE)-luciferase, have been described elsewhere 12, 13. Polymer molecular weights were determined by hydrolyzing all activated ester side chains on a polymer sample with 1M NaOH, neutralizing with aqueous HCl and then analyzing the size of the poly(methacrylic acid) with Gel Permeation Chromatography (GPC) using a PL-aquagel-OH-30 (Polymer Laboratories) column upstream of a refractive index detector using water as the eluent. Mw was determined using the Cirrus polymer analysis program based on poly(ethylene glycol) standards. Analytical HPLC was performed using a 4.6 × 150 mm Agilent Eclipse XDB-C8 5 μm reverse phase column with signal detection at 280 nm. Preparative HLC was performed using a 25 × 250 mm Vydac C8 15 μm semi-preparative column with signal detection at 280 nm. Nonlinear regression analysis of binding and reporter assay curves was performed using Prism 4 software.
Poly(methacrylic acid) conjugated to 4-hydroxytamoxifen analog with six-carbon linker (5)
N-hydroxysuccinimide-activated methacrylate was polymerized under atom transfer radical polymerization conditions following a previous procedure to give polymer 4 with Mw=12503 g/mol and Mw/Mn=1.07 14. After dissolving 20 mg of 4-hydroxytamoxifen analog 315 in DMF (1 mL), 10 mg of polymer 4 was added along with triethylamine (0.01 mL) and the reaction mixture was stirred for 16 h at 60°C. After cooling to room temperature, the organic solvent was removed under reduced pressure and the remaining brown residue was dissolved in 1M NaOH (1mL). The solid was sonicated and stirred until fully dissolved and then the remaining solution was neutralized with 6 M HCl. The conjugate was then purified using preparative reverse phase HPLC using a water:acetonitrile gradient starting at 20% acetonitrile and ending at 100% acetonitrile after 70 minutes. The eluent also contained 0.1% trifluoroacetic acid. The percent incorporation of 4-hydroxytamoxifen ligand onto the polymer was determined to be 50% of the total side chains by comparing the NMR integration of the methyl peak of 4-hydroxytamoxifen with the methyl peak of the methacrylic acid polymer (see supporting information for more details). Compound 3 exists as a 1:1 mixture of E and Z isomers that readily interconvert at room temperature. It has no impact on biological activity 15, 16. NMR indicates that a similar ratio of E and Z isomers of the 4-hydroxytamoxifen analog are coupled to the polymer. 1H NMR (500 MHz)(d6-DMSO): δ 8.05 (d), δ 7.95 (d), δ 7.12 (m),δ 7.05 (d), δ 6.88 (dd) δ 6.65 (dd) δ 6.55 (t) δ 6.32 (d), δ 4.05 (t), δ 3.86 (t), δ 3.5 (m), δ 3.0 (m), δ 2.35 (m), δ 1.9-1.5 (br m) δ 1.5-1.3 (br m), δ 1.1-0.8 (br m), δ 0.75 (t).
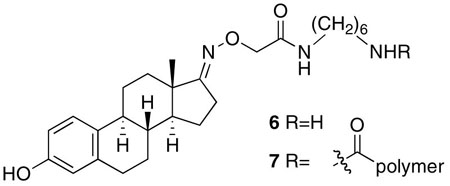
N-[6-aminohexyl]-2-({[3-hydroxyestra-1(10),2,4-trien-17-ylidene]amino}oxy)acetamide (6)
The synthesis of the estrone-17-(O-carboxymethyl)oxime attached to 1,6 diaminohexane was accomplished following previous reports.17 1H NMR (500 MHz, CDCl3/CD3OD (4:1 v/v): δ 7.28 (d, J=8.5 Hz, 1H), δ 6.78 (dd, J=8.5, 2.6 Hz, 1H), δ 6.72 (d, J=2.6 Hz, 1H) δ 4.62, (s, 2H), δ 3.50-3.40 (m, 4H), δ 3.1-2.7 (m, 8H), δ 2.6-2.35 (m, 2 H), δ 2.2-2.0 (m, 4H), δ 1.9-1.4 (m, 12H), δ 1.13 (s, 3H).
Poly(methacrylic acid) conjugated to estrone-17-(O-carboxymethyl)oxime analog with six-carbon linker (7)
The synthesis of conjugate 7 was identical to the synthesis of tamoxifen conjugate 5, but with 15 mg of NHS-activated poly(methacrylate) ester and 30 mg of the estrone-17-(O-carboxymethyl)oxime 6. Identical workup and hydrolysis conditions and purification by preparative reverse phase HPLC yielded 1 mg of pure conjugate. Comparative NMR integration of backbone protons to estrone protons revealed that 50% of the side chains were conjugated to the estrone analog. 1H NMR (500 MHz, (CD3)2SO (4:1 v/v): δ 7.2-6.9 (m), δ 6.6-6.4 (m), δ 5.7 (s), δ 3.0-2.0 (m), δ 1.4-1.2 (m), δ 0.8 (s).
pH stability studies
Conjugates were diluted to a concentration of 1 mM in 10 mM sodium phosphate buffer at 6 different pH values (1.2, 3.0, 7.0, 8.2, 11.33, 12.7) and incubated at 37 °C for 48 hours. At 0, 24 and 48 hours, aliquots at each pH were taken and diluted to 10 μM in water. The diluted samples were then analyzed for the release of compound 3 by analytical HPLC using the same gradient as described in the preparative HPLC purification of the conjugates above.
Radiolabeled ligand receptor binding assay
In each well of a 96 well plate, 25 μL of a solution containing binding buffer (100 mM potassium phosphate (pH 7.4), 100 μg/mL bovine gamma globulin, and 0.02% sodium azide) plus 2 nM [2,4,6,7,16,17-3H]estradiol (GE Healthcare) and different concentrations of competitor were added to a 25 μL solution containing 30 nM of full length recombinant estrogen receptor alpha or beta (Invitrogen) in binding buffer. After a two hour incubation at room temperature, the whole mixture was transferred to a MultiScreen™ HTS Filter Plate (Millipore) and unbound ligand was washed away with binding buffer. Plates were dried overnight, then Microscint 0™ scintillation fluid (Perkin Elmer) was added and the radioactivity was counted on a Packard TopCount scintillation counter. Data were analyzed using Prism software.
Cell Culture and Luciferase Reporter Assays
The ER-negative MDA-MB-231 breast cell line was grown in phenol red free RPMI 1640 medium supplemented with 0.876 g/L glutamine, 100 mg/L streptomycin sulfate, 100 units/mL of penicillin G and 10% fetal bovine serum (FBS) at 37 °C in an air/carbon dioxide (95:5) atmosphere. Transfection assays were run with the same media conditions except serum free media was used.
MDA-MB-231 cells were plated in 24 well plates (2 × 106 cells per plate). Transfections were performed according to the protocol for Lipofectamine 2000™. In order to normalize for the transfection efficiency in each well, the dual luciferase system was used in which a constitutively expressed, chemically orthogonal luciferase expression vector was also transfected. The total amount of DNA/well for each plasmid was as follows: pSG5-ERα or pSG5-ERβ 0.3 μg/well, ERE-luciferase 0.3 μg/well, and Renilla-luciferase 0.1 μg/well. The ratio of total DNA/Lipofectamine 2000™ ratio was 1:3. After transfection, the plates were incubated at 37 °C for 6 hours before replacing the media with serum free media for 24 hours. The serum free media was then replaced with fresh serum free media and the ligands. All ligands were delivered in DMSO or ethanol and the total concentration of organic solvent in each was 0.1% or less. For competition experiments, the ligand was added to media already containing 10 nM estradiol. After 18 hours, the cells were lysed and assayed for dual luciferase activity in a Packard TopCount luminometer according to the protocol provided by Promega (Madison, WI). The relative light units (RLU) were then calculated by dividing the output of the ERE-driven luciferase in each well by the output of the Renilla luciferase. Each drug concentration was tested in triplicate and each competition experiment was repeated at least three times.
Results and Discussion
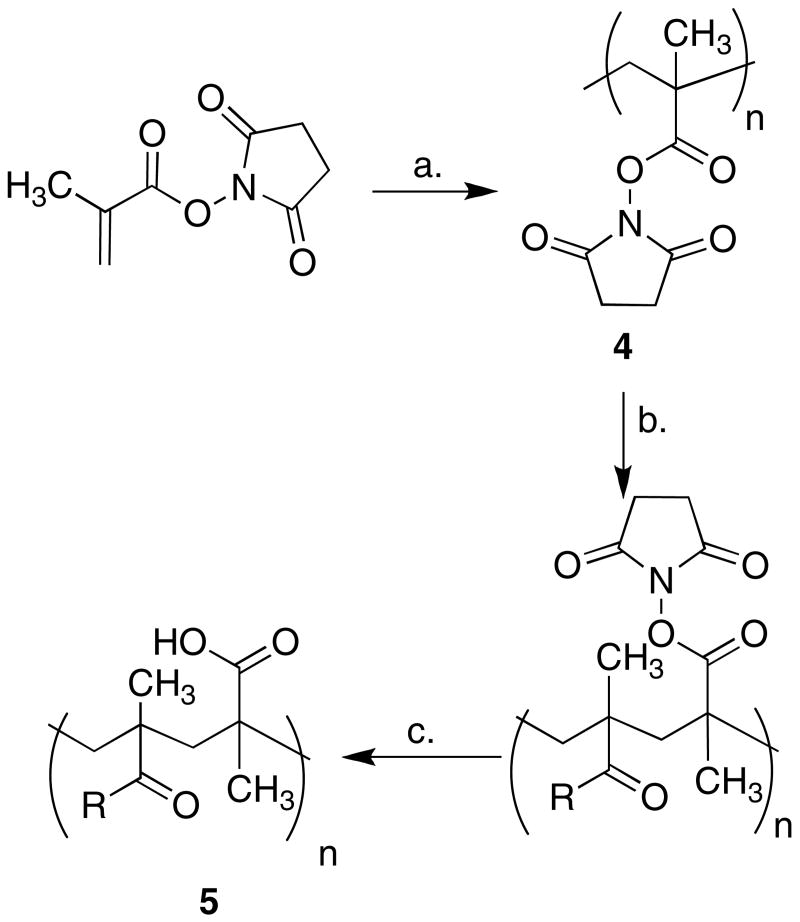
In designing the tamoxifen-polymer probes, we took inspiration from other bioactive polymer conjugates 7, 18, 19 and opted for a non-proteinaceous scaffold that could be synthesized with well-defined size and chemical reactivity. Poly(methacrylic acid) was chosen as the scaffold due to its ease in coupling reactions and its ability to be synthesized with a narrow molecular weight range using controlled radical polymerization 20. With a goal of preparing a polymer-tamoxifen conjugate that was approximately the same size as a small peptide hormone, a scaffold was synthesized from N-hydroxysuccinimide activated ester monomer units using atom transfer radical polymerization (ATRP)14. A polymer scaffold with a molecular weight of approximately 12,500 and a polydispersity index of 1.07 was synthesized with N-hydroxysuccinimide activated ester side chains well suited for facile attachment of ER ligands.
Following the general conjugation principle of attaching the ER ligands to the polymer through a flexible alkyl linker, analogs of the more potent tamoxifen metabolite, 4-hydroxytamoxifen (OHT), were synthesized with a diaminoalkyl group extending from the basic side chain. Previous work has shown that a six carbon diamine-containing compound (Figure 1, compound 3) was a high affinity ligand for the estrogen receptor in vitro and a potent antagonist of estrogen receptor transcription in cell-based experiments 15. Compound 3 had a Ki value of 10 ± 6 nM for binding to full-length recombinant ER alpha in a fluorescent ligand competition binding assay compared to 6.3 ± 0.2 nM for estradiol and 8 ± 5 nM for 4-hydroxytamoxifen. It also acted as an antagonist of estradiol-activated transcriptional activation in an estrogen response element-controlled luciferase reporter gene assay in the ER negative HeLa cell line transfected with an expression vector for ER alpha with an IC50 value of 80 ± 50 nM 15.
For the synthesis of conjugate 5, a roughly equimolar ratio of ligand was used compared to the number of reactive side chains on the polymer. The activated ester-containing polymer was conjugated to 4-hydroxytamoxifen analog 3 and the remaining unconjugated side chains were hydrolyzed to carboxylic acids to give conjugate 5 (Scheme 1). Since the free 4-hydroxytamoxifen analog is a potent antiestrogen on its own, great care was taken to ensure that no unconjugated ligand was present in the polymer samples. Preparative reverse phase HPLC was found to be the best purification method to remove all unconjugated ligand. After purification, the extent of conjugation of ligand was measured using NMR integration by comparing the integration of the peak corresponding to the methyl group of 4-hydroxytamoxifen with the integration of the peak corresponding to the methyl groups on the polymer backbone (see supporting information for more details). The resulting conjugate showed approximately 50% of the side chains were conjugated with ligand, indicating that either the coupling reaction was incomplete or that not all of the side chains on the polymer are reactive.
Scheme 1.
Synthesis of polymer conjugates.a
a Reagents and conditions: (a) 2-bromo-2-methyl-(2-hydroxyethyl) propanoate, CuBr, 2,2′-bipyridine, DMSO, 100 °C. (n=150); (b) 3, NEt3, DMF, 90 °C, 72 h; (c) 1M NaOH, 12 h.
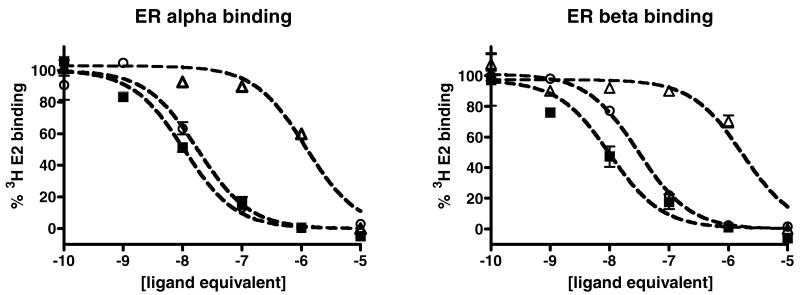
The polymer conjugate was then tested for its ability to bind to ER alpha and ER beta using a radiolabeled estradiol competition assay with purified recombinant receptor. In these assays, shown in Figure 2, the conjugate was able to bind to both estrogen nuclear receptors. The concentrations on the x-axis of the binding curves correspond to the concentration of the conjugated 4-hydroxytamoxifen analog as determined by comparing the NMR integration of ligand peaks to polymer backbone peaks (see Supplemental Information for more details). The IC50 values were 11 ± 9 nM for ER alpha and 35 ± 10 nM for ER beta. In the same assay, the unconjugated ligand (compound 3) had IC50 values of 15 ± 5 nM for ER alpha and 9 ± 5 nM for ER beta. For comparison, estradiol was found to have IC50 values of 0.5 ± 0.1 nM for ER alpha and 0.3 ± 0.3 for ER beta and 4-hydroxytamoxifen had IC50 values of 1 ± 0.3 nM for ER alpha and 8 ± 5 nM for ER beta. The values for estradiol and 4-hydroxytamoxifen are consistent with other reports in the literature 21. The conjugate shows dramatically improved affinity compared to protein-based steroid hormone conjugates. Commercially available bovine serum albumin (BSA)-estradiol conjugates have been used to target membrane estrogen receptors, but their use has been highly controversial with reports of high levels of unconjugated steroid, slow binding to the estrogen receptor, poor affinity for the receptor in vitro (IC50 levels greater than 100 nM) and unusual biological responses that are possibly due to the BSA portion of the conjugate 22, 23. The poly(methacrylic acid)-tamoxifen conjugate was purified by HPLC to prevent the presence of any unconjugated ligand and did not need to be pre-incubated with the receptor before the radioligand was added to see competition for binding. Overall, the binding affinity of the conjugates was equal or slightly worse than the binding affinity of the unconjugated analog and the affinity was sufficiently potent to be useful in biological assays. The lack of any loss of affinity suggests that the linker between the polymer and the conjugated ligand is long enough to prevent unfavorable interactions from occurring between the receptor and the polymer backbone.
Figure 2.
Binding of compound 3 (black squares), conjugate 5 (open circles) and control conjugate 7 (open triangles) to ER alpha (left) and ER beta (right) as measured using a radiolabled ligand competition binding assay. The lines represent the best fit to a one binding site competition model using non-linear regression analysis.
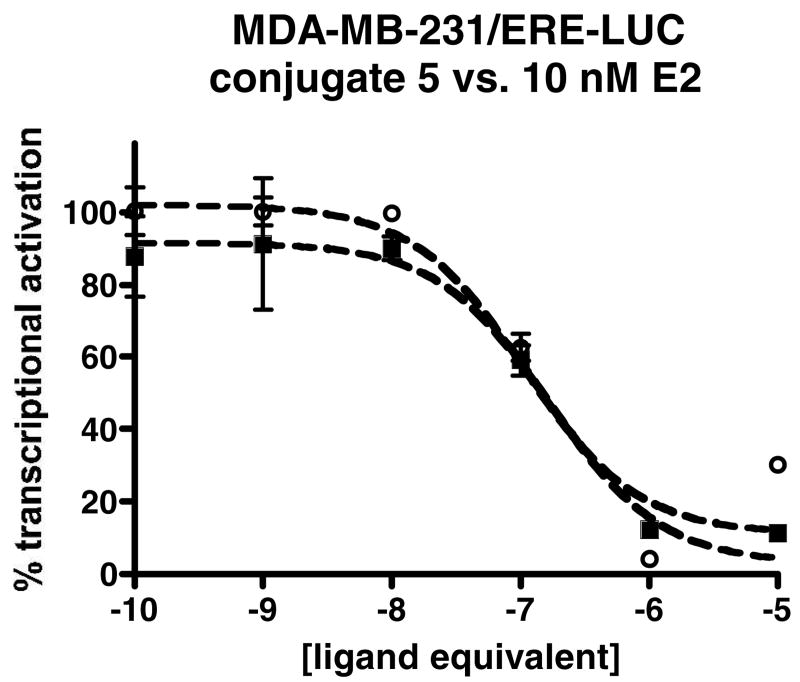
One interesting feature of the other conjugates of estrogen receptor ligands is their reported cellular activity. The estradiol-protein conjugates have been reported to localize to the plasma membrane and the estradiol-dendrimer conjugate localized to the cytoplasm in ER-positive cells 7,22-24. None of the conjugates were capable of activating estrogen-receptor mediated transcription, although their ER antagonist effects were not tested. To test the effect of the hydroxytamoxifen-polymethacrylate conjugate on ER-mediated transcription, the ER negative breast cancer cell line MDA-MB-231 was transiently transfected with an expression vector for human ERα or human ERβ and a reporter plasmid containing the luciferase gene controlled by a classic estrogen response element-containing promoter sequence. After transfection, the cells were treated for 18 hours with different concentrations of conjugate and then the amount of luciferase expression was measured using a luminescence assay. Compound 3, conjugate 5 and the unconjugated poly(methacrylic acid) were unable to activate transcription as agonists at an ERE-controlled promoter. However, conjugate 5 was able to act as an antagonist of estradiol-stimulated transcription (Figure 3). The conjugate inhibited transcriptional activity induced by 10 nM estradiol in ER alpha transfected cells with an IC50 of 80 ± 70 nM and in ER beta transfected cells with an IC50 of 25 ± 10 nM. This is roughly the same as the unconjugated ligand 3 alone and approximately 50 fold worse than 4-hydroxytamoxifen. No activity was seen in MDA-MB-231 cells transfected with only the reporter plasmid and no estrogen receptor expression vector, suggesting dependence on the presence of estrogen receptor. To the best of our knowledge, this is the first time that a macromolecular conjugate of a hormone has been reported to modulate a nuclear receptor's transcriptional activity.
Figure 3.
Competition of conjugate 5 versus 10 nM estradiol in transient transfection assays of MDA-MB-231 cells with an ERE driven luciferase reporter gene and either ER alpha (closed squares) or ER beta (open circles) The numbers are plotted on the y-axis as the percent signal compared to the activation with 10 nM estradiol alone. Curve represents the best fit to a single-site competition binding model. See experimental procedures section for more details.
Considering that unmodified polymethacrylic acid conjugates are usually not taken into the cell to a high degree, let alone transported to the nucleus, the antagonist activity of our hydroxytamoxifen-polymer conjugate was unexpected. The polyanionic nature of the polymer is usually masked as amides or by polycationic binding partners before the polymer can be taken efficiently into cells and then uptake-enhancing peptides are usually also included to get significant uptake 25-27. One alternative explanation for the transcriptional activity of the polymer conjugate is that free hydroxytamoxifen analog is present in the sample or is being released by conjugate degradation. Considering that HPLC analysis showed that no free ligand was present, it is unlikely that the biological activity of the polymers is due to unconjugated ligand present from the beginning of the experiment. It does not, however, rule out possible degradation of the conjugate by cells and release of the drug. Similar inhibitory profiles are seen with the conjugate under both serum-rich and serum-free conditions, suggesting that there are no serum components causing conjugate degradation. We have not yet been able to completely rule out the possibility that the 4-hydroxytamoxifen analog is released after cleavage by some sort of membrane-associated hydrolase. While amide bonds can be hydrolyzed, they are generally not considered to be a linkage of choice for biodegradable linkers, especially considering the relatively short time frame of the experiments 28. In addition, amide bonds have been used with other steroid conjugates that have not modulated transcriptional activity or shown any form of degradation 29-31. To test the overall stability of the conjugates at different pH values, the conjugate was incubated at 37 °C for 2 days at 6 different pH values ranging from 1.3 to 12.7 and then analyzed by HPLC for hydrolyzed ligand. There was no ligand release seen at any of the pH values except for 12.7, which showed complete breakdown of the conjugate into free 4-hydroxytamoxifen analog and poly(methacrylic acid). This suggests that the conjugates are very stable under cellular conditions present in our assays.
Even though the conjugate is stable, it does not necessarily have to bind to the receptor to exert its effects. The conjugate is generally amphiphilic in nature and it is possible that it could be sequestering free estradiol through some sort of noncovalent aggregation. To rule out this possibility, another conjugate was synthesized linking an estrone-17-(O-carboxymethyl)oxime analog (6) to the polymer through a six carbon linker. This analog has been shown previously to be an effective ligand for a biotin conjugate, but only when the two nitrogens on the alkyl linker were methylated.17 In the absence of N-methylation, the analog binds poorly to estrogen receptor. Analog 6 has a very similar predicted partition coefficient compared to 4-hydroxytamoxifen analog 3, so conjugates of analog 6 should be useful in testing the role of general hydrophobicity. If the conjugates do act by sequestering free estradiol, the potencies of the two conjugates should be approximately the same. If the conjugate is acting by direct ligand binding to the estrogen receptor, then conjugate 5 should be much better than the estrone-17-(O-carboxymethyl)oxime-containing conjugate. The polymer conjugate with the estrone-17-(O-carboxymethyl)oxime was synthesized and purified in a similar manner as before to generate conjugate 7 with a similar extent of ligand incorporation. When tested in the ER assays, conjugate 7 was much less potent than conjugate 5 with IC50 values in the binding assay of 1.4 ± 0.9 μM for ER alpha and 2.6 ± 1 μM for ER beta. This is approximately 100 fold worse than conjugate 5. In the luciferase assay, conjugate 7 was also less potent with IC50 values of 4 ± 1 μM for ER alpha transfected cells and 1 ± 0.6 μM for ER beta transfected cells. This difference in affinity that reflects the differences in affinity of the unconjugated ligands strongly suggests that the action of the conjugates on the receptor is specific. As a result, we believe that the conjugate somehow can enter cells and bind to the receptor intact or it is being cleaved by a novel enzymatic process and releasing drug inside the cell. In either case, this conjugate represents a new paradigm in delivering antiestrogens to tumor cells.
In summary, a new type of ER-targeting conjugate has been developed that is capable of rapid, high-affinity binding to ER alpha and ER beta in vitro. The conjugate is also a potent antagonist of estrogen receptor –mediated transcription in live cells. Studies to investigate the conjugate's mechanism of antagonism, uptake and localization as well as the possible therapeutic significance are ongoing. The highly adaptable nature of atom transfer radical polymerization to synthesize polymer backbones as well as the apparent ability of these polymers to antagonize responses associated with the nucleus makes this scaffold well-suited to make different steroid hormone-polymer conjugates for use as chemical probes in the growing area of steroid conjugate research.
Supplementary Material
NMR spectra of the polymer conjugates are available. Examples of the determination of the extent of conjugation and determination of purity are also included. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
The American Cancer Society (IRG-58-006-41A), the Army Breast Cancer Research Program (BC030507), the American Association of Colleges of Pharmacy and the National Institutes of Health (R01 DK075376) supported this work. An IRL Fellowship from the National Science Foundation supported JPT. Purdue Research Foundation supported ELR.
References
- 1.Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205–13. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5(3):207–13. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 3.Normanno N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, Perrone F. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer. 2005;12(4):721–47. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 4.Maillard S, Ameller T, Gauduchon J, Gougelet A, Gouilleux F, Legrand P, Marsaud V, Fattal E, Sola B, Renoir JM. Innovative drug delivery nanosystems improve the anti-tumor activity in vitro and in vivo of anti-estrogens in human breast cancer and multiple myeloma. J Steroid Biochem Mol Biol. 2005;94(13):111–21. doi: 10.1016/j.jsbmb.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Zeisig R, Teppke AD, Behrens D, Fichtner I. Liposomal 4-hydroxy-tamoxifen: effect on cellular uptake and resulting cytotoxicity in drug resistant breast cancer cells in vitro. Breast Cancer Res Treat. 2004;87(3):245–54. doi: 10.1007/s10549-004-8699-6. [DOI] [PubMed] [Google Scholar]
- 6.Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2(1):108–19. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 8.Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19(6):1606–17. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- 9.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 10.Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67(6):471–5. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281(36):26683–92. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- 12.Weatherman RV, Carroll DC, Scanlan TS. Activity of a tamoxifen-raloxifene hybrid ligand for estrogen receptors at an AP-1 site. Bioorg Med Chem Lett. 2001;11(24):3129–31. doi: 10.1016/s0960-894x(01)00646-1. [DOI] [PubMed] [Google Scholar]
- 13.Weatherman RV, Clegg NJ, Scanlan TS. Differential SERM activation of the estrogen receptors (ERalpha and ERbeta) at AP-1 sites. Chem Biol. 2001;8(5):427–36. doi: 10.1016/s1074-5521(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 14.Godwin A, Hartenstein M, Muller AH, Brocchini S. Narrow Molecular Weight Distribution Precursors for Polymer-Drug Conjugates. Angew Chem-Int Ed. 2001;40(3):594–597. doi: 10.1002/1521-3773(20010202)40:3<594::AID-ANIE594>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Trebley JP, Rickert EL, Reyes PT, Weatherman RV. Tamoxifen-based Probes for the Study of Estrogen Receptor-Mediated Transcription. In: Jaroch S, Hilmar W, editors. In Chemical Genomics: Small Molecule Probes to Study Cellular Function. Vol. 58. Springer; Berlin: 2006. pp. 76–87. [DOI] [PubMed] [Google Scholar]
- 16.Robertson DW, Katzenellenbogen JA, Hayes JR, Katzenellenbogen BS. Antiestrogen basicity--activity relationships: a comparison of the estrogen receptor binding and antiuterotrophic potencies of several analogues of (Z)-1,2-diphenyl-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-butene (tamoxifen, Nolvadex) having altered basicity. J Med Chem. 1982;25(2):167–71. doi: 10.1021/jm00344a015. [DOI] [PubMed] [Google Scholar]
- 17.Muddana SS, Peterson BR. Facile synthesis of CIDS: biotinylated estrone oximes efficiently heterodimerize estrogen receptor and streptavidin proteins in yeast three hybrid systems. Org Lett. 2004;6(9):1409–12. doi: 10.1021/ol0497537. [DOI] [PubMed] [Google Scholar]
- 18.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc. 2002;124(50):14922–33. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 19.Griffith BR, Allen BL, Rapraeger AC, Kiessling LL. A polymer scaffold for protein oligomerization. J Am Chem Soc. 2004;126(6):1608–9. doi: 10.1021/ja037646m. [DOI] [PubMed] [Google Scholar]
- 20.Lutz JF, Neugebauer D, Matyjaszewski K. Stereoblock copolymers and tacticity control in controlled/living radical polymerization. J Am Chem Soc. 2003;125(23):6986–93. doi: 10.1021/ja029517w. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JÅ. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 22.Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology. 1999;140(11):5455–5458. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi Y, Koslowski M, Bodenner DL. Binding of estrogen receptor with estrogen conjugated to bovine serum albumin (BSA) Nucl Recept. 2004;2(1):5. doi: 10.1186/1478-1336-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulayeva NN, Gametchu B, Watson CS. Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids. 2004;69(3):181–92. doi: 10.1016/j.steroids.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen KD, Nori A, Tijerina M, Kopeckova P, Kopecek J. Cytoplasmic delivery and nuclear targeting of synthetic macromolecules. J Control Release. 2003;87(13):89–105. doi: 10.1016/s0168-3659(02)00352-8. [DOI] [PubMed] [Google Scholar]
- 26.Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Deliv Rev. 2005;57(4):609–36. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Wolfert MA, Dash PR, Nazarova O, Oupicky D, Seymour LW, Smart S, Strohalm J, Ulbrich K. Polyelectrolyte vectors for gene delivery: influence of cationic polymer on biophysical properties of complexes formed with DNA. Bioconjug Chem. 1999;10(6):993–1004. doi: 10.1021/bc990025r. [DOI] [PubMed] [Google Scholar]
- 28.Ulbrich K, Subr V. Polymeric anticancer drugs with pH-controlled activation. Adv Drug Deliv Rev. 2004;56(7):1023–50. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 29.De Goeij AF, van Zeeland JK, Beek CJ, Bosman FT. Steroid-bovine serum albumin conjugates: molecular characterization and their interaction with androgen and estrogen receptors. J Steroid Biochem. 1986;24(5):1017–31. doi: 10.1016/0022-4731(86)90355-9. [DOI] [PubMed] [Google Scholar]
- 30.Godeau JF, Schorderetslatkine S, Hubert P, Baulieu EE. Induction of Maturation in Xenopus-Laevis Oocytes by a Steroid Linked to a Polymer. Proc Nat Acad Sci USA. 1978;75(5):2353–2357. doi: 10.1073/pnas.75.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussey SL, Peterson BR. Efficient delivery of streptavidin to mammalian cells: clathrin-mediated endocytosis regulated by a synthetic ligand. J Am Chem Soc. 2002;124(22):6265–73. doi: 10.1021/ja0258733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NMR spectra of the polymer conjugates are available. Examples of the determination of the extent of conjugation and determination of purity are also included. This material is available free of charge via the Internet at http://pubs.acs.org.